Cytotoxicity of Silver Nanoparticle and Chitin-Nanofiber Sheet Composites Caused by Oxidative Stress
Abstract
:1. Introduction
2. Results
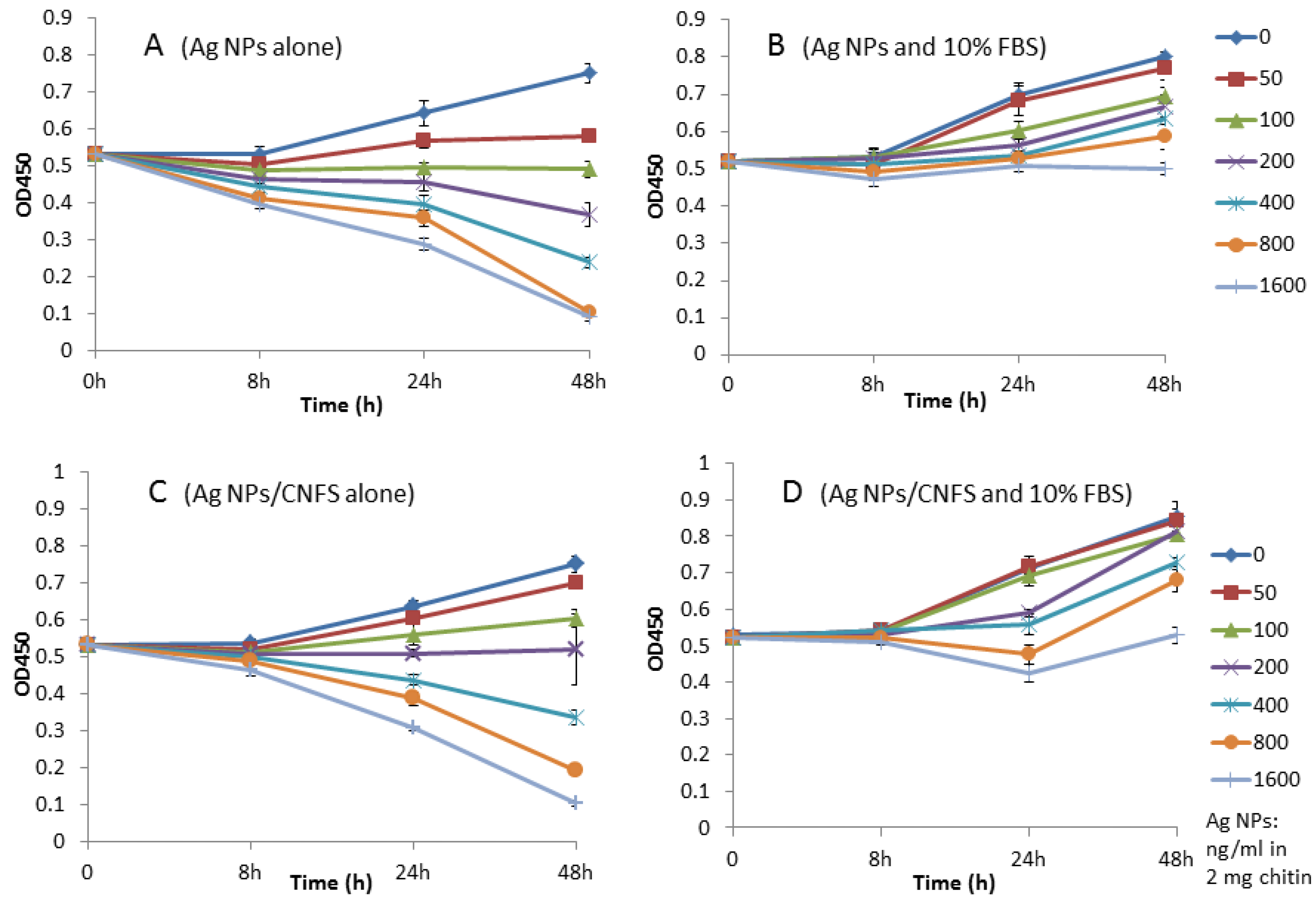
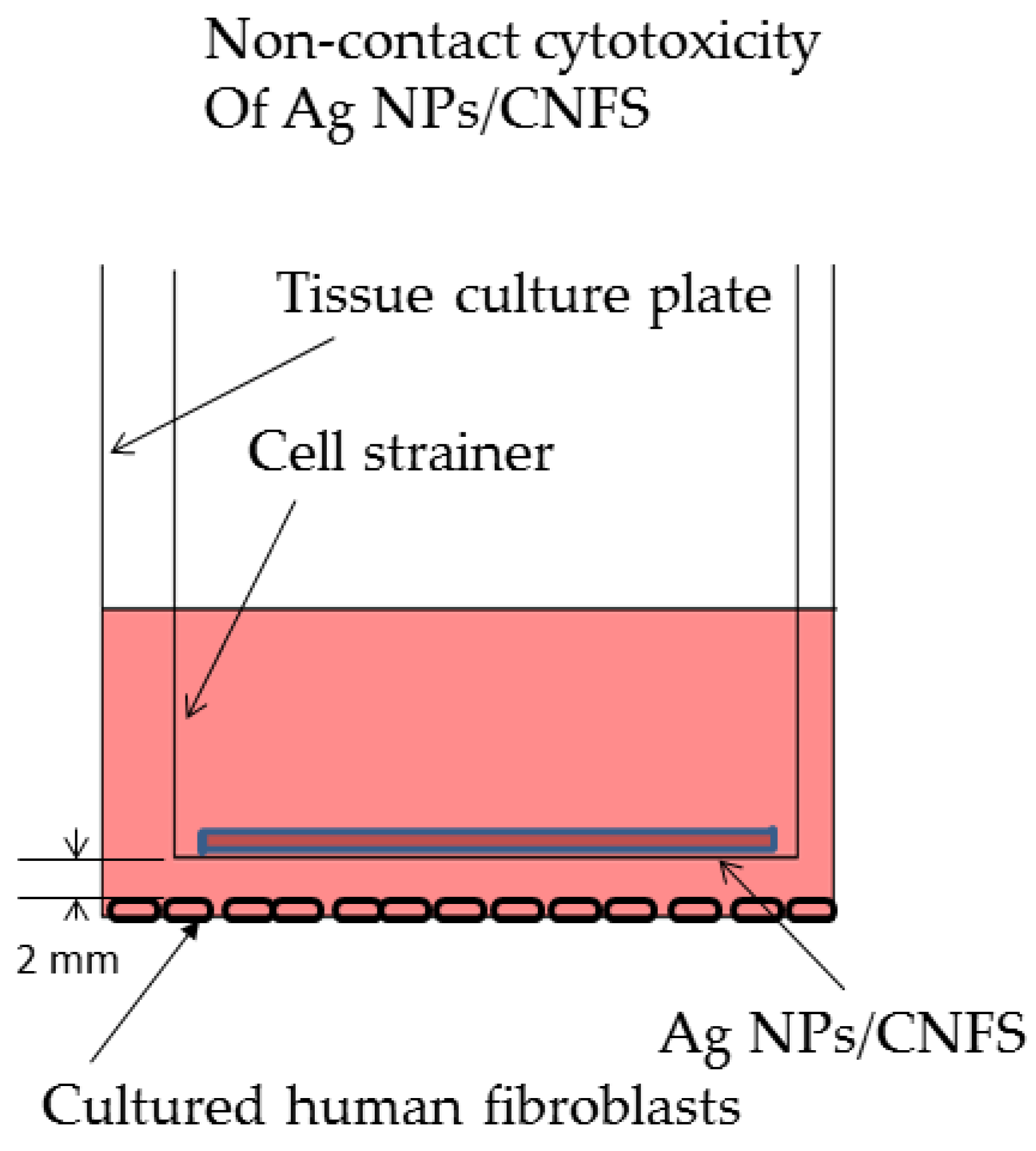
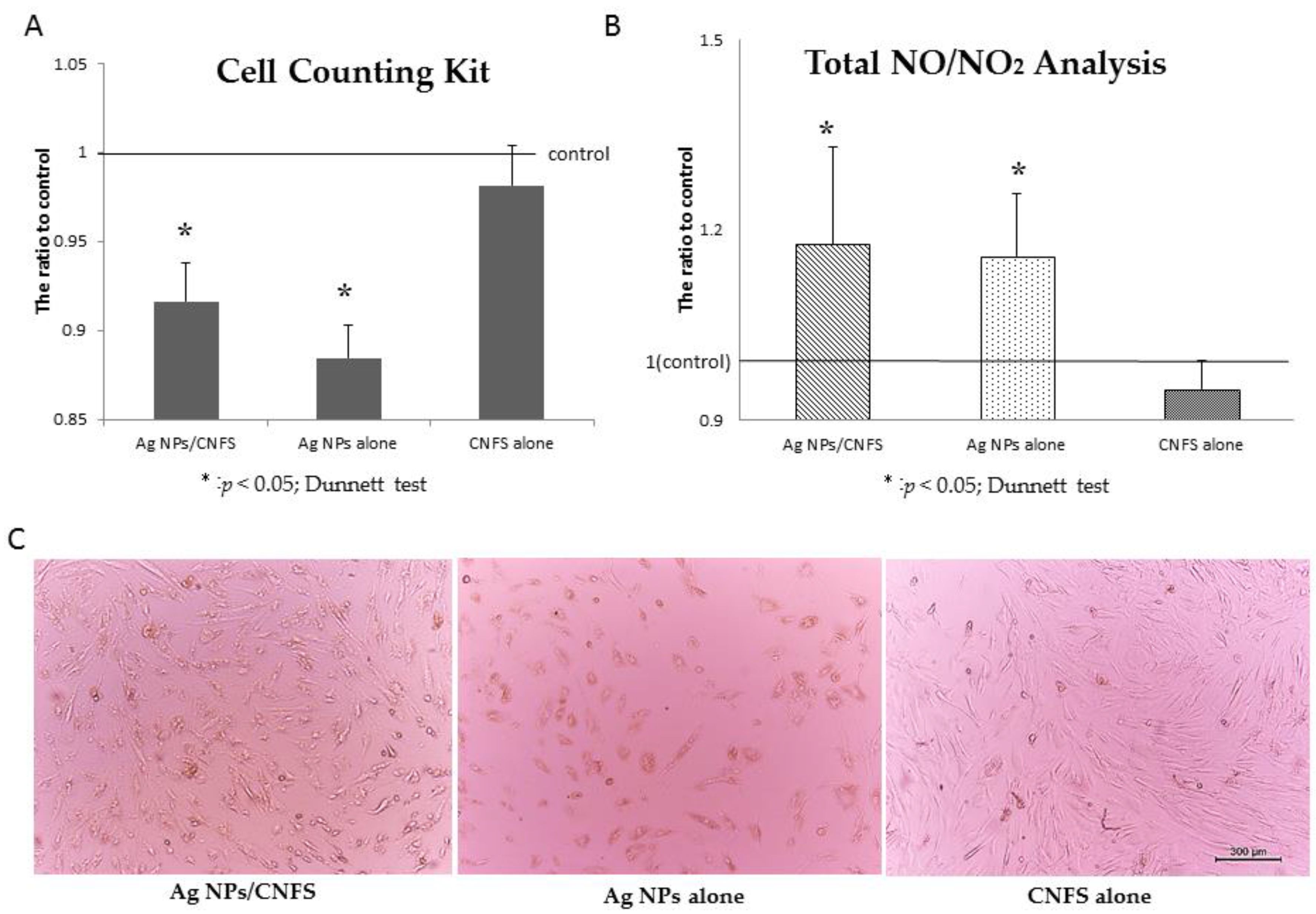
2.1. Cell Proliferation Assay and NO/NO2 Measurement In Vitro
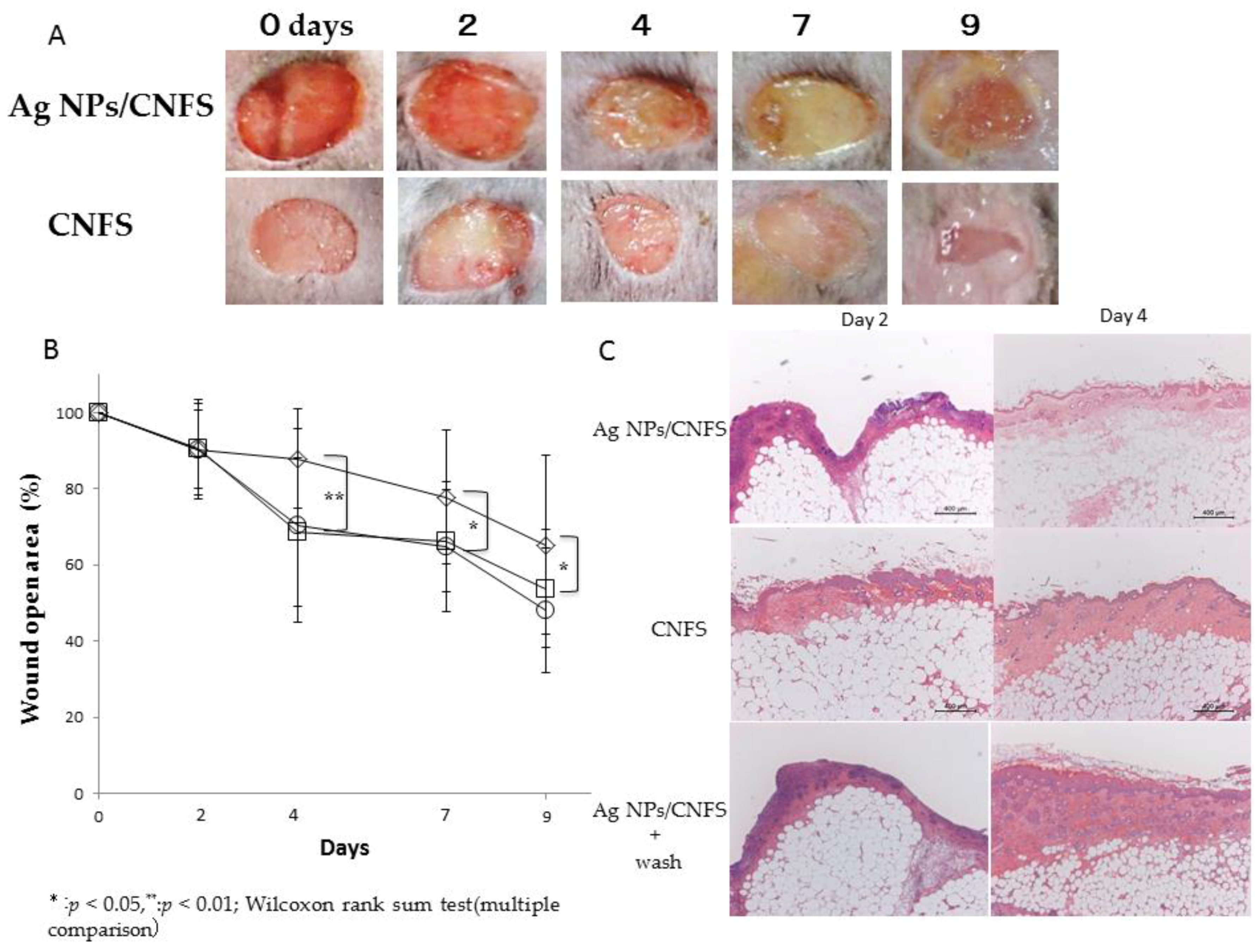
2.2. Wound Closure and Histochemical Analyses
2.3. Carbonyl Protein
3. Discussion
4. Materials and Methods
4.1. Preparation of Ag NPs and Ag NPs/CNFS
4.2. Cell Proliferation Assay and NO/NO2 Measurement In Vitro
4.3. In Vivo Study
4.3.1. Wound Closure Analyses
4.3.2. Histochemistry
4.3.3. Measurements of Carbonyl Protein
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ag NPs/CNFS | silver nanoparticles/chitin-nanofiber sheet composites |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| NO/NO2 | nitric oxide/nitrogen dioxide |
| FBS | heat-inactivated fetal bovine serum |
| DMEM | Dulbecco’s modified Eagle’s medium |
References
- Ahmed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmocol. 2008, 233, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Delima, R.; Seabra, A.B.; Duran, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Komarneni, S.; Li, D.; Newalkar, B.; Katsuki, H.; Bhalla, A.S. Microwave-polyol process for Pt and Ag nanoparticles. Langmuir 2002, 18, 5959–5962. [Google Scholar] [CrossRef]
- Mori, Y.; Tagawa, T.; Fujita, M.; Kuno, T.; Suzuki, S.; Matsui, T.; Ishihara, M. Simple and environmentally friendly preparation and size control of silver nanoparticles using an inhomogeneous system with silver-containing glass powder. J. Nanopart. Res. 2011, 13, 2799–2806. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Ishihara, M.; Mori, Y.; Nakamura, S.; Kishimoto, S.; Hattori, H.; Fujita, M.; Kanatani, Y.; Ono, T.; Miyahira, Y.; et al. Preparation of size-controlled silver nanoparticles and chitin-based composites and their antimicrobial activities. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Nguyen, V.Q.; Ishihara, M.; Nakamura, S.; Hattori, H.; Ono, T.; Miyahira, Y.; Matsui, T. Interaction of silver nanoparticles and chitin powder with different sizes and surface structures: The correlation with antimicrobial activities. J. Nanomater. 2013, 2013. [Google Scholar] [CrossRef]
- Wise, J.P.; Goodale, B.C.; Wise, S.S.; Craig, G.A.; Pongan, A.F.; Walter, R.B.; Thompson, W.D.; Ng, A.K.; Aboueissa, A.M.; Mitani, H. Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquat. Toxicol. 2010, 97, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Braydich-Stolle, L.K.; Lucas, B.; Schrand, A.; Murdock, R.C.; Lee, T.; Schlager, J.J.; Hussain, S.M.; Hofmann, M.C. Silver nanoparticles disrupt GDNF/Fyn kinase signaling in spermatogonial stem cells. Toxicol. Sci. 2010, 116, 577–589. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Mun, G.L.K.; Hande, P.; Valiyaveetti, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Dang, D.A.; Autrup, H. Cytotoxicity and genotoxicity of silver nanoparticles in human lung cancer cell line, A549. Arch. Toxicol. 2011, 85, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2008, 19, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Hackenberg, S.; Scherzed, A.; Kessier, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, J.E.; Cho, J.; Chung, K.H.; Park, K.; Yi, J.; Ryu, D.Y. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitro 2009, 23, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef] [PubMed]
- Vlachou, E.; Chip, E.; Shale, E.; Wilson, Y.T.; Papini, R.; Molemen, N.S. The safety of nanocrystalline silver dressings on burns: A study of systemic silver absorption. Burns 2007, 33, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Trop, M.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Silver coated dressing Acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J. Trauma 2006, 60, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Hattori, H.; Nakamura, S. A review on biomedical applications of chitosan-based biomaterials. Int. J. Pharm. Biol. Sci. 2015, 6, 162–178. [Google Scholar]
- Mi, F.L.; Tan, Y.C.; Liang, H.F.; Sung, H.W. In vivo biocompatibility and degradability of a novel injectable-chitosan-based implant. Biomaterials 2002, 23, 181–191. [Google Scholar] [CrossRef]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides. A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Ishihara, M.; Ozeki, Y.; Deguchi, H.; Sato, H.; Saito, Y.; Yura, H.; Sato, M.; Kikuchi, M.; Kurita, A.; et al. Experimental evaluation of photocrosslinkable chitosan as a biological adhesive with surgical application. Surgery 2001, 130, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Amano, Y.; Nogami, Y.; Takase, B.; Ishihara, M. Hemostasis for severe hemorrhage with photocrosslinkable chitosan hydrogel and calcium alginate. Ann. Biomed. Eng. 2010, 38, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Ishihara, M.; Fujita, M.; Kishimoto, S.; Kanatani, Y.; Ishizuka, T.; Nogami, Y.; Nakamura, S.; Tanaka, Y.; Maehara, T. Hemostatic effects of photocrosslinkable chitosan hydrogel-mixed photocrosslinked chitosan sponges (PCM-S) on hepatic bleeding in rats. Artif. Org. 2010, 34, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Yamada, H.; Tanaka, I.; Kaba, N.; Matsuura, M.; Okumura, M.; Kadosawa, T.; Fujinaga, T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials 1999, 20, 1407–1414. [Google Scholar] [CrossRef]
- Kiyozumi, T.; Kanatani, Y.; Ishihara, M.; Saitoh, D.; Shimizu, J.; Yura, H.; Suzuki, S.; Okada, Y.; Kikuchi, M. The effect of chitosan hydrogel containing DMEM/F12 medium on full-thickness skin defects after deep dermal burn. Burns 2007, 33, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Nguyen, V.Q.; Mori, Y.; Nakamura, S.; Hattori, H. Adsorption of silver nanoparticles onto different surface structures of chitin/chitosan and correlations with antimicrobial activities. Int. J. Mol. Sci. 2015, 16, 13973–13988. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; Ishihara, M.; Mori, Y.; Nakamura, S.; Kishimoto, S.; Fujita, M.; Hattori, H.; Kanatani, Y.; Ono, T.; Miyahira, Y.; et al. Preparation of size-controlled silver nanoparticles and chitosan-based composites and their anti-microbial activities. Biomed. Mater. Eng. 2013, 23, 473–483. [Google Scholar] [PubMed]
- Nguyen, V.Q.; Ishihara, M.; Kinoda, J.; Hattori, H.; Nakamura, S.; Ono, T.; Miyahira, Y.; Matsui, T. Development of antimicrobial biomaterials produced from chitin-nanofiber sheet/silver nanoparticle composites. J. Nanobiotechnol. 2014, 12. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Fujita, M.; Ishihara, M.; Tachibana, S.; Yamamoto, Y.; Kaji, T.; Kawauchi, T.; Kanatani, Y. Protective effect of inhalation of hydrogen gas on radiation-induced dermatitis and skin injury in rats. J. Radiat. Res. 2014, 55, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Dallea-Donne, I.; Rossi, R.; Giustarini, D.; Mizani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef]
- Crosswhite, P.; Sun, Z. Nitric acid, oxidative stress and inflammation in pulmonary arterial hypertension. J. Hypertens. 2010, 28, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, J.; Skoglund, S.; Karlsson, M.-E.; Wold, S.; Wallinder, I.O.; Hedberg, Y. Sequential studies of silver released from silver nanoparticles in aqueous media simulating sweat, laundry detergent solutions and surface water. Environ. Sci. Technol. 2014, 48, 7314–7322. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1764–1807. [Google Scholar] [CrossRef] [PubMed]
- Dobias, J.; Bernier-Latmani, R. Silver release from silver nanoparticles in natural waters. Environ. Sci. Technol. 2013, 47, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.L.K.; Zheng, C.; Cheung, G.S.P. Cytotoxicity of a novel nano-silver particle endodontic irrigant. Clin. Cosmet. Investig. Dent. 2015, 7, 65–74. [Google Scholar] [CrossRef] [PubMed]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoda, J.; Ishihara, M.; Hattori, H.; Nakamura, S.; Fukuda, K.; Yokoe, H. Cytotoxicity of Silver Nanoparticle and Chitin-Nanofiber Sheet Composites Caused by Oxidative Stress. Nanomaterials 2016, 6, 189. https://doi.org/10.3390/nano6100189
Kinoda J, Ishihara M, Hattori H, Nakamura S, Fukuda K, Yokoe H. Cytotoxicity of Silver Nanoparticle and Chitin-Nanofiber Sheet Composites Caused by Oxidative Stress. Nanomaterials. 2016; 6(10):189. https://doi.org/10.3390/nano6100189
Chicago/Turabian StyleKinoda, Jun, Masayuki Ishihara, Hidemi Hattori, Shingo Nakamura, Koichi Fukuda, and Hidetaka Yokoe. 2016. "Cytotoxicity of Silver Nanoparticle and Chitin-Nanofiber Sheet Composites Caused by Oxidative Stress" Nanomaterials 6, no. 10: 189. https://doi.org/10.3390/nano6100189





