2.1. Preparation of Precursor Gels
Precursor gels were prepared by mixing and drying silver acetate (CH
3COOAg) and strontium acetate hemihydrate (Sr(CH
3COO)
2·0.5H
2O) mixed aqueous solution and titanium tetraisopropoxide (Ti[(CH
3)
2CHO]
4) ethanolic solution.
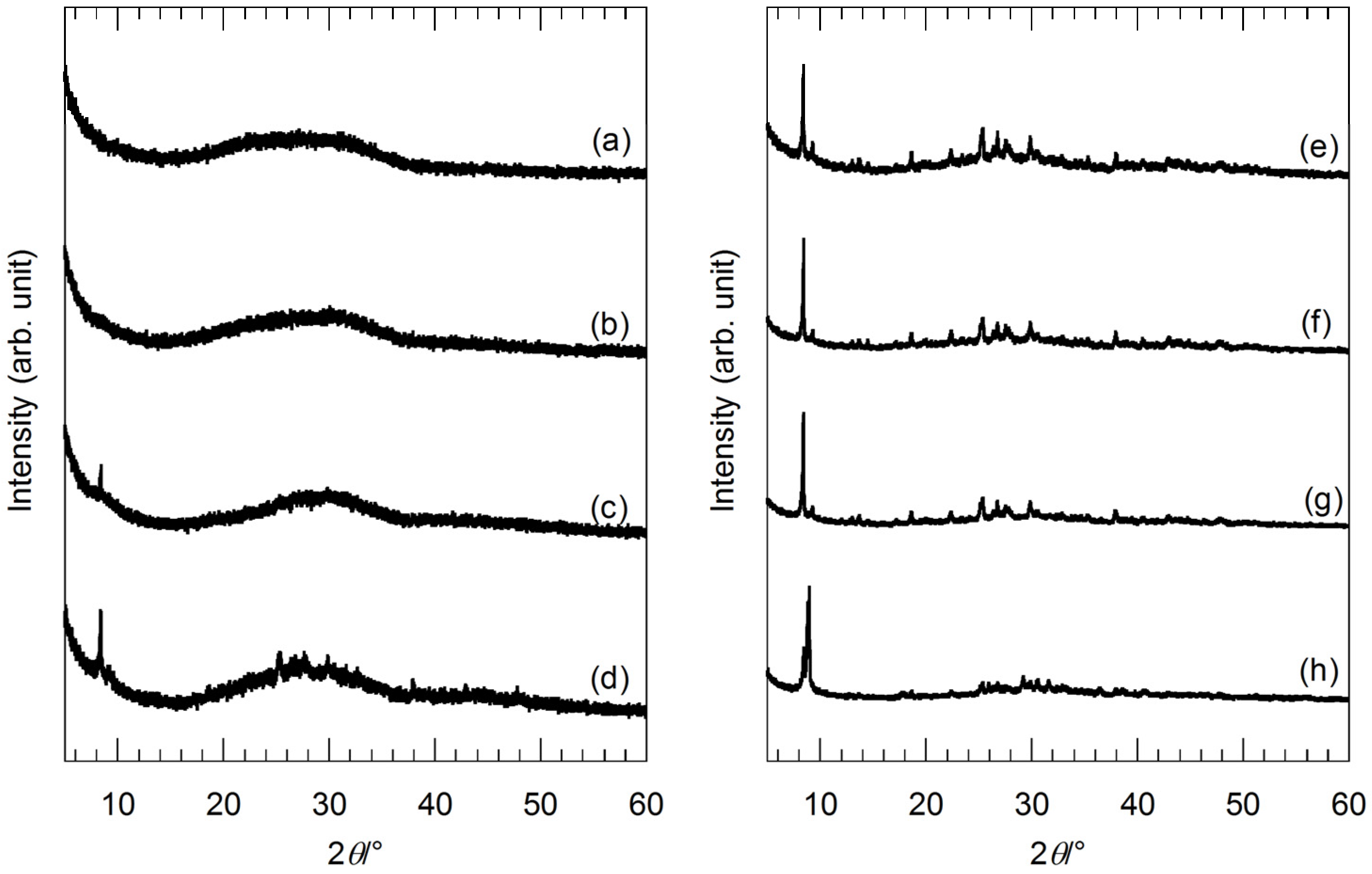
Figure 1 shows the X-ray diffraction (XRD) patterns of the precursor gels before the hydrothermal treatment. The XRD patterns of the precursor gels with molar ratios of Ag:Sr:Ti = 1:0:4 and 1:1:4 in
Figure 1a,b show an amorphous pattern, while the XRD peaks identified as Sr(CH
3COO)
2·0.5H
2O are found for the XRD patterns of the precursor gels with molar ratios of Ag:Sr:Ti = 1:2:4, 1:3:4 and 1:4:4 (
Figure 1c,d,f)). Since there is no peak identified as CH
3COOAg for these precursor gels regardless of the Sr molar fractions, Ag
+ ions are assumed to be preferentially incorporated into amorphous titanium oxide gel networks. In the case of the lower Sr molar fractions, Sr
2+ ions are completely incorporated into the amorphous gel networks with Ag
+ ions. However, for the higher Sr molar fractions (molar ratios of Ag:Sr:Ti = 1:3:4 and 1:4:4), excess Sr(CH
3COO)
2·0.5H
2O crystals were precipitated. On the other hand, XRD patterns of the series of precursor gels with the various Ag molar fractions (the molar ratio of Sr:Ti was fixed to 1:1) are shown in
Figure 1e–h. All of the XRD peaks are identified as Sr(CH
3COO)
2·0.5H
2O for the precursor gels with molar ratios of Ag:Sr:Ti = 0:4:4, 1:4:4 and 2:4:4 (
Figure 1e–g). In contrast, other than the Sr(CH
3COO)
2·0.5H
2O peaks, the peaks identified as CH
3COOAg are found for the precursor gel with a molar ratio of Ag:Sr:Ti = 4:4:4 (
Figure 1h). Therefore, Ag
+ ions are completely incorporated into the gel networks for the precursor gels with the lower Ag molar fractions (molar ratios of Ag:Sr:Ti = 1:4:4 and 2:4:4). For these precursor gels, a certain amount of Ag
+ and Sr
2+ ions are incorporated into the amorphous titanium oxide gel networks, and excesses of Sr(CH
3COO)
2·0.5H
2O and CH
3COOAg are precipitated.
Figure 1.
X-ray diffraction (XRD) patterns of the precursor gels with various molar ratios of (a) Ag:Sr:Ti = 1:0:4; (b) 1:1:4; (c) 1:2:4; (d) 1:3:4; (e) 0:4:4; (f) 1:4:4; (g) 2:4:4; and (h) 4:4:4.
Figure 1.
X-ray diffraction (XRD) patterns of the precursor gels with various molar ratios of (a) Ag:Sr:Ti = 1:0:4; (b) 1:1:4; (c) 1:2:4; (d) 1:3:4; (e) 0:4:4; (f) 1:4:4; (g) 2:4:4; and (h) 4:4:4.
2.2. Synthesis of Hybrid Particles from Precursor Gels with Various Sr Molar Fractions
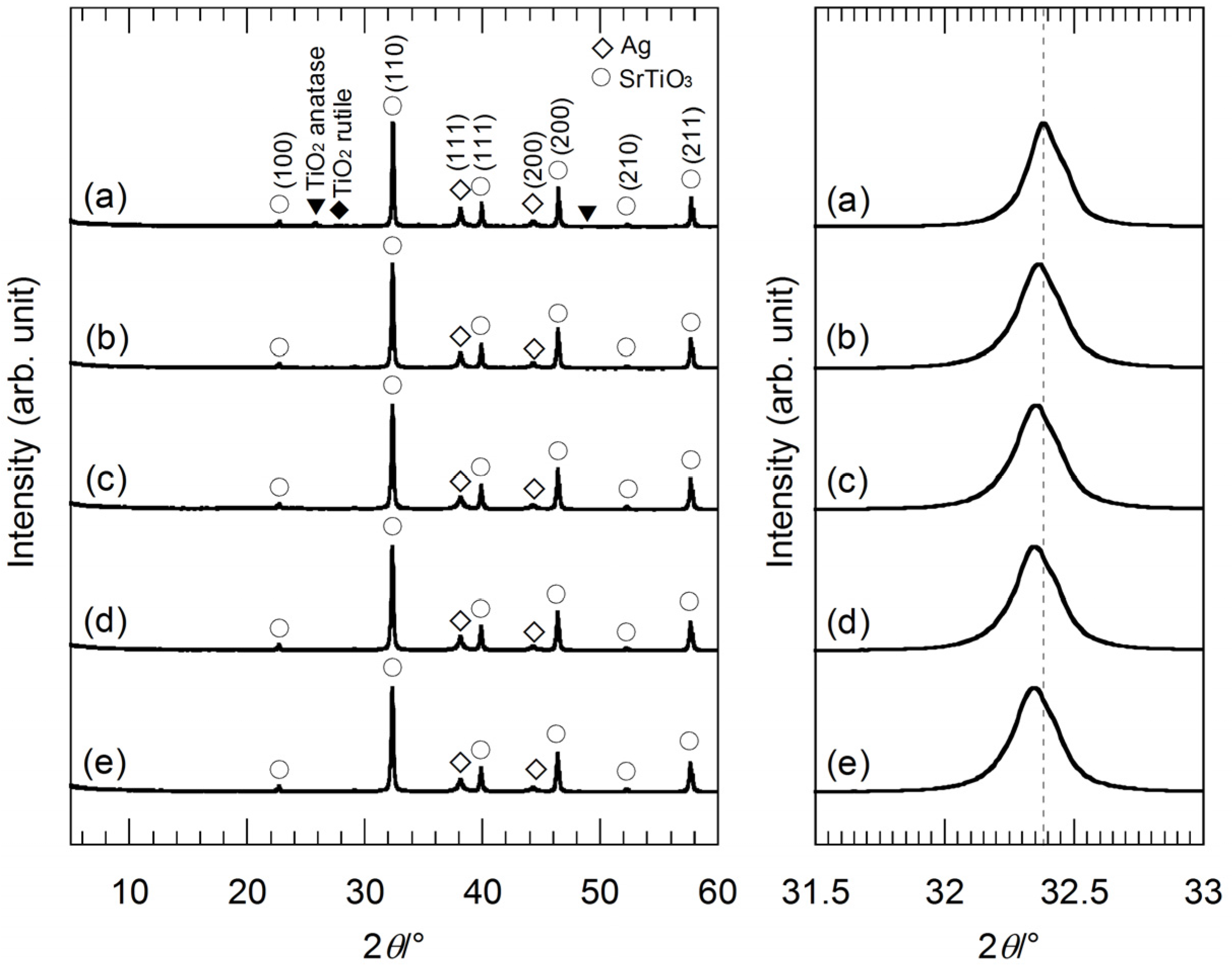
Figure 2 shows XRD patterns of the hybrid particles synthesized from the precursor gels with molar ratios of Ag:Sr:Ti = 1:0:4, 1:2:4, 1:3:4 and 1:4:4 by the hydrothermal treatment in strontium hydroxide (Sr(OH)
2) aqueous solutions. The hydrothermally-synthesized sample powder from the precursor gel without Sr
2+ ion (Ag:Sr:Ti = 1:0:4), which was reacted with Sr(OH)
2, mainly consists of Ag and SrTiO
3, though this sample contains impurities; anatase- and rutile-type TiO
2 (
Figure 2a). When the other precursor gels containing Sr
2+ ions were used, we could obtain the Ag-SrTiO
3 hybrid particles without impurity (
Figure 2b–e), because the Sr source included in the precursor gels relatively increases and the Ti source included in the gels relatively decreases. In the hydrothermal reaction, the Sr
2+ ions dissociated from the gel networks, and the excess Sr(CH
3COO)
2·0.5H
2O could be also reacted with titanium oxide gels other than Sr
2+ ions supplied by Sr(OH)
2. Thus, the formation reaction of SrTiO
3 is considered to be facilitated for the precursor gels with the higher Sr molar fractions. In
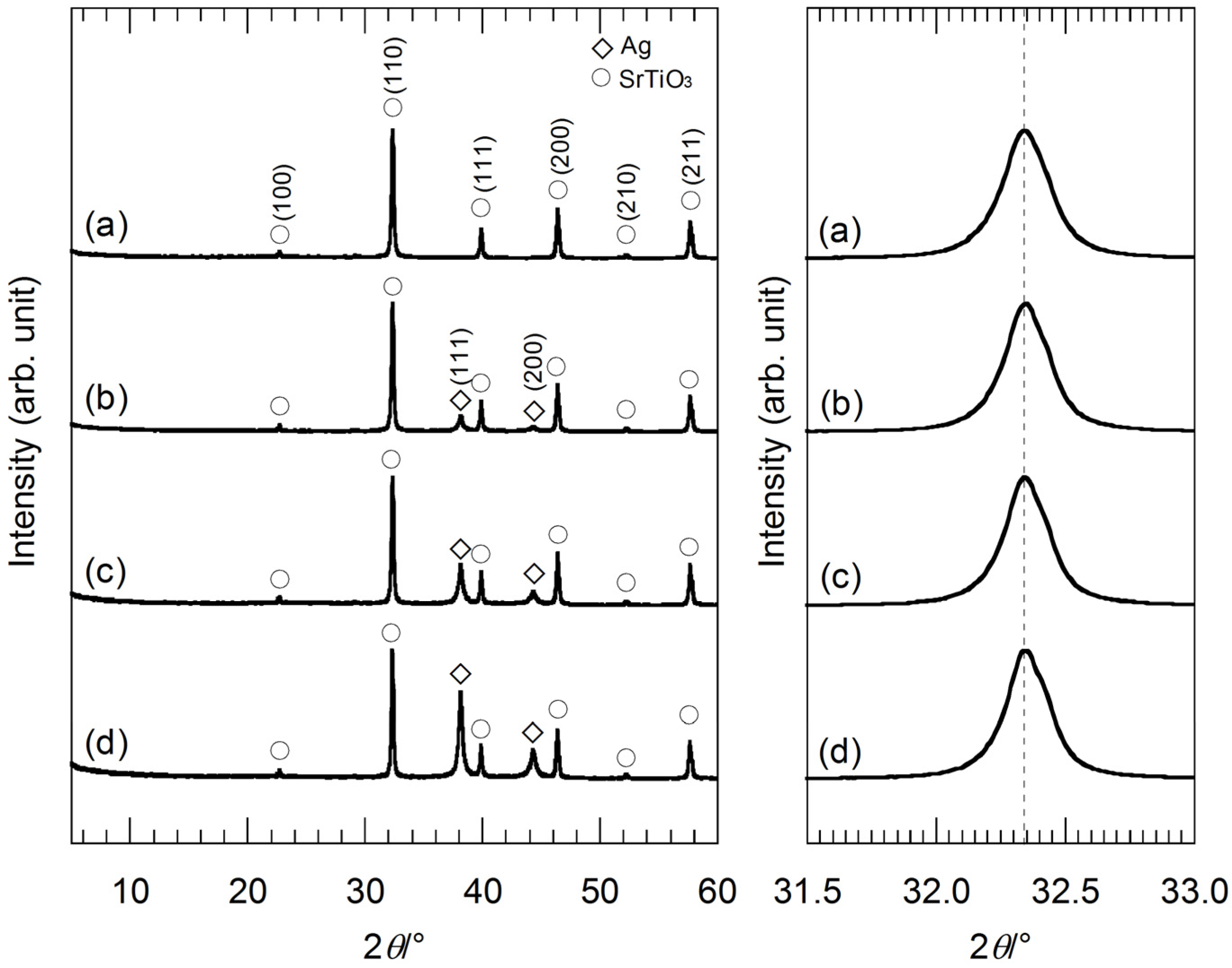
Figure 2, the XRD peaks originating from Ag tend to broaden with a decrease in the Ag molar fraction in the precursor gels. This suggests that the size of Ag nanoparticles becomes smaller by using the gels with the lower Ag molar fractions, because of a decrease in the supply of the Ag source. A comparison of the XRD peaks indexed as (110) of SrTiO
3 reveals a slight diffraction peak-shift. All of the XRD peaks originating from SrTiO
3 of the hybrid particles synthesized from the gels containing Sr
2+ ions (
Figure 2b–e) slightly shift toward a lower 2θ angle as compared to the hybrid particles from the precursor gel without Sr
2+ ions (
Figure 2a). Ag
+ ions can be doped into SrTiO
3, because the Ag
+ ion radius is comparable to the Sr
2+ ion radius [
20]. However, it is difficult to confirm the doping of Ag
+ by the XRD measurement due to the similarity of the ion radii. For the case of BaTiO
3 particles synthesized by the hydrothermal method, it was reported that an incorporation of hydroxyl ions in the crystal lattice enlarges the cell volume [
21,
22,
23,
24]. Therefore, we investigated the presence of the hydroxyl defects in the synthesized SrTiO
3 crystals by a thermogravimetric analysis.
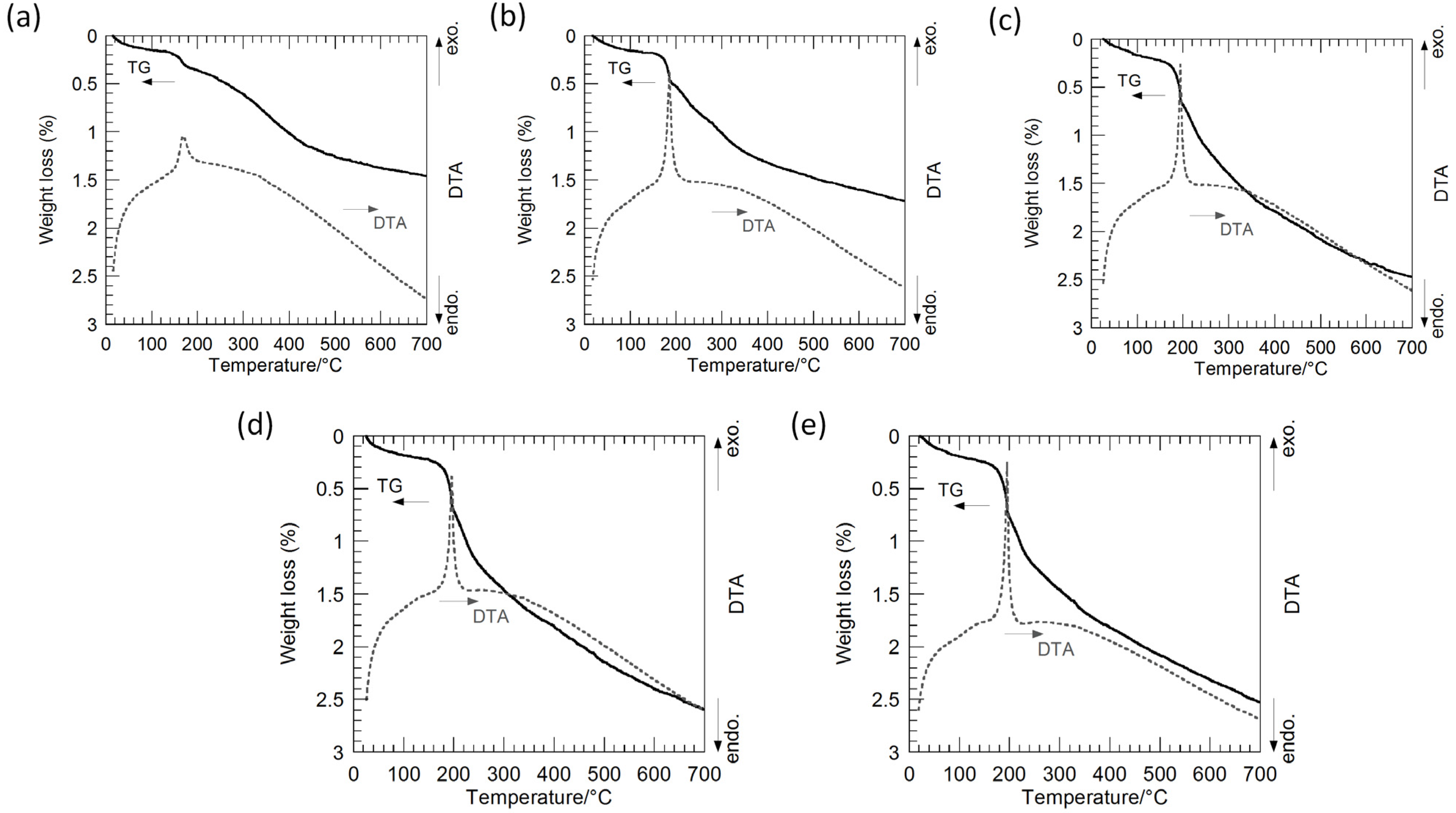
Figure 3 shows thermogravimetry-differential thermal analysis (TG-DTA) curves of the hybrid particles synthesized from the precursor gels with molar ratios of Ag:Sr:Ti = 1:0:4, 1:1:4, 1:2:4, 1:3:4 and 1:4:4 via the hydrothermal treatment at 230 °C for 6 h in Sr(OH)
2 aqueous solutions. The weight loss is up to approximately 2.5%, and these hybrid particles are considered to contain a small amount of hydroxyl groups. The slight weight loss accompanied by the sharp exothermic peak is found at approximately 180 °C for all of the hybrid particles. The weight loss of the hybrid particles synthesized by the hydrothermal treatment in a range from 200 to 700 °C, which may be attributed to the hydroxyl ions incorporated in the SrTiO
3 crystals, monotonically increases from 1.1% to 1.9% with the Sr molar fraction in the precursor gels. Accordingly, the shift of the SrTiO
3 XRD peaks is attributed to the incorporation of hydroxyl groups in the SrTiO
3 crystals.
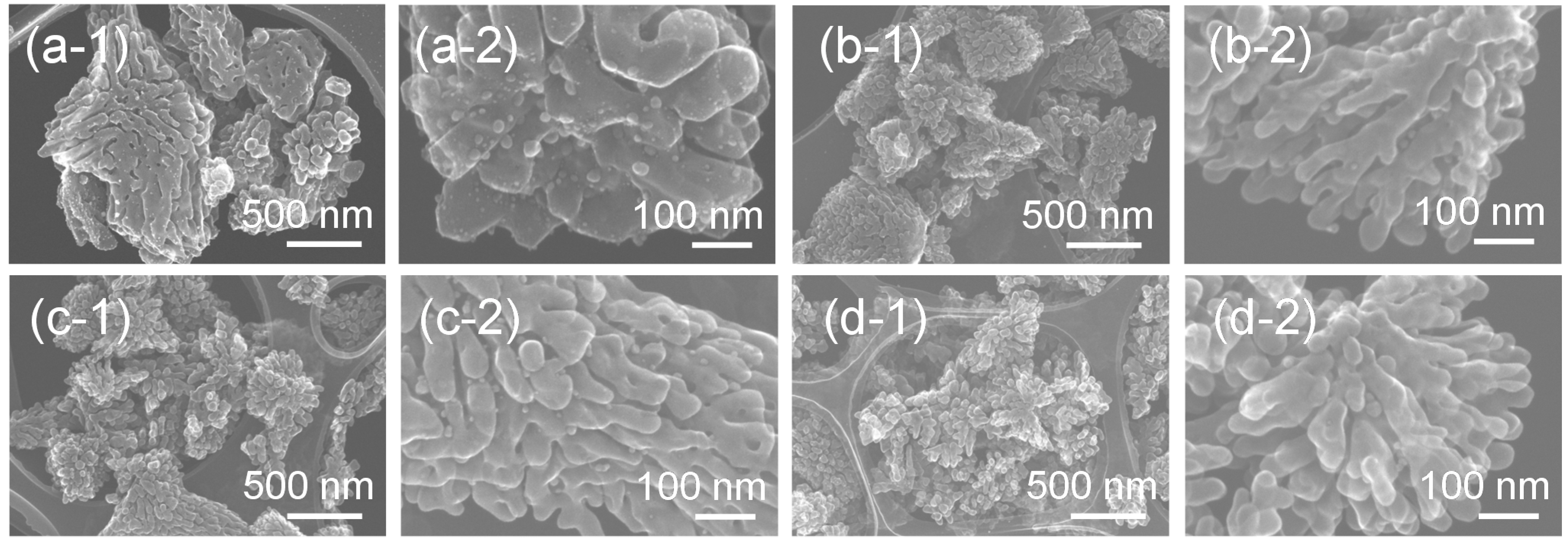
Secondary electron images, obtained by a scanning transmission electron microscope (STEM), of these hybrid particles synthesized from the gels with molar ratios of Ag:Sr:Ti = 1:0:4–1:3:4 via the hydrothermal treatment are shown in
Figure 4. The synthesized Ag-SrTiO
3 hybrid particles have a dendritic structure consisting of many branched nanoparticles. The particle size of the particles is sub-micro- to micro-meter order, and the particle size decreases with the Sr molar fraction in the precursor gels. The formation of the particles with similar hierarchical structures was reported for the other perovskite compounds. Maxim
et al. [
25] reported the hydrothermal synthesis of the dendritic BaTiO
3 particles by using layered titanate as a precursor. Daniels
et al. [
26] reported dendritic La
0.5Sm
0.5CrO
3 particles synthesized by the sol-gel-hydrothermal method. Other research groups reported the synthesis of single-crystal BaTiO
3 [
27], Ba
1−xSr
xTiO
3 [
28] and SrTiO
3 dendrites [
29]. We have also reported dendritic Ag-BaTiO
3 hybrid particles by using the gels derived by the sol-gel process [
15]. Such dendritic growth is promoted under the non-equilibrium condition, in which the growth rate of crystals sufficiently exceeds the mass transport rate of ions for the crystal growth (diffusion-limited aggregation phenomena) [
25,
27,
29,
30,
31,
32]. In our present study, we consider that the formation mechanism of the dendritic hybrid nanoparticles is explained by an analogy to these dendritic perovskite particles. During hydrothermal treatment, the amorphous titanium oxide gels with Ag
+ and Sr
2+ ions are locally dissolved in basic solutions, and SrTiO
3 nuclei immediately form by the reaction of the dissolved titanium oxide gels with Sr
2+ ions in the reaction solution and/or in the dried gel itself. As the sol-gel-derived amorphous titanium oxide gels can be considered to act as highly reactive precursors, the nucleation rate of SrTiO
3 is fast, and a large amount of nuclei is formed in the reaction solution [
22]. The high surface tension anisotropy induced in the nuclei, owing to a large number of defects, gives rise to dendritic growth in the diffusion-limited aggregation phenomena [
25]. On the other hand, the dissolution of the precursor gels is accompanied by a release of Ag
+ ions. Thus, the local concentration of Ag
+ ions around the growing SrTiO
3 dendrites increases, and these Ag
+ ions react with OH
− ions and immediately form nanocrystalline precipitates on the surface of SrTiO
3 dendrites. Ag
2O is expected to be formed in basic aqueous solutions at room temperature, but Ag
2O is then reduced under the hydrothermal conditions [
15]. As a result of competitive formation reactions of Ag and SrTiO
3 around the precursor gels, Ag nanoparticle-loaded SrTiO
3 hybrid nanoparticles can be obtained.
Figure 2.
(Left) XRD patterns of the hybrid particles synthesized from the precursor gels with molar ratios of (a) Ag:Sr:Ti = 1:0:4; (b) 1:1:4; (c) 1:2:4; (d) 1:3:4 and (e) 1:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions. (Right) XRD peaks of the hybrid particles indexed as (110) of SrTiO3 in the 2θ range of 31.5°–33.0°. The slight peak-shift can be clearly seen.
Figure 2.
(Left) XRD patterns of the hybrid particles synthesized from the precursor gels with molar ratios of (a) Ag:Sr:Ti = 1:0:4; (b) 1:1:4; (c) 1:2:4; (d) 1:3:4 and (e) 1:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions. (Right) XRD peaks of the hybrid particles indexed as (110) of SrTiO3 in the 2θ range of 31.5°–33.0°. The slight peak-shift can be clearly seen.
Figure 3.
Thermogravimetry-differential thermal analysis (TG-DTA) curves of the Ag-SrTiO3 hybrid particles synthesized from the precursor gels with molar ratios of (a) Ag:Sr:Ti = 1:0:4; (b) 1:1:4; (c) 1:2:4; (d) 1:3:4; and (e) 1:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions.
Figure 3.
Thermogravimetry-differential thermal analysis (TG-DTA) curves of the Ag-SrTiO3 hybrid particles synthesized from the precursor gels with molar ratios of (a) Ag:Sr:Ti = 1:0:4; (b) 1:1:4; (c) 1:2:4; (d) 1:3:4; and (e) 1:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions.
Figure 4.
Secondary electron images of the Ag-SrTiO3 hybrid particles synthesized from the gels with a molar ratio of (a) Ag:Sr:Ti = 1:0:4, (b) 1:1:4, (c) 1:2:4 and (d) 1:3:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions obtained by the STEM at (a-1–d-1) low and (a-2–d-2) high magnifications.
Figure 4.
Secondary electron images of the Ag-SrTiO3 hybrid particles synthesized from the gels with a molar ratio of (a) Ag:Sr:Ti = 1:0:4, (b) 1:1:4, (c) 1:2:4 and (d) 1:3:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions obtained by the STEM at (a-1–d-1) low and (a-2–d-2) high magnifications.
2.3. Synthesis of Hybrid Particles from Precursor Gels with Various Ag Molar Fractions
Figure 5 shows the XRD patterns of the hybrid particles synthesized from the precursor gels with molar ratios of Ag:Sr:Ti = 0:4:4, 1:4:4, 2:4:4 and 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)
2 aqueous solutions. All of the XRD peaks are identified as Ag and SrTiO
3, suggesting the formation of the Ag-SrTiO
3 hybrid particles. The broadness of the XRD peaks originating from Ag is almost the same for all the hybrid particles, and thus, the crystalline size of the Ag nanoparticles is assumed to be similar. In
Figure 5, the shift of the XRD peaks of the hybrid particles indexed as (110) of SrTiO
3 in the 2θ range of 31.5°–33.0° is not found. The XRD peak-shift is related to the Sr molar fractions in the precursor gels.
Figure 5.
(Left) XRD patterns of the hybrid particles synthesized from the precursor gels with molar ratios of (a) Ag:Sr:Ti = 0:4:4; (b) 1:4:4; (c) 2:4:4; and (d) 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions; (Right) XRD peaks of the hybrid particles indexed as (110) of SrTiO3 in the 2θ range of 31.5°–33.0°.
Figure 5.
(Left) XRD patterns of the hybrid particles synthesized from the precursor gels with molar ratios of (a) Ag:Sr:Ti = 0:4:4; (b) 1:4:4; (c) 2:4:4; and (d) 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions; (Right) XRD peaks of the hybrid particles indexed as (110) of SrTiO3 in the 2θ range of 31.5°–33.0°.
Figure 6 shows the TG-DTA curves of these hybrid particles synthesized from the precursor gels with the various Ag molar fractions, and a weight loss of 2.2%–2.6% up to 700 °C is found for all the hybrid particles. The sharp exothermic peaks appear at around 180 °C in the DTA curves of the Ag-SrTiO
3 hybrid particles, while such exothermic peaks cannot be found for the SrTiO
3 particles without Ag. Accordingly, it is suggested that the precursor gels were almost completely converted into Ag and SrTiO
3 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)
2 solutions, but a small amount of the hydroxyl groups might be present. The slightly large weight loss of around 1.7%–2.0% in the range from 200 to 700 °C, which may be attributed to the hydroxyl ions incorporated in the SrTiO
3 crystals, is found for these SrTiO
3 and Ag-SrTiO
3 hybrid particles synthesized from the gels with molar ratios of Ag:Sr:Ti = 0:4:4, 1:4:4, 2:4:4 and 4:4:4 at 230 °C for 6 h in Sr(OH)
2 aqueous solutions. It is considered that these hydroxyl defects induce the enlargement of the SrTiO
3 lattices and result in the XRD peak-shift toward a lower 2θ angle (
Figure 5a–d) as compared to the hybrid particles from the precursor gel without Sr
2+ ions (
Figure 2a).
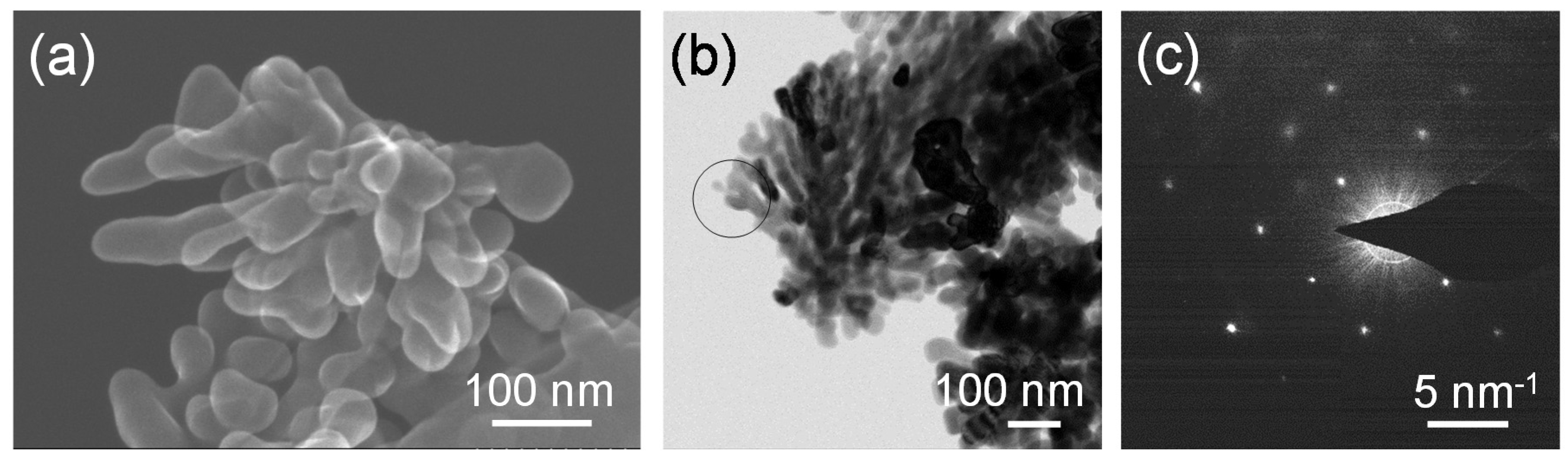
Figure 7a,b shows a secondary electron image obtained by the STEM and field-emission transmission electron microscope (FE-TEM) images of the SrTiO
3 particles synthesized from the gel with the molar ratio of Ag:Sr:Ti = 0:4:4 at 230 °C for 6 h in Sr(OH)
2 solutions, respectively. It can be clearly seen that dendritic nanoparticles with a size of a few hundreds of nanometers were formed. The selected-area electron diffraction (SAED) analysis was performed for the dendritic SrTiO
3 particle shown in
Figure 7b, and the spot pattern was obtained for the corresponding area (
Figure 7c). A dendritic SrTiO
3 particle is not a single crystal, but a part of the branches may have a single-crystalline nature.
Figure 6.
TG-DTA curves of the SrTiO3 and the Ag-SrTiO3 hybrid particles synthesized from the precursor gels with molar ratios of Ag:Sr:Ti = (a) 0:4:4; (b) 2:4:4; and (c) 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions.
Figure 6.
TG-DTA curves of the SrTiO3 and the Ag-SrTiO3 hybrid particles synthesized from the precursor gels with molar ratios of Ag:Sr:Ti = (a) 0:4:4; (b) 2:4:4; and (c) 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions.
Figure 7.
(a) A secondary electron image obtained by the STEM; and (b) Field-emission transmission electron microscope (FE-TEM) images of the SrTiO3 particles synthesized from the gel with a molar ratio of Ag:Sr:Ti = 0:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions; (c) electron diffraction pattern of the SrTiO3 particles synthesized from the gels without Ag (the molar ratio of Ag:Sr:Ti = 0:4:4) obtained by the selected area shown in (b).
Figure 7.
(a) A secondary electron image obtained by the STEM; and (b) Field-emission transmission electron microscope (FE-TEM) images of the SrTiO3 particles synthesized from the gel with a molar ratio of Ag:Sr:Ti = 0:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions; (c) electron diffraction pattern of the SrTiO3 particles synthesized from the gels without Ag (the molar ratio of Ag:Sr:Ti = 0:4:4) obtained by the selected area shown in (b).
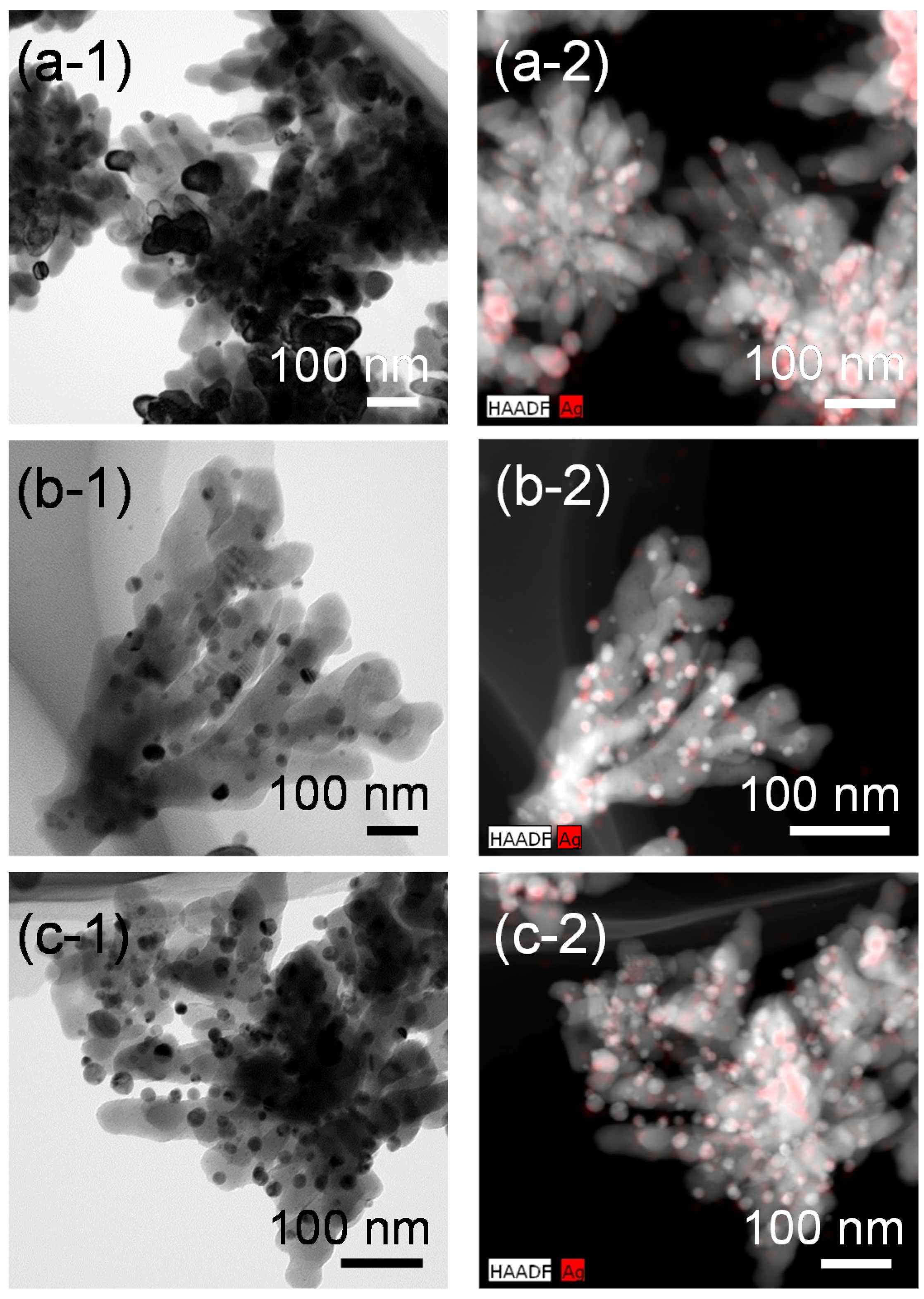
Figure 8 shows FE-TEM images and the corresponding high-angle annular dark field (HAADF)-STEM images with STEM-energy dispersive X-ray spectroscopy (EDX) mapping of the Ag-SrTiO
3 hybrid particles synthesized from the gels with the various Ag fractions by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)
2 solutions. We can see the Ag nanoparticles are loaded on the SrTiO
3 dendritic nanoparticles, which may have a high specific surface area. There is a wide size distribution of the adsorbed Ag nanoparticles, but the Ag nanoparticles with a size of a few tens of nanometers are distributed without severe agglomeration in these observation areas. By using the sol-gel-derived amorphous titanium oxide gels incorporated with the metal source as the precursors, we could obtain the Ag nanoparticle-loaded SrTiO
3 dendritic nanoparticles by the hydrothermal treatment at low temperature. These Ag-SrTiO
3 hybrid nanoparticles are expected to be applied to the high-capacitance Ag/SrTiO
3 composite materials or the photocatalysts.
Figure 8.
FE-TEM images of the Ag-SrTiO3 hybrid particles synthesized from the gels with molar ratios of (a-1) Ag:Sr:Ti = 1:4:4; (b-1) 2:4:4 and (c-1) 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions. (a-2–c-2) Corresponding high-angle annular dark field (HAADF)-STEM images with STEM-energy dispersive X-ray spectroscopy (EDX) mapping of the hybrid particles shown in (a-1–c-1), respectively. Red-colored regions correspond to the Ag nanoparticles.
Figure 8.
FE-TEM images of the Ag-SrTiO3 hybrid particles synthesized from the gels with molar ratios of (a-1) Ag:Sr:Ti = 1:4:4; (b-1) 2:4:4 and (c-1) 4:4:4 by the hydrothermal treatment at 230 °C for 6 h in Sr(OH)2 aqueous solutions. (a-2–c-2) Corresponding high-angle annular dark field (HAADF)-STEM images with STEM-energy dispersive X-ray spectroscopy (EDX) mapping of the hybrid particles shown in (a-1–c-1), respectively. Red-colored regions correspond to the Ag nanoparticles.