1. Introduction
Biomolecular templating is fundamental in the development of advanced biosensors, bioreactors, affinity chromatographic separation materials, and many diagnostics such as those used in cancer therapeutics [
1,
2,
3]. In this regard, biomolecules are used to precisely position nanoscale materials onto substrates. Since single molecules are by far too small to direct the formation of (supramolecular) complex shapes and patterns, the ordered structures of biological molecules and assemblies are excellent templates for constructing inorganic nanostructures and devices. In addition, genetic control over the template properties and coupling could bring to the forefront new platforms and material functionalities that will advance both biotechnology and nanoscale engineering.
Bacterial cell surface layers (S-layers) are two dimensional protein lattices that cover the cell surface of many Bacteria and Archaea [
4]. These lattices are composed of identical (glyco)protein subunits, exhibit oblique (p1, p2), square (p4), or hexagonal (p3, p6) symmetry and are very porous. Due to the monomolecular structure of the lattices, the pores are identical in size and morphology, ranging between 2 and 8 nm [
5].
The conditions required for extraction and disintegration have shown that S-layers are held together and onto the supporting envelope by non-covalent forces including hydrogen or ionic bonds, and hydrophobic or electrostatic interactions [
6,
7,
8,
9]. Isolated S-layer subunits maintain this inherent property of self-assembling in protein lattices. After detachment from the cell surface and disruption into monomers using chaotropic (e.g., guadinium hydroclorid or urea in the case of Gram-positive bacteria) agents, S-layers are able to reassemble into regular lattices, identical to those observed on intact cells upon removal of the chemical [
10,
11]. Reassembly can occur in solution, on solid supports such as silicon wafers, gold chips, and glass, on lipid films, and at the air/water interface [
12]. The property of regular structure formation as well as the metal binding properties of S-layers have been considered for bionanotechnological applications [
13]. Moreover, their utilization can be extended by the design of functional recombinant S-layer fusion proteins. By genetic modifications, S-layers have been given specific functions. For instance, an S-layer fusion protein consisting of the S-layer protein SbpA and a synthetic analogue of the B-domain of protein A capable of binding the fragment crystallisable (Fc) part of immunoglobulin G (IgG) was constructed and used for coating biocompatible microbeads for generating a specific adsorbent, which should find clinical application in the treatment of various autoimmune diseases [
14]. Furthermore, for the treatment of IgE-mediated allergies by construction of vaccines, Bet v1 allergen was fused to the S-layer protein SbsC of
Bacillus stearothermophylus ATCC 12980 and the fusion protein proved to be fully functional [
15].
The mature S-layer protein SslA of
Sporosarcina ureae ATCC 13881 is processed from a 1097 amino acid (aa) long precursor by the cleavage of a 30 aa signal peptide and self-assembles into protein lattices that exhibit square (p4) symmetry with a lattice constant of 12.2 nm [
16,
17]. The S-layer has a complex pattern of pores and gaps that are approximately 2 nm wide [
18]. In vitro, the recombinant mature SslA self-assembles into micrometer sized, crystalline monolayers on silicon wafers, while in solution not only sheets but tube like structures have also been observed [
17]. With various truncated SslA forms cloned, expressed, and purified from
E. coli, it could be demonstrated that the central SslA domain of this S-layer protein is self-sufficient for the self-assembly [
17]. In a previous study, a fusion protein consisting of streptavidin (aa 16-133) and the central SslA part (aa 341-925) was briefly presented [
19]. In this work the cloning and expression of this chimeric protein (SslA
341-925streptavidin) is described in detail and the protein is further characterized with respect to its self-assembling properties in solution and on a silicon wafer. Moreover, the electrophoretic mobility shift assay (EMSA) and transmission electron microscopy (TEM) are used to prove the accessibility of the streptavidin domain in the fusion protein lattice.
3. Discussion
Recombinant fusion proteins incorporating functional domains of distinct proteins could be relevant not only in drug development, but also in biotechnological applications such as the bio-functionalization of surfaces. The ability of isolated bacterial S-layer protein subunits to self-assemble into protein layers on solid surfaces makes them an almost ideal biological template for bio(nano)patterning. In this study an S-layer fusion protein that combines the remarkable self-assembling property of the S-layer moiety with the ligand binding function i.e., biotin binding function, of the streptavidin molecule is described and characterized.
In the design of this fusion protein, an important prerequisite was the preserved self-assembly potential of the S-layer part. A previous study about this S-layer protein showed that the central part of SslA (aa 341-925) is sufficient for in vitro self-assembly into monolayers exhibiting the p4 lattice structure [
17]. Furthermore, the insertion of the functional domain has to be done at favourable positions in the polypeptide chain so that the whole fusion protein preserves the self-assembling ability and presents the additional function of the fused protein domain. The position for a functional fusion was selected based on the results of previous studies showing that usually the C-terminus of S-layers can be modified without affecting the protein properties and function, and on the fact that SslA despite the lack of its C-terminal part, has a preserved self-assembly potential [
20]. In this context, streptavidin was fused to the C-terminus of the SslA
341-925. Fusion of streptavidin to a truncated SslA version—in this case to the central part of SslA—constitutes an advantage over the fusion to the whole SslA sequence because it is shorter and in this sense, the chance that the fusion protein will be correctly folded is higher. Previous studies have shown that residues 16-133 of streptavidin are enough for biotin binding [
21]. For fusion, this minimal sized form of streptavidin, that still retained the full biotin-binding activity, was used.
The SslA
341-925streptavidin fusion protein could be stably expressed in
E. coli and isolated from the soluble fraction of these cells. In the literature it was reported that streptavidin fused to the C-terminal of the S-layer protein of
Geobacillus stearothermophilus PV72/p2 (SbsB) accumulated in the soluble fraction as well [
22]. Without the S-layer fusion partner, streptavidin was found in inclusion bodies in
E. coli cells. The His
6 tag on the N-terminal of the SslA
341-925streptavidin allowed an easy purification of this protein via Ni affinity chromatography. In addition to the band corresponding to the fusion protein, another one was also detected after purification. This might represent a degradation product. Similar results have been reported by Posseckardt J. [
23].
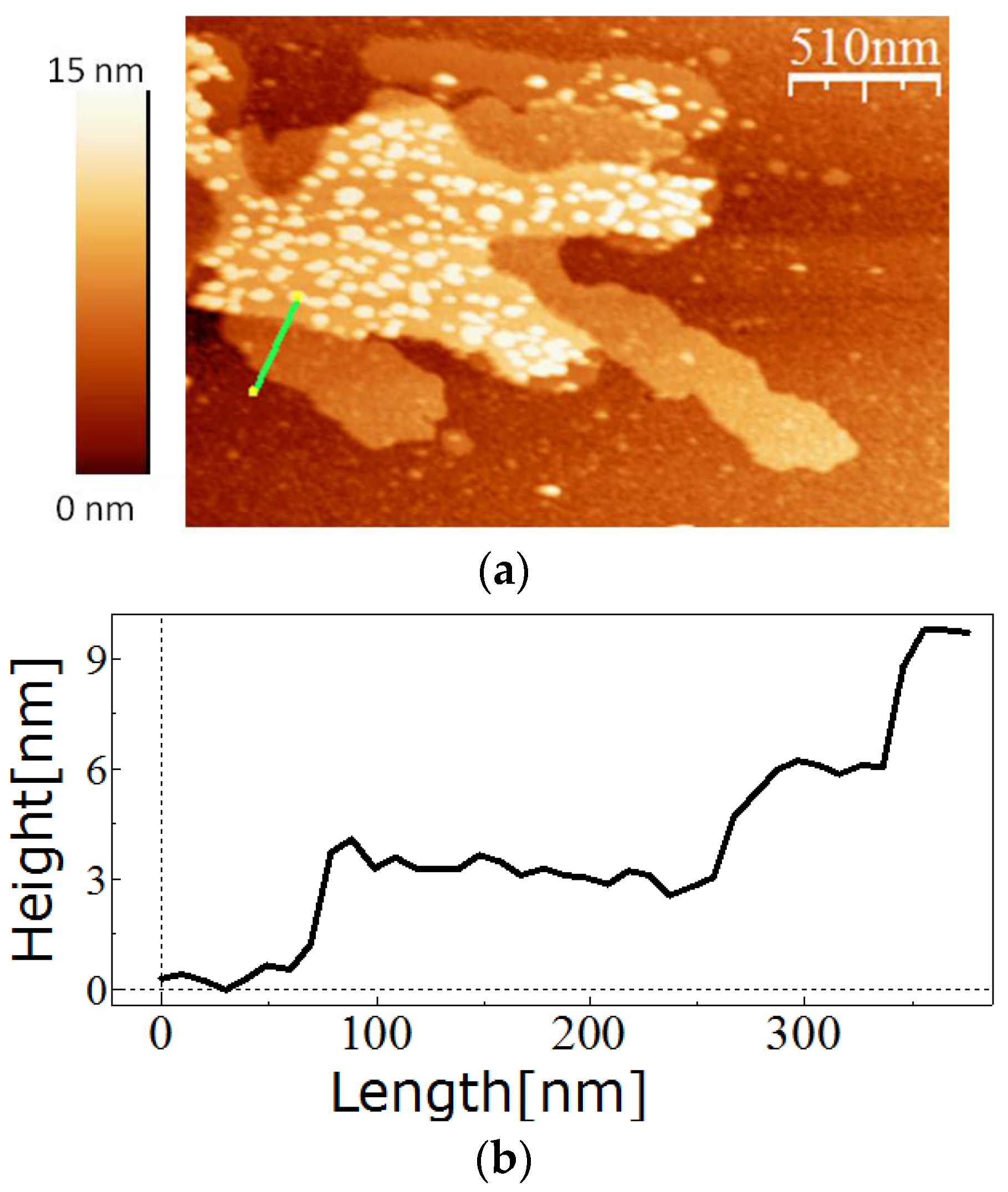
Similar to the wild-type or full length recombinant SslA, the SslA341-925streptavidin chimeric protein could self-assemble in solution and on functionalised silicon surfaces demonstrating that fusion of the streptavidin domain does not interfere with the self-assembling properties of this S-layer. The monitored self-assembly products had the form of multi-layered sheets or monolayer cylinders.
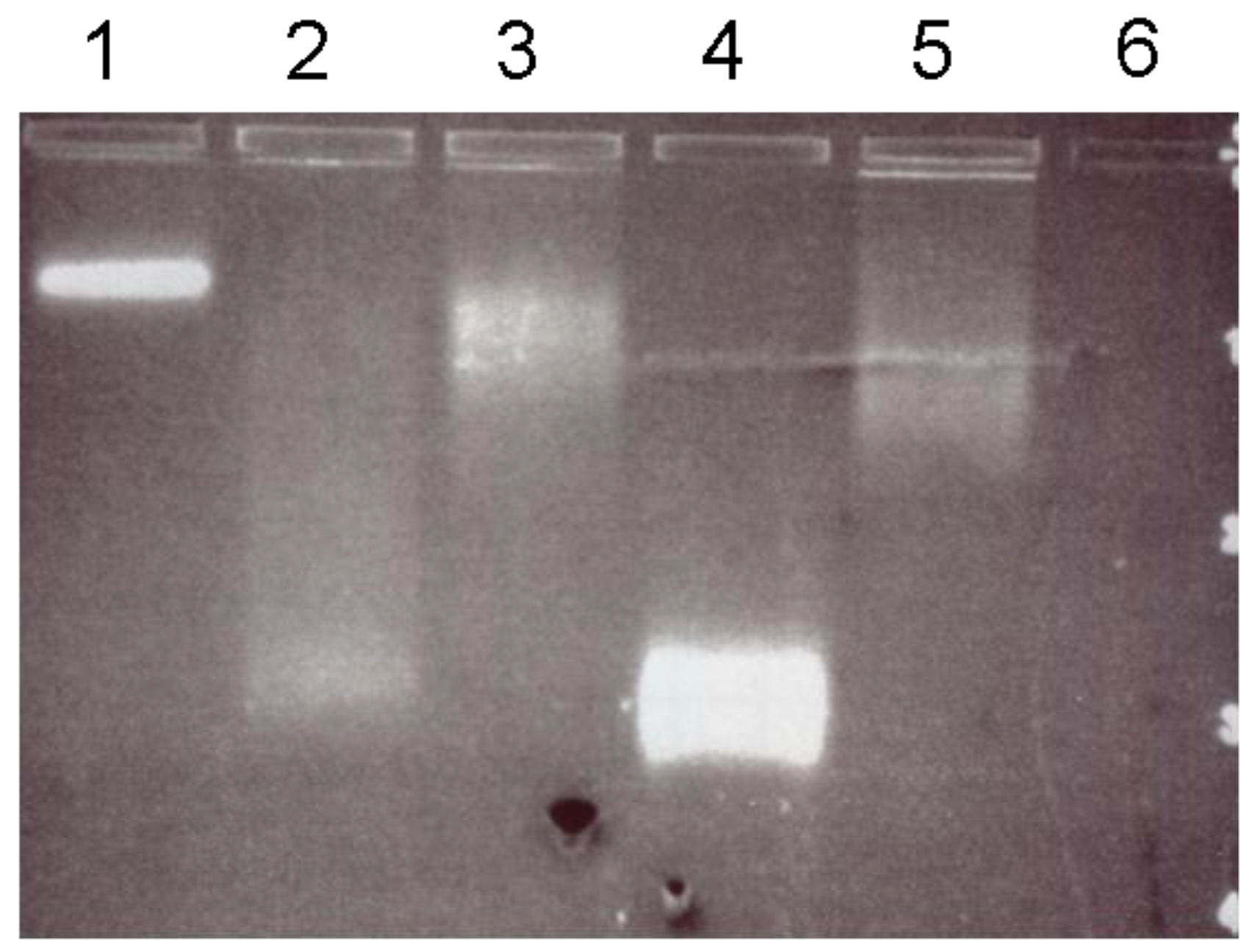
Since the streptavidin domain used for fusion descended from wild-type streptavidin, it was assumed to show the typical high affinity for biotin. However, it remained to be investigated whether the biotin binding sites remain exposed and sterically available after the fusion or if they are blocked by the S-layer partner. In the mobility assay (gel electrophoresis technique) the interaction between SslA
341-925streptavidin protein monomers and biotinylated quantum dots was monitored by applying a constant electric field and following the diffusion of the charged molecules in the porous gel matrix. Interestingly, the quantum dot/SslA
341-925streptavidin fusion protein complexes were not too large to enter the gel. Other studies reporting about the EMSA of QD/DNA complexes have evidenced that QDs completely inhibited the DNA from moving towards the positive electrode when the QDs/DNA was just at or excessive stoichiometry [
24]. The phenomenon was accounted to the negative charges of DNA that were counteracted by the positively charged QDs or to the large size of the newly formed complexes unable to enter the gel. This problem was not experienced during the EMSA of SslA
341-925streptavidin and biotinylated quantum dots; the difference in the migration pattern of these moieties proves a successful binding reaction. Another work implying the fusion of the same streptavidin domain to the S-layer protein of
Bacillus stearothermophylus ATCC 12980 (SbsC) could not determine the functionality of the fused streptavidin domain despite testing with EMSA of biotinylated DNA and the S-layer/streptavidin chimeric protein [
23]. The reasons suggested included the inability of the fusion protein to bind biotinylated molecules, the large size of the DNA molecules (4300 bps long) in comparison to the fusion protein, and the tetrameric structure of streptavidin with the biotin binding site lying at the interface of the 4 subunits [
25,
26].
A further prove of the preserved biotin binding ability of the SslA
341-925streptavidin are the TEM images of the negatively stained, patterned protein layers, which reveal that the biotinylated quantum dots are bound to the protein template. On tubular structures, a more accentuated marginal deposition of quantum dots is observed. The substrate onto which the fusion protein has been immobilised might also play a role. Due to the O
2 plasma treatment step, the carbon coated Cu grid surface can be regarded as being more hydrophilic, therefore the most important driving force for the protein adsorption are the long range electrostatic interactions occurring between the positively charged sites on the fusion protein and the negative charges of the substrate. As a result, the SslA
341-925streptavidin adsorbs in a way such that the streptavidin moiety remains exposed. However when the protein lattice folds into tube like structures, the streptavidin domain becomes buried and no longer accessible to the biotinylated quantum dots. Therefore no particles are bound onto these protein regions. Marginal deposition is possible though, either due to the fact that the streptavidin domains are located at the edges of the protein sheets, or because during folding, tubes do not close completely, but the layer edges simply meet each other. It is further interesting to note that only the S-layer part of the fusion protein did not fold into tube like structures [
17]. Coupling the S-layer to the streptavidin domain indeed results in extension of the protein. This extended protein structure allows the adoption of a certain curvature when assembled into protein sheets.
In conclusion, the SslA341-925streptavidin fusion protein self-assembles into uniform, coherent layers in solution and on silicon wafers. This remarkable property facilitates the formation of a two-dimensional protein array with repetitive features in the nanometer range that exposes streptavidin. Due to the high affinity between streptavidin and biotin and the fact that nearly each molecule can be biotinylated, the SslA341-925streptavidin protein template can be considered a functional biomolecular matrix for immobilizing biotinylated molecules in a controlled manner.
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
Sporosarcina ureae ATCC 13881 cells were grown at 30 °C in S ureae growth medium I (17.01 g Na3PO4·12H2O, 1.28 g glucose, 1.0 g yeast extract, 10 g peptone, 5.28 g (NH4)2SO4) while continuously agitating at 200 rpm until OD600 = 1.5, which is within the logarithmic growth phase.
E. coli cells were grown in liquid at 37 °C under continuous agitation at 140 rpm. For selection of bacterial transformants, ampicillin was added to the media (final concentration 110 μg/mL). For bacterial gene expression in E. coli expression strains, cultures were grown until an optical density OD600 = 0.4 and were afterwards induced with isopropyl β-D-1 thiogalactopyranoside (IPTG) (1 mM final concentration).
4.2. Cloning of SslA341-925streptavidin
The SslA341-925streptavidin was created by overlap extension PCR. In a first step, the DNA sequence encoding SslA341-925 was amplified using the oligonucleotide primers Afor 5′-CGCGGCCATATGGGCGTTAAAAAAGCAGGAAT and Crev 5′-GTACCAGGTGCCGGTGATGCCCGAACTAATAACTAATGCATTTGCAGTTG. Next, streptavidin (aa 16-133) was amplified by PCR with primers Bfor 5′-ATGCATTAGTTATTAGTTCGGGCATCACCGGCACCTGGTA and Drev 5′-CACTAGCTCGAGCACCTTGGTGAAGGTGTCGTGG. The two amplified DNA fragments were mixed in a third PCR reaction for obtaining the chimeric gene SslA341-925streptavidin, which was then cloned into the pET46 Ek/Lic (Novagen, Darmstadt, Germany) vector using the Ek/Lic cloning strategy. The recombinant plasmid pET46Ek/Lic-SslA341-925streptavidin was transformed by electroporation with a Gene Pulser II (voltage: 2.5 kV; capacity: 25 μF; resistance: 200 Ω, BIORAD, München, Germany) into E. coli Nova Blue Giga Singles (Novagen, Darmstadt, Germany) competent cells as a non-expression host.
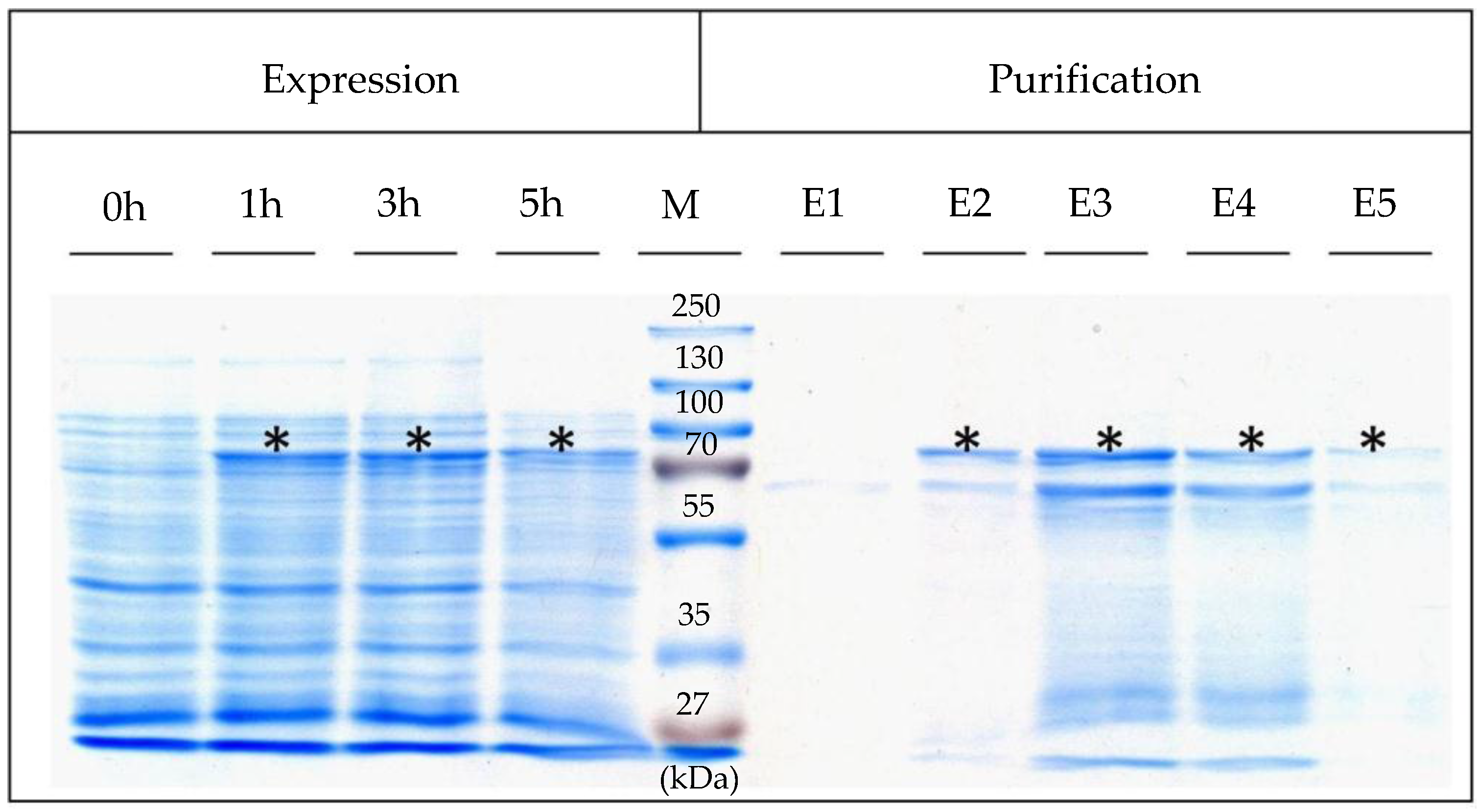
4.3. Expression, Isolation, and Purification of SslA341-925streptavidin
For heterologous expression, the pET46Ek/Lic-SslA
341-925streptavidin plasmid was transformed into
E. coli Rosetta Blue (DE3) cells. For expression, 0.5 L of Luria-Bertani (LB) medium was inoculated with 5 mL of the LB-grown preculture of
E. coli Rosetta Blue (DE3). The cultures were grown at 37 °C until an optical density OD
600 = 0.4, and then recombinant gene expression was induced with 1 mM IPTG. 5 h after the induction of expression, cells were harvested by centrifugation (10,000×
g for 10 min), washed twice with ddH
2O, and the pellet resuspended in 20 mL buffer consisting of 20 mM Tris/HCl (pH = 7.9), 0.5 M NaCl, 5 mM imidazol (AppliChem, Darmstadt, Germany), 10 mg lysozyme (New England Biolabs, Frankfurt, Germany) and 1 mM AEBSF (AppliChem, Darmstadt, Germany). After incubation at 30 °C for 2 h under continuous shaking at 300 rpm, the cell suspension was chilled on ice for 15 min. Then, Triton X-100 (Sigma-Aldrich, München, Germany) was added in a final concentration of 0.5% (
v/
v) and the cells were broken up by sonication (Bandelin Sonopuls UW 2070, Berlin, Germany). Following sonication, RNase A and DNase I (Roth, Karlsruhe, Germany) were given to the mixture and incubated at 30 °C for 15 min under continuous shaking followed by centrifugation at 13,000×
g for 10 min in order to separate the insoluble cell fraction. The supernatant was applied to a Ni affinity chromatography purification column (Macherey-Nagel, Düren, Germany) and purified as described in [
19]. Five elution fractions were collected and immediately dialysed against ultrapure water at 4 °C overnight. Expression was controlled by SDS-PAGE of cell extracts performed according to Laemmli (1970) [
27].
4.4. In Vitro Recrystallisation in Solution and on a Silicon Wafer of SslA341-925streptavidin
In vitro recrystallisation experiments were conducted in solution and on a Si substrate. In the first case, the purified SslA341-925streptavidin fusion protein (at a concentration of 1 mg/mL) was monomerized with 5 M GuHCl which was subsequently removed by dialysis against distilled water for 3 h at 4 °C. After dialysis, the protein solution was centrifuged at 14,000× g for 10 min and the supernatant containing the SslA341-925streptavidin monomers was diluted to 0.1 mg/mL with 0.5 mM Tris/HCl buffer pH = 9 and 0.1 mM CaCl2 until a total volume of 10 mL. 5 mL of this mixture was pipetted into a cell culture well and the fusion protein was allowed to recrystallize for 48 h. After recrystallisation, protein samples were taken out and prepared for TEM.
For in vitro recrystallisation on a silicon substrate, 0.5 mg of the purified SslA341-925streptavidin protein was dissolved in 5 M GuHCl, and then 1/5 of the solution was dialysed against Tris/HCl buffer (pH = 3) for 2 h at 4 °C in the presence of an APTES-functionalised silicon substrate. After dialysis, the silicon substrate was washed with distilled water, dried on air, and the self-assembly structures analysed by AFM.
4.5. Preparation of APTES Functionalised Silicon Substrates
Silicon pieces were prepared according to [
19].
4.6. Investigation of Biotin-Binding Ability of SslA341-925streptavidin
Qdot Biotin Conjugates (CdSe/ZnS coated with a polymer shell and conjugated to biotin) in H2O were purchased from Invitrogen, Carlsbad, CA, USA and were used as received. According to the manufacturer’s specifications, the size of the Qdot Biotin conjugate is 10–12 nm and the emission wavelength maximum is near 605 nm.
For binding of biotinylated quantum dots to the SslA341-925streptavidin monomers, 1 mg of purified fusion protein was monomerized with 5 M GuHCl followed by removal of the chemical by a dialysis step against distilled water for 2 h at 4 °C. After dialysis, the protein solution was centrifuged for 10 min at 14,000× g and the supernatant containing the fusion protein monomers was mixed with quantum dots. Mixtures containing different amounts of the fusion protein (5, 15 and 50 μg), 20 mM quantum dots (CdSe/ZnS coupled to biotin, purchased from Invitrogen), and 10 μL 20 mM KHPO4 (pH = 6.4) were incubated for 2 h at room temperature, then separated on a 1% agarose gel at 80 V for 2 h.
For the binding studies, at first chimeric protein layers were formed by in vitro recrystallisation in solution and afterwards were immobilised onto plasma treated carbon-coated copper grids and allowed to adsorb for 1 h. Biotinylated quantum dots (at a concentration of 0.02 nM in 20 mM KHPO4 buffer) were allowed to adsorb for 60 min onto the S-layer sheets. Excess particles were washed away with several drops of TBS buffer (0.2M Tris/HCl, pH = 7.4, 1.37 M NaCl) and the samples were additionally stained with 2% uranyl formiate for 20 s.
4.7. Atomic Force Microscopy
The measurements were performed in tapping mode in air using the Extended Multimode AFM with Nanoscope IIIa controller system Digital Instruments, Inc. Veeco Metrology, Santa Barbara, CA, USA). Silicon tips that were 130 µm long were used for imaging. The images were analyzed using the software WsxM (Nanotech Electronica S.L., Madrid, Spain).
4.8. Transmission Electron Microscopy
Self-assembly products were prepared for imaging as follows: formvar/carbon films on copper grids (PLANO, Wetzlar, Germany) were plasma treated (O2 SPI Plasma Prep II, West Chester, USA) for 10 s in order to make them hydrophilic. Directly thereafter, 10 µL of fusion protein solution was applied onto the grid and left to adsorb for 1 h. Afterwards, the grid was washed with distilled water and the protein stained with 7 µL of 2% uranyl formiate. After staining, the whole grid was blotted with filter paper and dried on air. Transmission electron micrographs were obtained on a Zeiss LIBRA200FEG (Oberkochen, Germany) using 200 kV acceleration voltage.