Recent Advances on Carbon Nanotubes and Graphene Reinforced Ceramics Nanocomposites
Abstract
:1. Introduction
2. CNTs-Reinforced Ceramics Nanocomposites
2.1. Pre-Processing for Good Dispersion
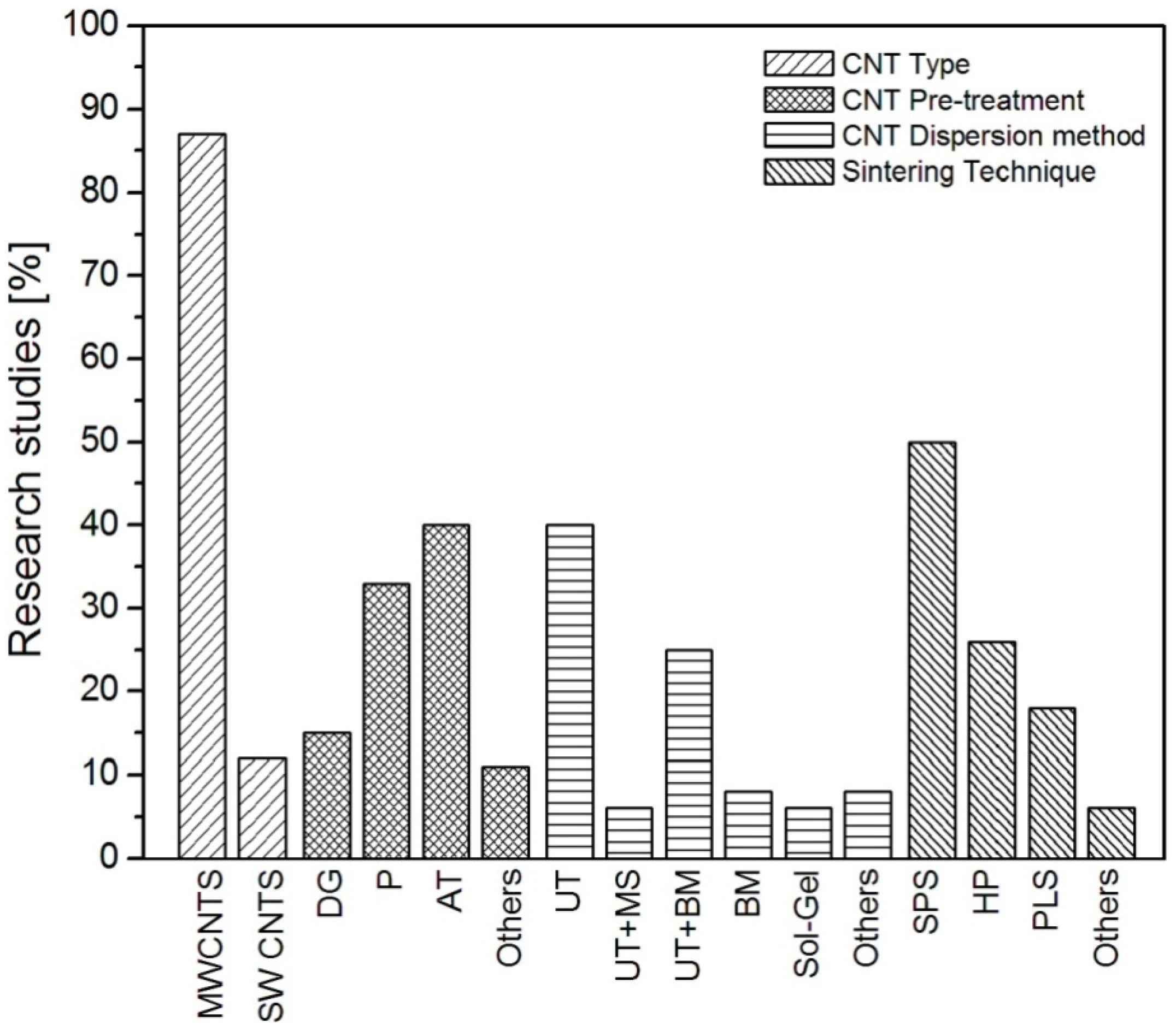
| Reference | Matrix | CNT types | Purification methods | Dispersion procedures | Sintering techniques |
|---|---|---|---|---|---|
| [10] | Si3N4 | SW | P | UT of CNTs with surfactant (C16TAB) and Si3N4 | SPS under vacuum |
| [12] | Al2O3 | MW | Oxidation at 500 °C for 90 min | UT of CNTs in ethanol | SPS at 1500 °C for 10 min under 50 MPa |
| [32] | Al2O3 | MW | AT (H2SO4 + HNO3) | UT of CNTs into water and SDS then incubation for 2 weeks | HP at 1600 °C, 60 min, 40 MPa |
| [33] | Al2O3 | MW | AT (H2SO4 + HNO3) for 3 h | 24 h BM of ball Al2O3 powder and 30 min UT of CNTs in water and then BM of CNTs/Al2O3 mixture | PLS at 1500–1600 °C, 120–240 min, Ar |
| [34] | Al2O3 | MW | Pristine | UT of CNTs for 1 h in alcohol | CIP at150MPa and PLS at 1500 °C, and 1700 °C with 2 h |
| [35] | Al2O3 | MW | AT (heating in 65% HNO3 at 80 °C for 8 h) | BM and Surfactant (Darvan C–N) | PLS at 1500 °C for 2 h using Ar |
| [36] | Mulite | MW | P | CNTs dispersion into ethanol by MS and UT | HP at 1600 °C for 60 min under Ar atmosphere at 30 MPa |
| [37] | Si3N4 | MW | P | 24 h ball milling the CNTs and Si3N4 slurry | HP at 1750 °C for 60 min under 30 MPa |
| [38] | ZrB2–SiC | MW | P | 20 min UT of CNTs and matrix with subsequent 24 h ball milling | HP at 1900 °C for 60 min under 30 MPa |
| [39] | BaTiO3 | MW | P | - | HP, 1200 °C, 60 min |
| [40] | Al2O3 | MW | - | DG (CVD at 750 °C for 15 min for direct CNTs growth on Al2O3 nano-particles) | SPS at 1150 °C for 10 min under 100 MPa |
| [41] | Al2O3 | SW | Pristine | UT of CNTs in ethanol | SPS at 1520 °C under 80 MPa |
| [42] | Al2O3 | MW | P | 35 h UT in water | SPS at 1300 °C, 20 min, 90 MPa |
| [43] | Al2O3 | MW | AT | UT of CNTs and Al2O3 in water followed by 2 h and BM of CNTs/Al2O3 | PLS at 1600 °C, 15 min, Ar |
| [44] | Al2O3 | MW | AT (HNO3 for 30 min) | 5 h BM of CNTs and 1 h UT of CNTs. 5 h BM of CNT/Al2O3 in ethanol | PLS at 1550 °C, Ar |
| [45] | Al2O3 | MW | AT (H2SO4 + HNO3 in 3:1 for 7 h) | surfactant (SDS) using combination of UT and 24 h BM | HP at 1550 °C for 1 h under 30 MPa using Ar gas |
| [46] | Al2O3 + ZrO | MW | AT (heating in 65% HNO3 at 80 °C for 8 h)) | 2 min UT of CNTs with surfactant (SDS)and 24 BM then freezing with Nitrogen | HP at 1500 °C for 2 h under 30 MPa in Ar atmosphere |
| [47] | Al2O3 | SW | AT (H2SO4 + HNO3) | UT for 24 h | SPS at 1300 °C for 5 min under 75 MPa |
2.2. Densification Processes

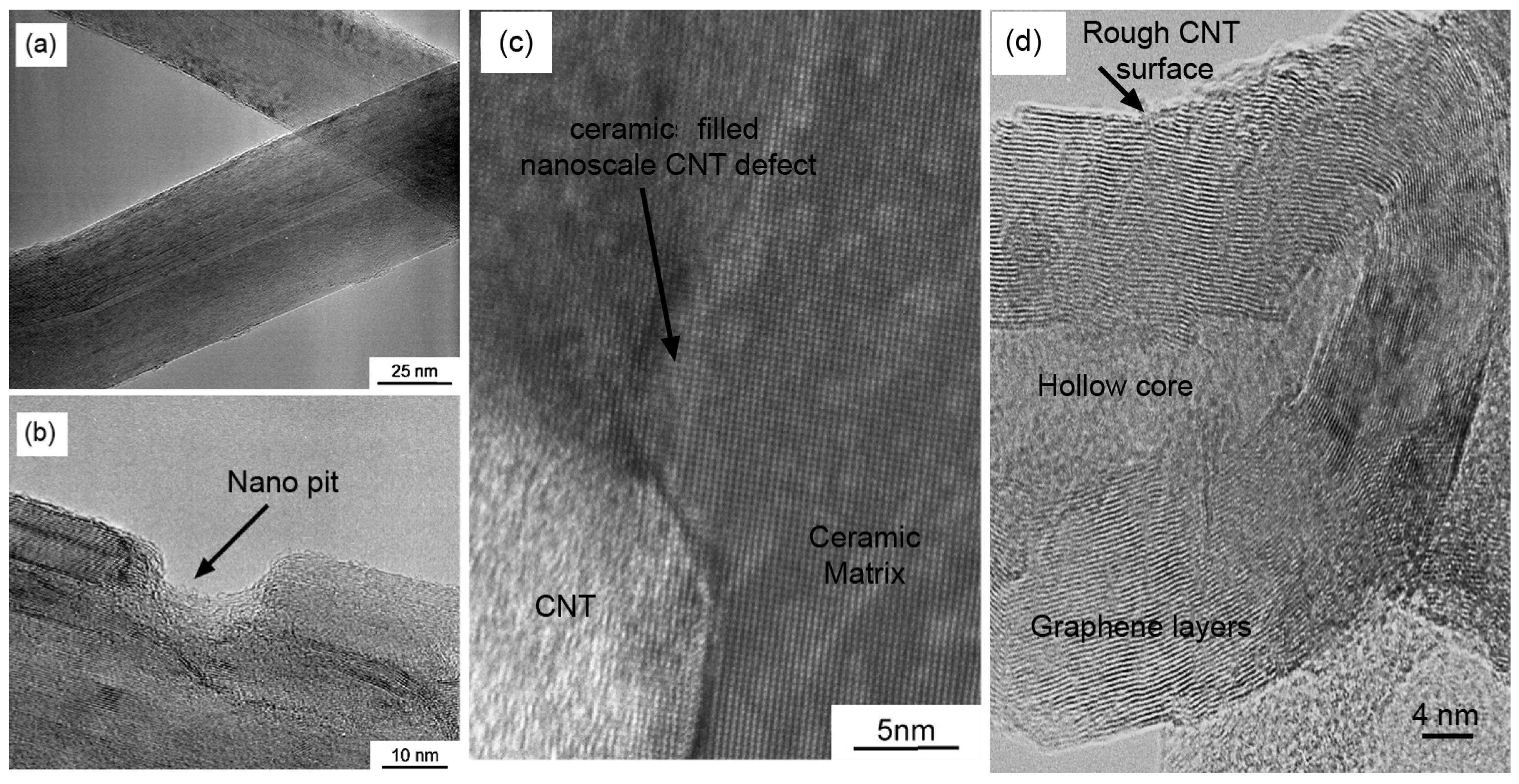
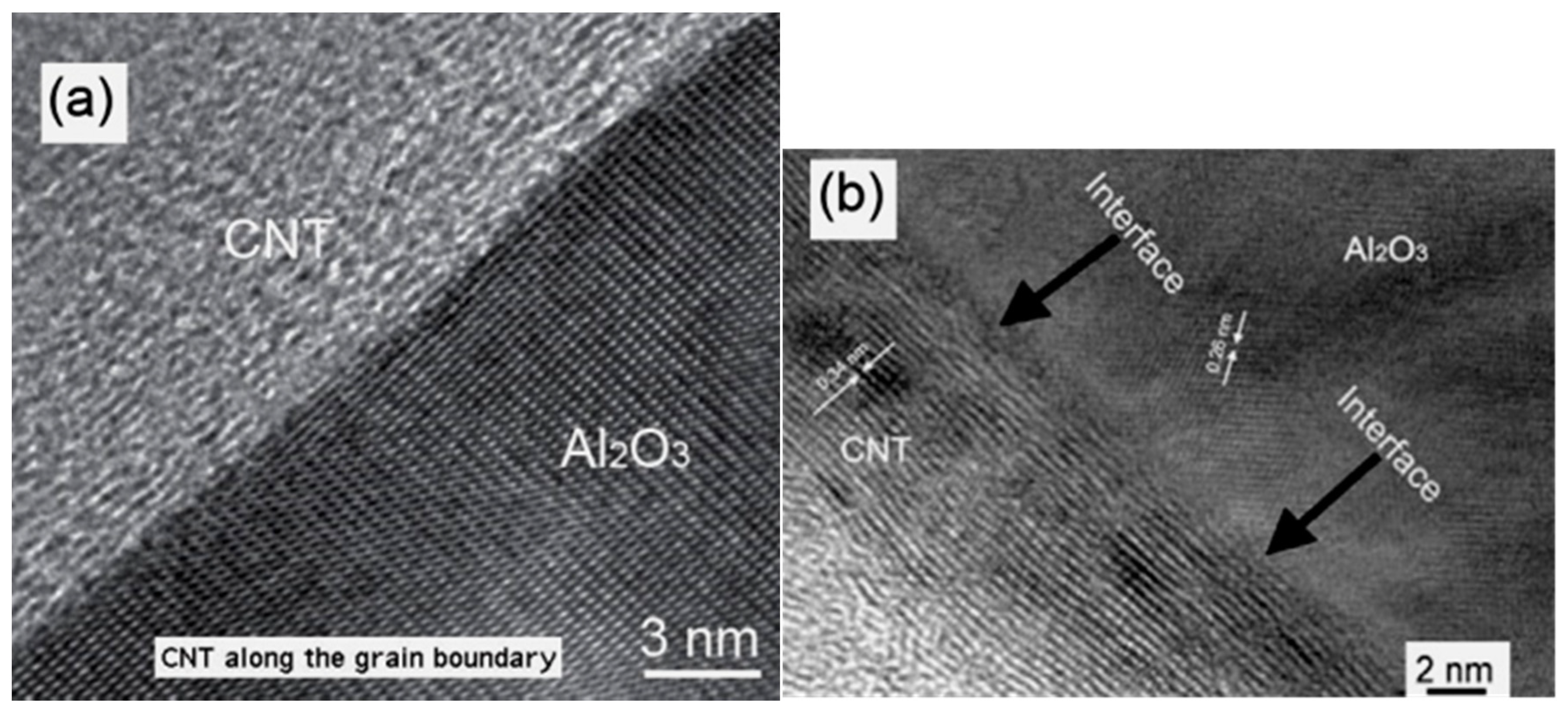
2.3. Microstructural Analysis
2.4. Mechanical and Functional Properties
| Reference | Matrix | CNT contents | Relative density (%) | Hardness (GPa) | Flexural strength (MPa) | Fracture toughness (MPa. m1/2) |
|---|---|---|---|---|---|---|
| [10] | Si3N4 | 0 | 99.2 | 15.7 | 1046 | 4.8 |
| 1 wt%MWCNTs | 98.7 | 15.0 | 996 | 6.6 | ||
| [12] | Al2O3 | 0 | 95.6 | 17.3 | 500 | 4.4 |
| 0.5 wt% MWCNTs | 99.2 | 16.8 | 685 | 5.9 | ||
| 1 wt% MWCNTs | 98.9 | 15.9 | 650 | 5.7 | ||
| [15] | Al2O3 | 0 | - | - | - | 3.3 |
| 3 wt% SWCNTs | - | - | - | 7.9 | ||
| [27] | Al2O3 | 0 | 97.7 | - | 326 | 3.08 |
| 6 wt% MWCNTs | 95.4 | - | 314 | 5.55 | ||
| [32] | Al2O3 | 0 | 99.8 | 16 | 356 | 3.5 |
| 2 wt% MWCNTs | 99.5 | 18 | 402 | 6.8 | ||
| 5 wt% MWCNTs | 99.1 | - | 423 | 5.7 | ||
| [34] | Al2O3 | 0 | 99.5 | 17.5 | 222 | 3.92 |
| 0.15 vol% MWCNTs | 98.4 | 21.4 | 242 | 5.27 | ||
| [35] | Al2O3 | 0 | - | 16.9 | - | 5.5 |
| 1 vol% MWCNTs | - | 13.5 | - | 6.0 | ||
| [36] | Mulite (3Al2O3 + 2SiO2) | 0 | - | - | 466 | 2.0 |
| 2 wt% MWCNTs | - | - | 512 | 3.3 | ||
| [69] | SiC | 0 | 939 | - | 303 | 3.3 |
| 10 wt% MWCNTs | 94.7 | - | 321 | 3.8 | ||
| [38] | ZrB2-SiC | 0 | - | 15.8 | 582 | 4 |
| 2 wt% MWCNTs | - | 15.5 | 616 | 4.6 | ||
| [39] | BaTiO3 | 0 | 98.5 | - | - | 0.7 |
| 98.50 | 98.5 | 0.7 | ||||
| 0.5 wt% MWCNTs | 97.3 | 1.1 | ||||
| 1 wt% MWCNTs | 99.2 | 1.5 | ||||
| 3 wt% MWCNTs | 98.6 | 3.0 | ||||
| [73] | Al2O3 | 0 | - | - | 395 | 4.41 |
| 20 vol% MWCNTs | - | - | 403 | 4.62 | ||
| [74] | Al2O3 | 0 | - | - | - | 3 |
| 1 wt% MWCNTs | - | - | - | 5 | ||
| [75] | Al2O3 | 0 | - | 15.71 | - | 3.24 |
| 5 wt% MWCNTs | - | 0.72 | - | 4.14 | ||
| [76] | Al2O3 | 0 | - | 18.2 | - | 4.5 |
| 2.5 wt% MWCNTs | - | 15.75 | - | 11.4 | ||
| [77] | Al2O3 | 0 | 99.9 | 22.9 | - | 3.54 |
| 10 vol% MWCNTs | 97.4 | 11 | - | 2.76 |
2.5. CNTs/Ceramic Interface and the Toughening Mechanism
3. Graphene Reinforced Ceramic Nanocomposites
3.1. Raw Materials
3.2. GNS Dispersions Processes
3.3. Sintering Techniques
3.4. Structural Features, Mechanical Properties and Toughening Mechanisms
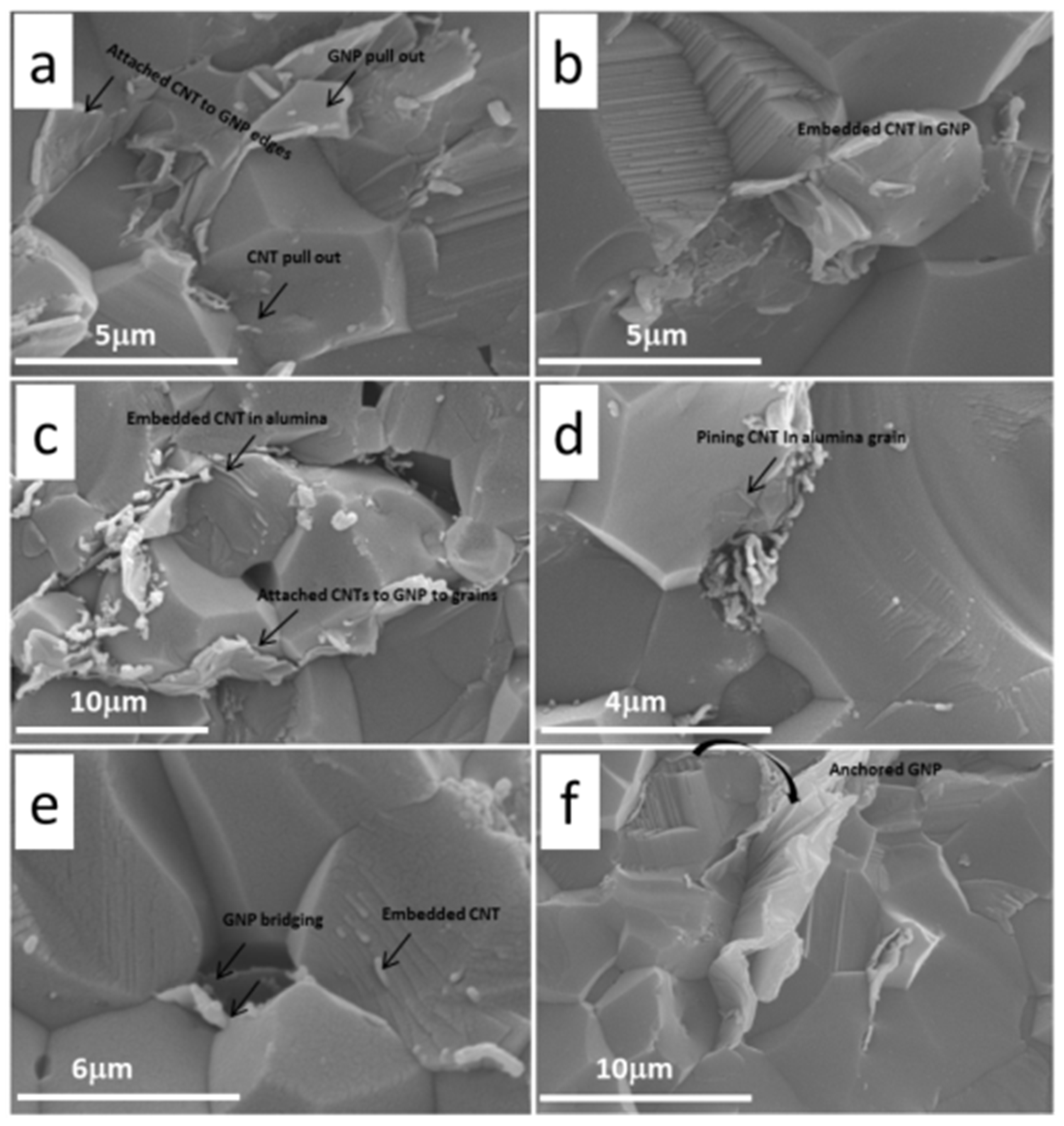
4. Potential Applications
| References | Ceramic matrix | Reinforcing agent | Key properties | Parts/Components | Potential industries |
|---|---|---|---|---|---|
| [101] | Al2O3 | CNTs/graphene | Wear resistance, high toughness, electrical properties, thermal properties | Cutting tools, corrosion/erosion resistance pipes, electrical contacts, armour plates | Automobile, petrochemical industry, electric component manufacturing, defence industry |
| [106] | Si3N4 | CNTs/graphene | Excellent mechanical, chemical, and thermal properties | Gas turbines, aircraft engine components and bearings | Power generation, aerospace, automobile sector |
| [107] | BaTiO3 | CNTs/graphene | Ferroelectrics, piezoelectric and colossal magnetoresistor properties | Electric generator, computer hard disks, sensors | Renewable energy, power generation, electronic, computer manufacturing, data storage, aerospace industry |
| [108,109,110] | ZrO2 | CNTs/graphene | High mechanical properties, excellent fracture toughness, elevated temperature stability, high breakdown electrical field and large energy bandgap | Solid oxide fuel cells, oxygen sensors and ceramic membranes | Renewable energy, chemical industry, water desalination sectors |
| [111,112,113] | TiN and FeN | CNTs/graphene | Excellent electrical properties | Capacitors, electronic conductor in electronic devices | Electrochemical industry, power and electronic sector, aerospace and automobile industries |
| [114] | Mulite | CNTs/graphene | High in electric and optical properties | Sensor | Electronic industry, aerospace sector and automobile industry |
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Niihara, K. New design concept of structural ceramics-ceramics nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Osayande, L.; Okoli, O.I. Fracture toughness enhancement for Al2O3 system: A review. Int. J. Appl. Ceram. Technol. 2008, 5, 313–323. [Google Scholar] [CrossRef]
- Ohnabe, H.; Masaki, S.; Sasa, T. Potential application of ceramics matrix composites to aero-engine components. Compos. A 1999, 30, 489–496. [Google Scholar] [CrossRef]
- Llorca, J.; Elices, M.J.; Celemin, A. Toughness and microstructural degradation at high temperature in SiC fiber-reinforced ceramics. Acta Mater. 1998, 46, 2441–2453. [Google Scholar] [CrossRef]
- Yongqing, F.; Gu, Y.W.; Hejun, D. SiC whisker toughened Al2O3-(Ti,W)C ceramic matrix composites. Scr. Mater. 2001, 44, 111.D–116.D. [Google Scholar] [CrossRef]
- Garcıa, E.; Schicker, S.; Bruhn, J.; Janssen, R.; Claussen, N. Processing and mechanical properties of pressureless-sintered niobium—Al2O3-matrix composites. J. Am. Ceram. Soc. 1998, 81, 429–432. [Google Scholar] [CrossRef]
- Padture, N.P. Multifunctional composites of ceramics and single-walled carbon nanotubes. Adv. Mater. 2009, 21, 1767–1770. [Google Scholar] [CrossRef]
- Sheldon, B.W.; Curtin, W.A. Nanoceramics composites tough to test. Nat. Mater. 2004, 3, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Peigney, A. Tougher ceramics with nanotubes. Nat. Mater. 2003, 2, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Corral, E.; Bell, N.; Stuecker, J.; Perry, N.; Garay, J.; Barrera, E.V. Engineered nanostructures for multifunctional single-walled carbon nanotube reinforced silicon nitride nanocomposites. J. Am. Ceram. Soc. 2008, 91, 3129–3137. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, D.; Song, J. Preparation and microstructure of multi-walled carbon nanotubes toughened Al2O3 composite. J. Am. Ceram. Soc. 2006, 89, 750–753. [Google Scholar] [CrossRef]
- Yamamoto, G.; Omori, M.; Hashida, T.; Kimura, H. A novel structure for carbon nanotube reinforced Al2O3 composites with improved mechanical properties. Nanotechnology 2008, 19, 315708. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.H.; Lou, J.; Curtin, W.A. A multiscale experiment on the tribological behavior of aligned carbon nanotube/ceramic composites. Scr. Mater. 2008, 58, 223. [Google Scholar] [CrossRef]
- Wie, T.; Fan, Z.; Wie, F. A new structure for multi-walled carbon nanotubes reinforced Al2O3 nanocomposite with high strength and toughness. Mater. Lett. 2008, 62, 641–644. [Google Scholar] [CrossRef]
- Zhan, G.D.; Mukherjee, A. Processing and characterization of nanoceramic composites with interesting structural and functional properties. Rev. Adv. Mater. Sci. 2005, 10, 185–196. [Google Scholar]
- Costa, J.; Flacker, A.; Nakashima, M.; Fruett, F.; Zampieri, M.; Longo, E. Integration of microfabricated capacitive bridge and thermistor on the alumina substrates. ECS Trans. 2012, 49, 451–458. [Google Scholar]
- Martin, C.A.; Lee, G.F.; Fedderly, J.J. Composite Armor System Including a Ceramic-Embedded Heterogeneously Layered Polymeric Matrix. U.S. Patent 8387510 B1, 2013. [Google Scholar]
- Baron, B.; Kumar, C.; le Gonidec, G.; Hampshire, S. Comparison of different alumina powders for the aqueous processing and pressureless sintering of Al2O3-SiC nanocomposites. J. Eur. Ceram. Soc. 2002, 22, 1543–1552. [Google Scholar] [CrossRef]
- Ipek, M.; Zeytin, S.; Bindal, C. An evaluation of Al2O3-ZrO2 composites produced by coprecipitation method. J. Alloys Compd. 2011, 509, 486–489. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.F.; Fan, Z.J.; Yan, J.; Wei, T. Preparation of composites by spark plasma sintering. Mater. Res. Bull. 2011, 46, 315–318. [Google Scholar] [CrossRef]
- Ramirez, C.; Garzón, L.; Miranzo, P.; Osendi, M.; Ocal, C. Electrical conductivity maps in graphene nanoplatelet/silicon nitride composites using conducting scanning force microscopy. Carbon 2011, 49, 3873–3880. [Google Scholar] [CrossRef]
- Balazsi, C. Silicon nitride composites with different nanocarbon additives. J. Korean Ceram. Soc. 2012, 49, 352–362. [Google Scholar] [CrossRef]
- Kvetkova, L.; Duszova, A.; Hvizdos, P.; Dusza, J.; Kun, P.; Balazsi, C. Fracture toughness and toughening mechanisms in graphene platelet reinforced Si3N4 composites. Scr. Mater. 2012, 66, 793–796. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Reece, M.J.; Jiang, K. Toughening of zirconia/alumina composites by the addition of graphene platelets. J. Eur. Ceram. Soc. 2012, 32, 4185–4193. [Google Scholar] [CrossRef]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in graphene ceramic composites. ACS Nano 2011, 5, 3182–3190. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, B.; Xia, Y.; Ahmad, I.; Zhu, Y. Graphene and carbon nanotube (GNT)-reinforced alumina nanocomposites. J. Eur. Ceram. Soc. 2015, 35, 179–186. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L.; Li, J.; Sun, S.; Chen, F.; Chen, L.; Jiang, W. Preparation and electrical properties of graphene nanosheet/Al2O3 composites. Carbon 2010, 48, 743–1749. [Google Scholar]
- Ebbesen, T.W.; Ajyan, P.M. Large scale synthesis of carbon nanotubes. Nature, 1992; 358, 220–222. [Google Scholar]
- Hiura, H.; Ebbesen, T.W.; Tanigaki, K. Opening and purification of carbon nanotubes in high yields. Adv. Mater. 1995, 7, 275–276. [Google Scholar] [CrossRef]
- Tohji, K.; Takashaki, H.; Nishina, Y. Purification procedure for single-walled nanotubes. J. Phys. Chem. 1997, B101, 1974–1978. [Google Scholar] [CrossRef]
- Ahmad, I.; Islam, M.; Almajid, A.; Yazdani, B.; Zhu, Y.Q. Investigation of yttria-doped Al2O3 nanocomposites reinforced by multi-walled carbon nanotubes. Ceram. Int. 2014, 40, 9327–9335. [Google Scholar] [CrossRef]
- Ahmad, I.; Kennedy, A.; Zhu, Y.Q. Carbon nanotubes reinforced Al2O3 nanocomposites: Mechanical properties and interfacial investigations. J. Comput. Sci. Technol. 2010, 70, 1199–1206. [Google Scholar] [CrossRef]
- Zhang, S.C.; William, G.; Hilmas, G.E.; Edward, J.Y. Pressureless sintering of carbon nanotube-Al2O3 composites. J. Eur. Ceram. Soc. 2010, 30, 1373–1380. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, P.K. Microstructure and physic-mechanical properties of pressure-less sintered multi-walled carbon nanotubes/Al2O3 nanocomposites. Ceram. Int. 2012, 38, 423–432. [Google Scholar] [CrossRef]
- Michalek, M.; Lkova, M.; Sedla, J.; Galusek, D. Al2O3/MWCNTs composites by aqueous slip casting and pressureless sintering. Ceram. Int. 2013, 139, 6543–6550. [Google Scholar] [CrossRef]
- Wang, J.; Kou, H.; Liu, X.; Pan, Y.; Guo, J. Reinforcement of mullite matrix with multi-walled carbon nanotubes. Ceram. Int. 2007, 33, 719–722. [Google Scholar] [CrossRef]
- Pasupuleti, S.; Peddetti, R.; Halloran, J.P. Toughening behavior in a carbon nanotube reinforced silicon nitride composite. Mater. Sci. Eng. 2008, A491, 224–229. [Google Scholar] [CrossRef]
- Tian, W.; Kan, Y.; Wang, P. Effect of carbon nanotubes on the properties of ZrB2-SiC ceramics. Mater. Sci. Eng. 2008, 487, 568–573. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, L.; Sun, J. Effect of adding carbon nanotubes on microstructure, phase transformation and mechanical properties of BaTiO3 ceramics. J. Am. Ceram. Soc. 2005, 88, 3515–3518. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.H.; Li, W.Z.; Wang, Q.W.; Datye, A.; Wu, K.H. Synthesis, microstructure and electrical conductivity of carbon nanotube–alumina nanocomposites. Ceram. Int. 2009, 35, 1775–1781. [Google Scholar] [CrossRef]
- Echeberria, J.; Rodríguez, N.; Bocanegra-Bernal, M.H. Hard and tough carbon nanotube-reinforced zirconia-toughened Al2O3 composites prepared by spark plasma sintering. Carbon 2012, 50, 706–717. [Google Scholar] [CrossRef]
- Kim, S.W.; Chung, W.S.; Sohn, K.S.; Son, C.Y.; Lee, S. Improvement of flexure strength and fracture toughness in alumina matrix composites reinforced with carbon nanotubes. Mater. Sci. Eng. 2009, A517, 293–299. [Google Scholar] [CrossRef]
- Bakhsh, N.; Khalid, F.A.; Hakeem, A.S. Effect of sintering temperature on densification and mechanical properties of pressureless sintered CNT–Al2O3 nanocomposites. Mater. Sci. Eng. 2014, 60, 012059. [Google Scholar]
- Li, T. Improving the antistatic ability of polypropylene fibers by inner antistatic agent filled with carbon nanotubes. Comput. Sci. Tech. 2004, 64, 2089–2096. [Google Scholar] [CrossRef]
- Hanzel, O.; Sedlácek, J.; Sajgalík, P. New approach for distribution of carbon nanotubes in Al2O3 matrix. J. Eur. Ceram. Soc. 2014, 34, 1845–1851. [Google Scholar] [CrossRef]
- Michalek, M.; lkova, M.; Sedla, J.; Galusek, D. Mechanical properties and electrical conductivity of Al2O3/MWCNT and Al2O3/zirconia/MWCNT composites. Ceram. Int. 2014, 40, 1289–1295. [Google Scholar] [CrossRef]
- Poyato, R.; Gallardo-López, A.; Gutiérrez-Mora, F.; Morales-Rodríguez, A.; Muñoz, A.; Domínguez-Rodríguezb, A. Effect of high SWNT content on the room temperature mechanicalproperties of fully dense 3YTZP/SWNT composites. J. Eur. Ceram. Soc. 2014, 34, 1571–1579. [Google Scholar] [CrossRef]
- Kyotani, T.; Tsai, L.F.; Tomita, A. Preparation of ultrafine carbon tubes in nanochannels of an anodic aluminum oxide film. Chem. Mater. 1996, 8, 2109–2113. [Google Scholar] [CrossRef]
- Sui, Y.C.; Acosta, D.R.; Cui, B.Z. Structure, thermal stability, and deformation of multibranched carbon nanotubes synthesized by CVD in the AAO template. J. Phys. Chem. 2001, B105, 1523–1527. [Google Scholar] [CrossRef]
- Bae, E.J.; Choi, W.B.; Park, G.S.; Song, S. Selective growth of carbon nanotubes on pre-patterned porous anodic aluminum oxide. Adv. Mater. 2002, 14, 277–279. [Google Scholar] [CrossRef]
- Parhama, H.; Bates, S.; Xia, Y.; Zhu, Y. A highly efficient and versatile carbon nanotube/ceramic composite filter. Carbon 2013, 54, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Parhama, H.; Kennedy, A.; Zhu, Y. Preparation of porous Al2O3—Carbon nanotube composites via direct growth of carbon nanotubes. J. Comput. Sci. Technol. 2011, 71, 1739–1745. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L. Development of a dispersion process for carbon nanotubes in ceramic matrix by hetero-coagulation. Carbon 2003, 41, 1063–1068. [Google Scholar] [CrossRef]
- Chan, B.; Seung, I. Fabrication of CNT-reinforced Al2O3 matrix nanocomposites by sol-gel. Mater. Sci. Eng. 2005, 395, 124–128. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L. Reinforcement of Al2O3 matrix with multi-walled CNT. Ceram. Int. 2004, 893–896. [Google Scholar]
- Gao, L.; Jiang, L.; Sun, J. Carbon nanotube-ceramic composites. J. Electroceram. 2006, 17, 51–55. [Google Scholar] [CrossRef]
- Coble, R.L. Diffusion Models for hot pressing with surface energy and pressure effects as driving forces. J. Appl. Phys. 1970, 41, 4798–4808. [Google Scholar] [CrossRef]
- Legorreta, G.; Estournes, C.; Peigney, A.; Weibel, A.; Flahaut, E.; Laurent, C. Spark-plasma-sintering of double-walled carbon nanotube–magnesia nanocomposites. Scr. Mater. 2009, 60, 741–744. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Dar, M.A. Structure and properties of Y2O3-doped Al2O3-MWCNT nanocomposites prepared by PL-sintering and hot-pressing. J. Mater. Eng. Perform. 2014, 23, 2110–2119. [Google Scholar] [CrossRef]
- Ghobadi, H.; Ali, N.; Ebadzade, T.; Sadeghian, Z.; Barzegar-Bafrooei, H. Improving CNT distribution and mechanical properties of MWCNT reinforced Al2O3 matrix. Mater. Sci. Eng. 2014, A617, 110–114. [Google Scholar] [CrossRef]
- Tatami, J.; Katashima, T.; Komeya, K.; Meguro, T.; Wakihara, T. Electrically conductive CNT-dispersed silicon nitride ceramics. J. Am. Ceram. Soc. 2005, 88, 2889–2893. [Google Scholar] [CrossRef]
- Katz, J.D.; Blake, R.D. Microwave sintering of multiple alumina and composite components. J. Am. Ceram. Soc. 1991, 70, 1304. [Google Scholar]
- Sheppard, L.M. Firing technology heats up for the 90s. J. Am. Ceram. Soc. 1988, 67, 1656. [Google Scholar]
- De, A.; Ahmad, I.; Whitney, E.D.; Clark, D.E. Microwaves theory and applications. Mater. Process. 1991, 21, 329–339. [Google Scholar]
- Fujitsu, S.; Ikegami, M.; Hyashi, T. Sintering of partially stabilized zirconia by microwave heating using ZnO–MnO2–Al2O3 plates in a domestic microwave oven. J. Am. Ceram. Soc. 2000, 83, 2085–2087. [Google Scholar] [CrossRef]
- Ahmad, I.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y. Carbon nanotube toughened aluminium oxide nanocomposites. J. Eur. Ceram. Soc. 2009, 30, 865–873. [Google Scholar] [CrossRef]
- Valecillos, M.C.; Hirota, M.; Brite, M.E.; Hirao, K. Sintering of alumina by 2.45 GHz microwave heating. J. Eur. Ceram. Soc. 2004, 24, 387–391. [Google Scholar] [CrossRef]
- Thostenson, P.G.; Karandikar, T.W. Fabrication and characterization of reaction bonded silicon carbide/carbon nanotube composites. J. Appl. Phys. 2005, 38, 3962–3965. [Google Scholar]
- Wang, Y.; Voronin, G.A.; Zerda, T.W.; Winiarski, A. SiC–CNT nanocomposites: High pressure reaction synthesis and characterization. J. Phys. Condens. Matter. 2006, 18, 275–282. [Google Scholar] [CrossRef]
- Kristen, H.B.; Gary, L.M.; Dinesh, K.A. Microwave sintering of alumina at 2.45 GHz. J. Am. Ceram. Soc. 2003, 86, 1307–1312. [Google Scholar] [CrossRef]
- Lopez, A. Hardness and flexural strength of single-walled carbon nanotubes/Al2O3 composites. J. Mater. Sci. 2014, 49, 7116–7123. [Google Scholar] [CrossRef]
- Zaman, A.C.; Kaya, F.; Kaya, C. OH and COOH functionalized single walled carbon nanotubes-reinforced alumina ceramic nanocomposites. Ceram. Int. 2012, 38, 1287–1293. [Google Scholar] [CrossRef]
- Estili, M.; Kawasaki, A.; Sakka, Y. Highly concentrated 3D macrostructure of individual carbon nanotubes in a ceramic environment. Adv. Mater. 2012, 24, 4322–4326. [Google Scholar] [CrossRef] [PubMed]
- Martinlli, J.R.; Sene, F.F. Electrical resistivity of ceramic-metal composite materials: Application in crucibles for induction furnaces. Ceram. Int. 2000, 26, 325–335. [Google Scholar] [CrossRef]
- Puchy, V.; Hvizdos, P.; Dusza, J.; Kovac, F.; Inam, F.; Reece, M.J. Wear resistance of Al2O3–CNT ceramic nanocomposites at room and high temperature. Ceram. Int. 2013, 39, 5821–5826. [Google Scholar] [CrossRef]
- Lee, K.; Mo, C.B.; Park, S.B.; Hong, S.H. Mechanical and electrical properties of multiwalled CNT–alumina nanocomposites prepared by a sequential two-step processing of ultrasonic spray pyrolysis and spark plasma sintering. J. Am. Cream. Soc. 2011, 94, 3774–3779. [Google Scholar] [CrossRef]
- Thomson, K.E.; Jiang, D.; Yao, W.; Ritchie, R.O.; Mukherjee, A.K. Characterization and mechanical testing of alumina-based nanocomposites reinforced with niobium and/or carbon nanotubes fabricated by spark plasma sintering. Acta Mater. 2012, 60, 622–632. [Google Scholar] [CrossRef]
- Ahmad, I.; Kennedy, A.; Zhu, Y. Wear resistance properties of multi-walled carbon nanotubes reinforced Al2O3 nanocomposite. Wear 2010, 269, 71–78. [Google Scholar] [CrossRef]
- Zhan, G.D.; Mukherjee, A.K. Carbon nanotube reinforced alumina-based ceramics with novel mechanical, electrical, and thermal properties. Int. J. Appl. Ceram. Technol. 2004, 1, 161–171. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, P.K. Effect of sintering temperature and nanotube concentration on microstructure and properties of carbon nanotube/alumina nanocomposites. Ceram. Int. 2014, 40, 7449–7458. [Google Scholar] [CrossRef]
- Bi, S.; Su, X.; Hou, G.; Liu, C.; Song, W.L.; Cao, M.S. Electrical conductivity and microwave absorption of shortened multi-walled carbon nanotube/alumina ceramic composites. Ceram. Int. 2013, 39, 5979–5983. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Balani, K.; Agarwal, A. Thermal conductivity of plasma-sprayed aluminum oxide—Multiwalled carbon nanotube composites. J. Am. Cream. Soc. 2008, 91, 942–947. [Google Scholar] [CrossRef]
- Laurent, C.; Peigney, A.; Rousset, A. Carbon nanotubes-Fe-Al2O3 nanocomposites. Part II: Microstructure and mechanical properties of the hot-pressed composites. J. Eur. Ceram. Soc. 1998, 18, 2005–2013. [Google Scholar] [CrossRef]
- Siegel, R.W.; Chang, S.K.; Ajayan, P.M. Mechanical behaviour of polymer and ceramic matrix nanocomposite. Scr. Mater. 2001, 44, 2061–2064. [Google Scholar] [CrossRef]
- Hoagland, R.G. A treatment of inelastic deformation around a crack tip due to micro cracking. J. Am. Ceram. Soc. 1980, 63, 404–410. [Google Scholar] [CrossRef]
- Kawamura, H.; Yamamoto, S. Improvement of Diesel Engine Startability by Ceramic Glow Plug Start System; Society of Automotive Engineers: New York, NY, USA, 1983. [Google Scholar]
- Zheng, G.; Sano, H.; Cheng, H.M. A TEM study of the microstructure of carbon fiber/polycarbosilane-derived SiC composites. Carbon 1999, 37, 2057–2062. [Google Scholar] [CrossRef]
- Estili, M. The homogeneous dispersion of surfactantless, slightly disordered, crystalline, multiwalled carbon nanotubes in α-alumina ceramics for structural reinforcement. Acta Mater. 2008, 56, 4070–4079. [Google Scholar] [CrossRef]
- Knieke, C.; Berger, A.; Voigt, M.; Taylor, R.N.K.; Röhrl, J.; Peukert, W. Scalable production of graphene sheets by mechanical delamination. Carbon 2010, 48, 3196–3204. [Google Scholar] [CrossRef]
- Hummers, J.; William, S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Inam, F.; Yan, H.; Reece, M.J.; Peijs, T. Dimethylformamide: an effective dispersant for making ceramic-carbon nanotube composites. Nanotechnology 2008, 19, 195710. [Google Scholar] [CrossRef] [PubMed]
- Balázsi, C.; Wéber, F.; Arató, P.; Fényi, B.; Hegman, N.; Kónya, Z.; Kiricsi, I.; Vértesy, Z.; Biró, L.P. Development of CNT-silicon nitrides with improved mechanical and electrical properties. Adv. Sci. Technol. 2006, 45, 1723–1728. [Google Scholar] [CrossRef]
- Milsom, B.; Viola, G.; Gao, Z.; Inam, F.; Peijs, T.; Reece, M.J. The effect of carbon nanotubes on the sintering behaviour of zirconia. J. Eur. Ceram. Soc. 2012, 32, 4149–4156. [Google Scholar] [CrossRef]
- Guo, S.; Sivakumar, R.; Kitazawa, H.; Kagawa, Y. Electrical properties of silica-based nanocomposites with multiwall carbon nanotubes. J. Am. Ceram. Soc. 2007, 90, 1667–1670. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Jiang, K. Mechanical properties of graphene platelet-reinforced alumina ceramic composites. Ceram. Int. 2013, 39, 6215–6221. [Google Scholar] [CrossRef]
- Ramirez, C.; Osendi, M.I. Characterization of graphene nanoplatelets-Si3N4 composites by Raman spectroscopy. J. Eur. Ceram. Soc. 2013, 33, 471–477. [Google Scholar] [CrossRef]
- Kvetková, L.; Duszová, A.; Kašiarová, M.; Orčáková, F.; Dusza, J.; Balázsi, C. Influence of processing on fracture toughness of Si3N4 + graphene platelet composites. J. Eur. Ceram. Soc. 2013, 33, 2299–2304. [Google Scholar] [CrossRef]
- Inam, F.; Vo, T.; Bhat, B.R. Structural stability studies of graphene in sintered ceramic nanocomposites. Ceram. Int. 2014, 40, 16227–16233. [Google Scholar] [CrossRef]
- Rutkowski, P.; Stobierski, L.; Górny, G. Thermal stability and conductivity of hotpressed Si3N4–graphene composites. J. Therm. Anal. Calorim. 2014, 116, 321–328. [Google Scholar] [CrossRef]
- Tapaszto, O.; Kun, P.; Weber, F.; Gergely, G.; Balazsi, K.; Pfeifer, J.; Arato, P.; Kidari, A.; Hampshire, S.; Balazsi, C. Silicon nitride based nanocomposites produced by two different sintering methods. Ceram. Int. 2011, 37, 3457–3461. [Google Scholar] [CrossRef]
- Evans, A.G. Perspective on the development of high-toughness ceramics. J. Am. Ceram. Soc. 1990, 73, 187–206. [Google Scholar] [CrossRef]
- Ritchie, R.O. The quest for stronger tougher materials. Science 2008, 320, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Curtin, W.A.; Sheldon, B.W. CNT-reinforced ceramics and metals. Mater. Today 2004, 7, 44–49. [Google Scholar] [CrossRef]
- Kramer, P.; White, K. Effect of sintering parameters and composition on the resistivity of a cermet used as an electrical feed through. Ceram. Eng. Sci. Proc. 1982, 3, 512–518. [Google Scholar]
- Tajima, Y. Development of high performance silicon nitride ceramics and their applications. Mater. Res. Soc. Symp. Proc. 1993, 287, 98–201. [Google Scholar]
- Komeya, H.K. Development of ceramic antifriction bearing. JSAE Rev. 1986, 7, 72–79. [Google Scholar]
- Huang, Q.; Gao, L. Manufacture and electrical properties of multiwalled carbon nanotube/BaTiO3 nanocomposite ceramics. J. Mater. Chem. 2004, 14, 2536–2541. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.; McIntyre, P.C.; Saraswat, K.C.; Byun, J.S. Atomic layer deposition of ZrO2 on W for metal-insulator-metal capacitor application. Appl. Phys. Lett. 2003, 823, 2874–2876. [Google Scholar] [CrossRef]
- Dusza, J.; Tomasek, K.; Blugan, G.; Kuebler, J. Microstructure and properties of carbon nanotube/zirconia composite. J. Eur. Ceram. Soc. 2008, 28, 1023–1027. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S. Optical, electronic, and transport properties of nanocrystalline titanium nitride thin films. J. Appl. Phys. 2001, 90, 4725–4734. [Google Scholar] [CrossRef]
- Carmalt, C.J.; Whaley, S.R.; Lall, P.S.; Cowley, A.H.; Jones, R.A. Titanium (IV) azido and imido complexes as potential precursors to titanium nitride. J. Chem. Soc. Dalton Trans. 1998, 1998, 553–558. [Google Scholar] [CrossRef]
- Janes, R.A.; Aldissi, M.; Kaner, R.B. Controlling surface area of titanium nitride using metathesis reactions. Chem. Mater. 2003, 15, 4431–4435. [Google Scholar] [CrossRef]
- Kim, S.; Kumta, P.N. Hydrazide sol–gel synthesis of nanostructured titanium nitride: Precursor chemistry and phase evolution. J. Mater. Chem. 2003, 13, 2028–2035. [Google Scholar] [CrossRef]
- Cao, M.S.; Wang, R.G.; Fang, X.Y.; Cui, Z.X.; Chang, T.J.; Yang, H.J. Preparing γ′-Fe4N ultrafine powder by twice-nitriding method. Powder Technol. 2001, 115, 96–98. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.; Yazdani, B.; Zhu, Y. Recent Advances on Carbon Nanotubes and Graphene Reinforced Ceramics Nanocomposites. Nanomaterials 2015, 5, 90-114. https://doi.org/10.3390/nano5010090
Ahmad I, Yazdani B, Zhu Y. Recent Advances on Carbon Nanotubes and Graphene Reinforced Ceramics Nanocomposites. Nanomaterials. 2015; 5(1):90-114. https://doi.org/10.3390/nano5010090
Chicago/Turabian StyleAhmad, Iftikhar, Bahareh Yazdani, and Yanqiu Zhu. 2015. "Recent Advances on Carbon Nanotubes and Graphene Reinforced Ceramics Nanocomposites" Nanomaterials 5, no. 1: 90-114. https://doi.org/10.3390/nano5010090





