Alumina Matrix Composites with Non-Oxide Nanoparticle Addition and Enhanced Functionalities
Abstract
:1. Introduction
2. Preparation of Nanocomposites
2.1. Homogeneous Distribution of Nanoparticles
2.1.1. Al2O3-SiC
2.1.2. Al2O3-CNT, CNF
2.2. Densification
2.2.1. Al2O3-SiC
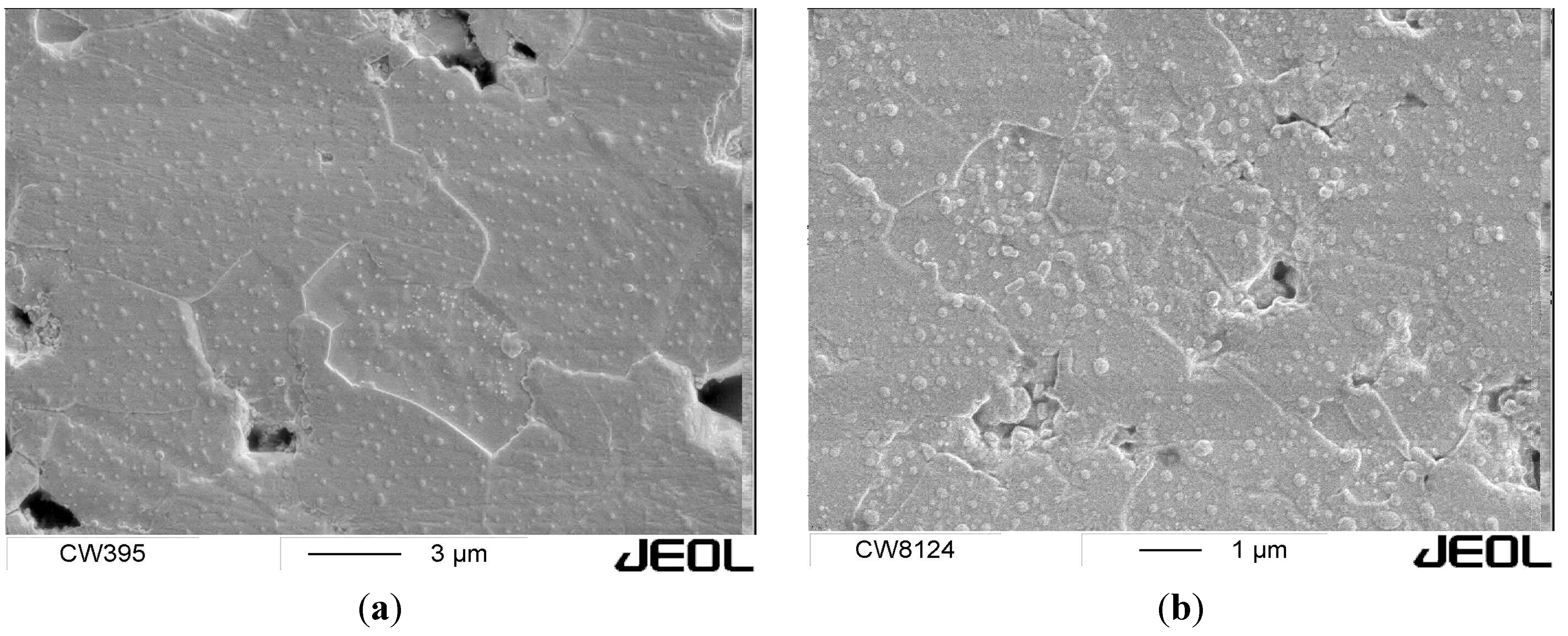
2.2.2. Al2O3-CNT, CNF
3. Room and High-Temperature Mechanical Properties
3.1. Al2O3-SiC

3.2. Al2O3-CNT, CNF
3.2.1. Elastic Modulus
3.2.2. Hardness
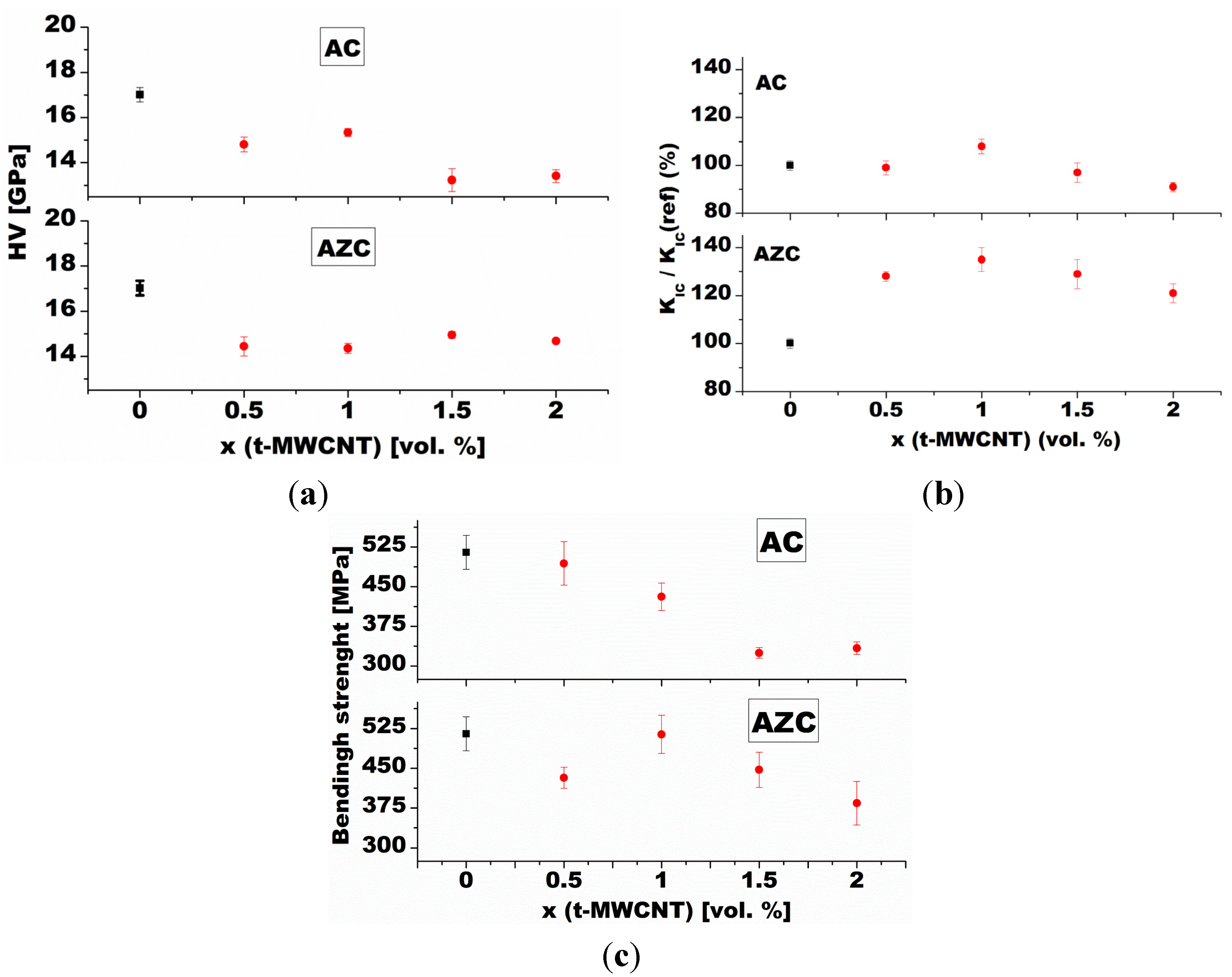
3.2.3. Fracture Toughness and Strength

3.2.4. Tribological Properties
3.2.5. Carbon Nanofibers
4. Functional Properties
4.1. Al2O3-SiC
4.2. Al2O3-CNT, CNF
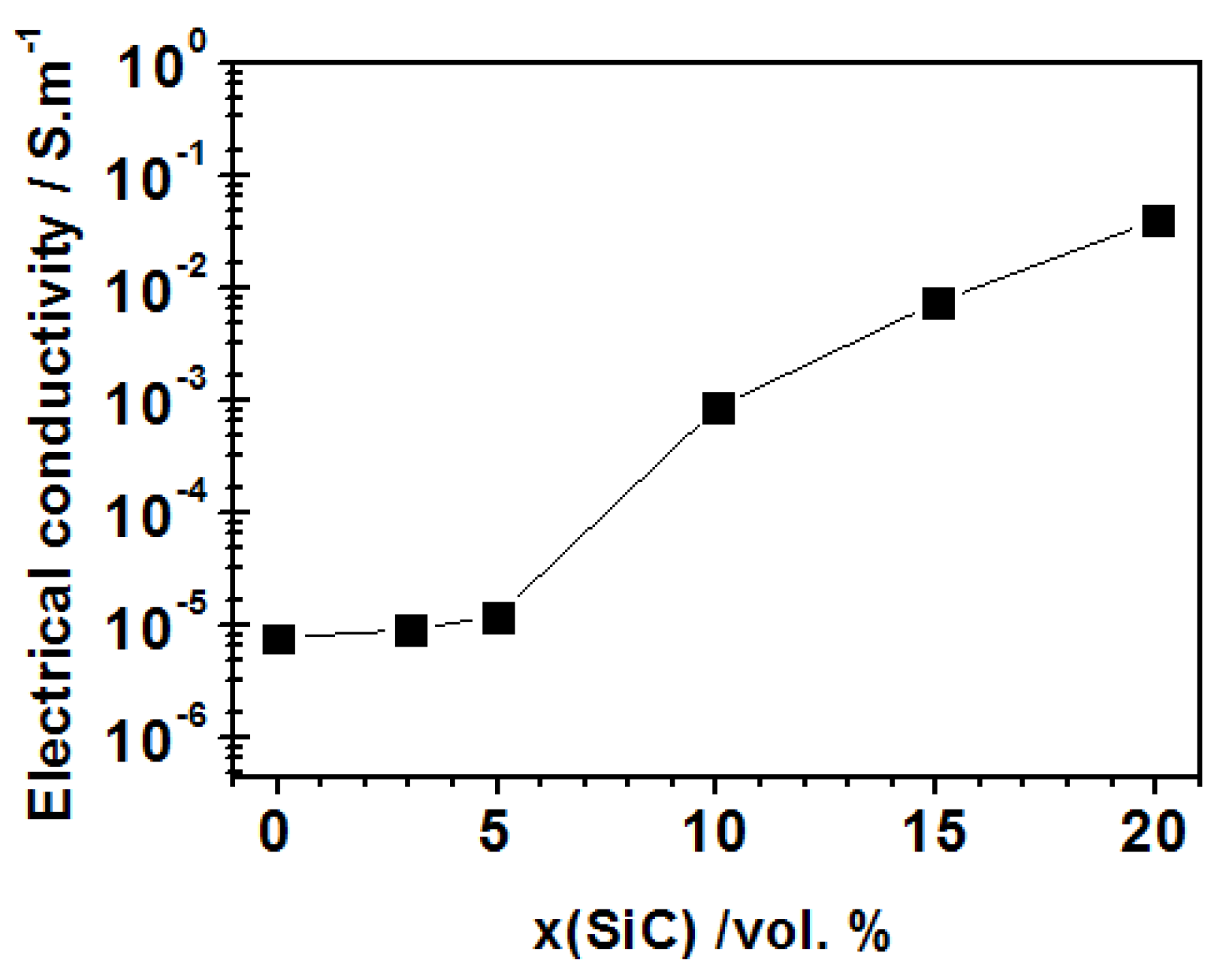
4.2.1. Electric Properties
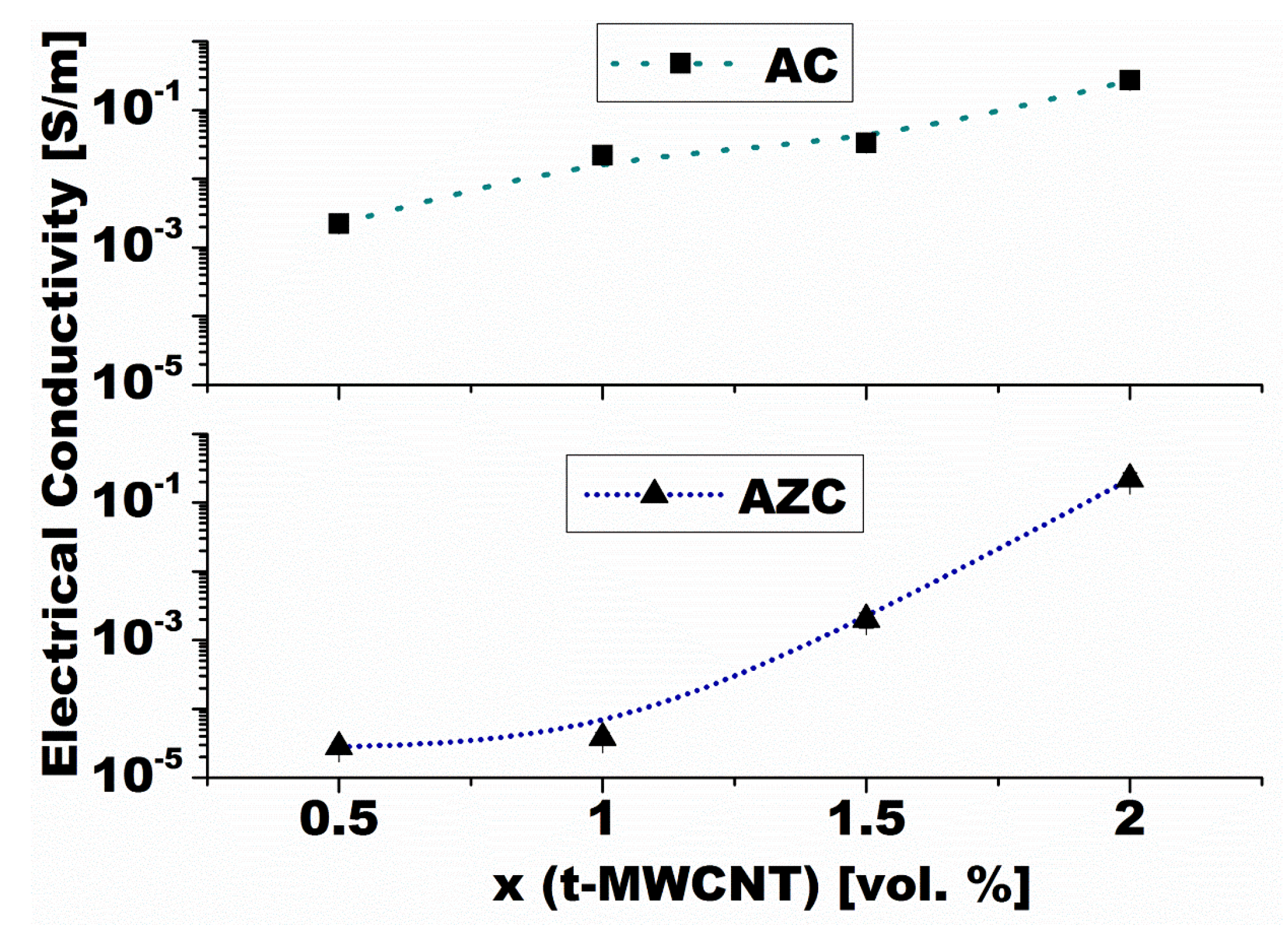
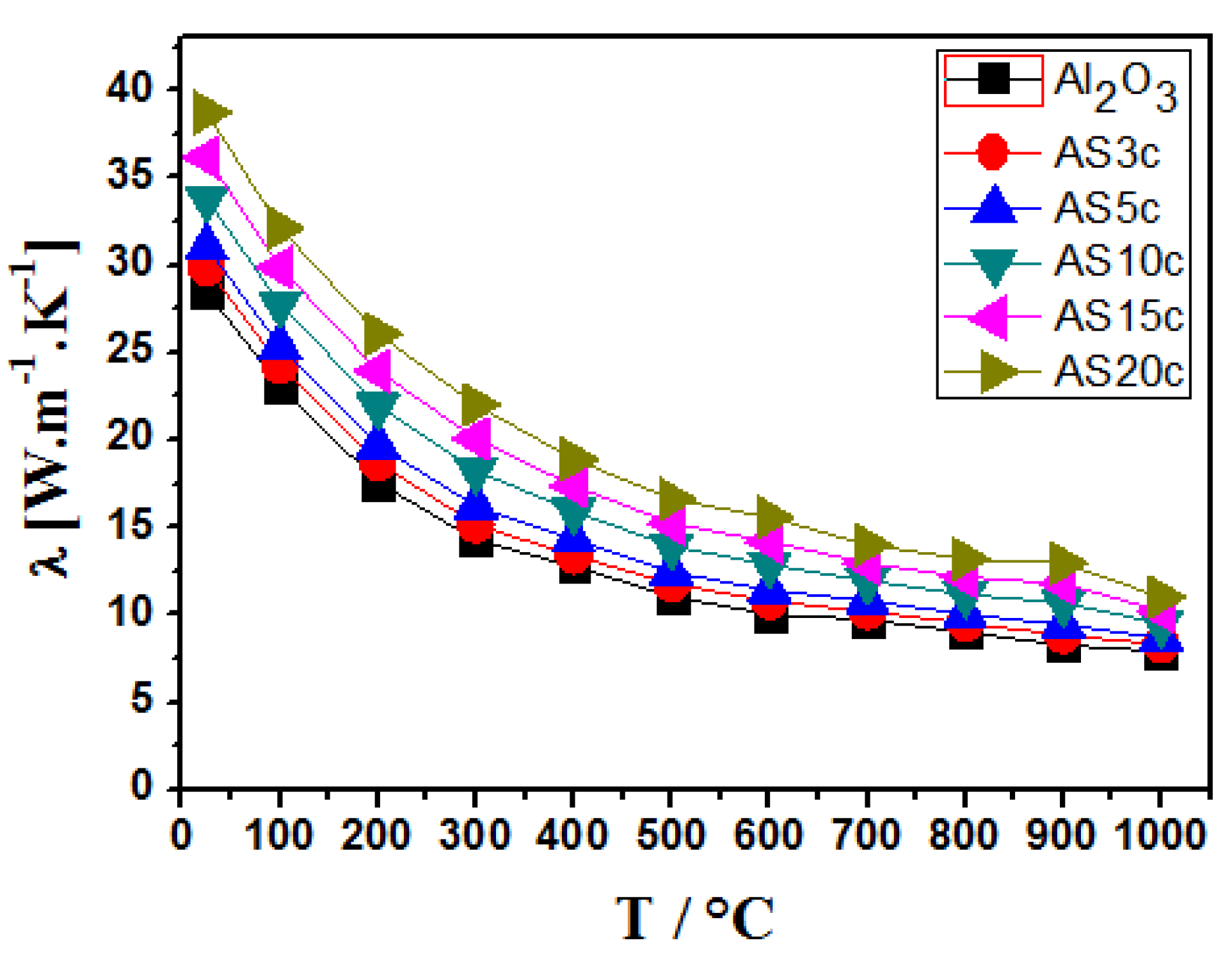
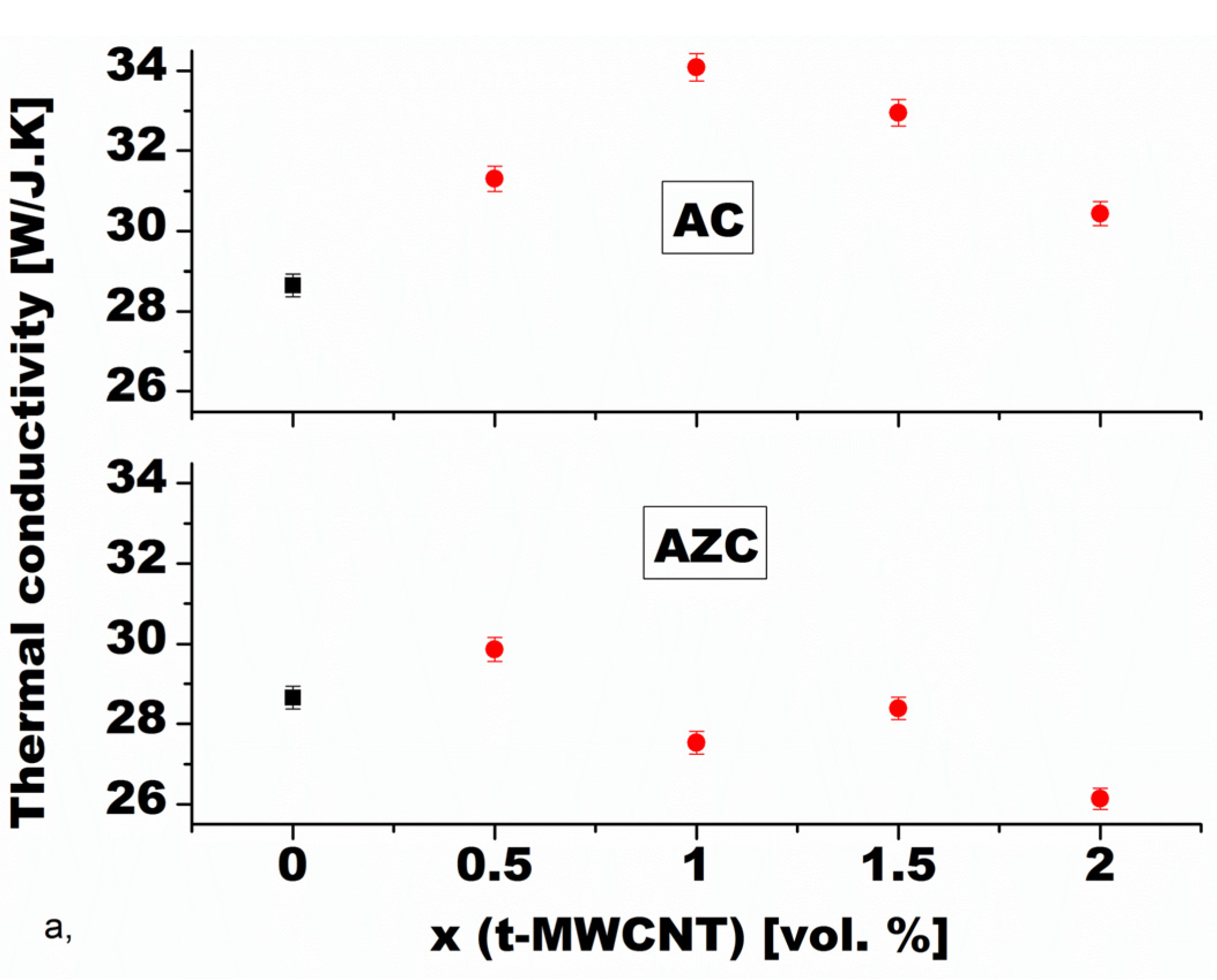
4.2.2. Thermal Conductivity
5. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Niihara, K. New design concept of structural ceramics—Ceramic nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Davidge, R.W.; Brook, R.J.; Cambier, F.; Poorteman, M.; Leriche, A.; O’Sullivan, D.; Hampshire, S.; Kennedy, T. Fabrication, properties, and modelling of engineering ceramics reinforced with nanoparticles of silicon carbide. Br. Ceram. Trans. 1997, 96, 121–127. [Google Scholar]
- Perez-Rigueiro, J.; Pastor, J.Y.; Llorca, J.; Elices, M.; Miranzo, P.; Moya, J.S. Revisiting the mechanical behavior of alumina silicon carbide nanocomposites. Acta Mater. 1998, 46, 5399–5411. [Google Scholar] [CrossRef]
- Wu, H.Z.; Lawrence, C.W.; Roberts, S.G.; Derby, B. The strength of Al2O3/SiC nanocomposites after grinding and annealing. Acta Mater. 1998, 46, 3839–3848. [Google Scholar] [CrossRef]
- Zhao, J.; Stearns, L.C.; Harmer, M.P.; Chan, H.M.; Miller, G.A. Mechanical behavior of alumina silicon-carbide nanocomposites. J. Am. Ceram. Soc. 1993, 76, 503–510. [Google Scholar] [CrossRef]
- Jiang, D.L.; Huang, Z.R. SiC whiskers and particles reinforced Al2O3 matrix composites and N2-HIP post-treatment. Key Eng. Mater. 1999, 159, 379–386. [Google Scholar] [CrossRef]
- Collin, M.I.K.; Rowcliffe, D.J. Influence of thermal conductivity and fracture toughness on the thermal shock resistance of alumina-silicon-carbide-whisker composites. J. Am. Ceram. Soc. 2001, 84, 1334–1340. [Google Scholar] [CrossRef]
- Akatsu, T.; Suzuki, M.; Tanabe, Y.; Yasuda, E. Effects of whisker content and dimensions on the R-curve behavior of an alumina matrix composite reinforced with silicon carbide whiskers. J. Mater. Res. 2001, 16, 1919–1927. [Google Scholar] [CrossRef]
- Davidge, R.W.; Twigg, P.C.; Riley, F.L. Effects of silicon carbide nano-phase on the wet erosive wear of polycrystalline alumina. J. Eur. Ceram. Soc. 1996, 16, 799–802. [Google Scholar] [CrossRef]
- Sternitzke, M.; Dupas, E.; Twigg, P.C.; Derby, B. Surface mechanical properties of alumina matrix nanocomposites. Acta Mater. 1997, 45, 3963–3973. [Google Scholar] [CrossRef]
- Chen, H.J.; Rainforth, W.M.; Lee, W.E. The wear behaviour of Al2O3-SiC ceramic nanocomposites. Scripta Mat. 2000, 42, 555–560. [Google Scholar] [CrossRef]
- De Arellano-Lopez, A.R.; Dominguez-Rodriguez, A.; Routbort, L. Microstructural constraints for creep in SiC-whisker-reinforced Al2O3. Acta Mater. 1998, 46, 6361–6373. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Shi, J.-L.; Zhang, Y.-F.; Lai, T.-R.; Guo, J.-K. Creep and creep-recovery behavior in silicon-carbide-particle-reinforced alumina. J. Am. Ceram. Soc. 1999, 82, 944–952. [Google Scholar] [CrossRef]
- Tai, Q.; Mocellin, A. Review: High temperature deformation of Al2O3-based ceramic particle or whisker composites. Ceram. Int. 1999, 25, 395–408. [Google Scholar] [CrossRef]
- Gao, G.; Cagin, T.; Goddard, W.A., III. Energetics, structure, thermodynamic and mechanical properties of nanotubes. Nanotechnology 1998, 9, 183–191. [Google Scholar] [CrossRef]
- Krishnan, A.; Dujardin, E.; Ebbesen, T.W.; Yianilos, P.N.; Treacy, M.M.J. Young’s modulus of single-walled nanotubes. Phys. Rev. B 1998, 58, 14013–14019. [Google Scholar] [CrossRef]
- Hernández, E.; Goze, C.; Bernier, P.; Rubio, A. Elastic properties of single-wall nanotubes. Appl. Phys. A 1999, 68, 287–292. [Google Scholar]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Berber, S.; Kwon, Y.-K.; Tománek, D. Unusually high thermal conductivity of carbon nanotubes. Phys. Rev. Lett. 2000, 84, 4613–4616. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.N.; Borsa, C.E.; Todd, R.I.; Davidge, R.W.; Brook, R.J. Fabrication, Characterisation and Properties of Alumina Matrix Nanocomposites. In British Ceramic Proceedings, No. 53: Novel Synthesis and Processing of Ceramics; Sale, F.R., Ed.; The Institute of Materials: London, UK, 1994; pp. 249–264. [Google Scholar]
- Timms, L.A.; Ponton, C.B. Processing of Al2O3/SiC nanocomposites—Part 1: Aqueous colloidal processing. J. Eur. Ceram. Soc. 2002, 22, 1553–1567. [Google Scholar] [CrossRef]
- Panda, P.K.; Mariappan, L.; Kannan, T.S. The effect of various reaction parameters on carbothermal reduction of kaolinite. Ceram. Int. 1999, 25, 467–473. [Google Scholar] [CrossRef]
- Amroune, A.; Fantozzi, G. Synthesis of Al2O3-SiC from kyanite precursor. J. Mater. Res. 2001, 16, 1609–1613. [Google Scholar] [CrossRef]
- Amroune, A.; Fantozzi, G.; Dubois, J.; Deloume, J.-P.; Durand, B.; Halimi, R. Formation of Al2O3-SiC powder from andalusite and carbon. Mater. Sci. Eng. 2000, A290, 11–15. [Google Scholar] [CrossRef]
- Xu, Y.; Nakahira, A.; Niihara, K. Characteristics of Al2O3-SiC nanocomposite prepared by sol-gel processing. J. Ceram. Soc. Jap. 1994, 102, 312–315. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.Z.; Hong, J.S.; Miyamoto, H.; Miyamoto, K.; Nishikawa, Y.; Torre, S.D.D.L. Mechanical properties and microstructure of nano-SiC-Al2O3 composites densified by spark plasma sintering. J. Eur. Ceram. Soc. 1999, 19, 609–613. [Google Scholar] [CrossRef]
- Wang, H.Z.; Gao, L.; Guo, J.K. The effect of nanoscale SiC particles on the microstructure of Al2O3 ceramics. Ceram. Int. 2000, 26, 391–396. [Google Scholar] [CrossRef]
- Abovyan, L.S.; Nersisyan, H.H.; Kharatyan, S.L.; Orru, R.; Saiu, R.; Cao, G.; Zedda, D. Synthesis of alumina-silicon carbide composites by chemically activated self-propagating reactions. Ceram. Int. 2001, 27, 163–169. [Google Scholar] [CrossRef]
- Tago, T.; Kawase, M.; Morita, K.; Hashimoto, K. Fabrication of silicon carbide whisker/alumina composite by thermal-gradient chemical vapor infiltration. J. Am. Ceram. Soc. 1999, 82, 3393–3400. [Google Scholar] [CrossRef]
- Su, B.; Sternitzke, M. Fourth Euro-Ceramics; Basic Science and Trends in Emerging Materials and Applications; Bellosi, A., Ed.; Grupp Editoriale Faenza Editrice: Faenza, Italy, 1995; Volume 4, pp. 109–116. [Google Scholar]
- Sternitzke, M.; Derby, B.; Brook, R.J. Alumina/silicon carbide nanocomposites by hybrid polymer/powder processing: Microstructures and mechanical properties. J. Am. Ceram. Soc. 1998, 81, 41–48. [Google Scholar] [CrossRef]
- Sawai, Y.; Yasutomi, Y. Effect of high-yield polycarbosilane addition on microstructure and mechanical properties of alumina. J. Ceram. Soc. Jpn. 1999, 107, 1146–1150. [Google Scholar] [CrossRef]
- Narisawa, M.; Okabe, Y.; Okamura, K.; Kurachi, Y. SiC-based fibers synthesized from hybrid polymer of polycarbosilane and polyvinylsilane. Key Eng. Mat. 1999, 164, 101–106. [Google Scholar] [CrossRef]
- Galusek, D.; Sedláček, J.; Riedel, R. Al2O3-SiC composites by warm pressing and sintering of an organosilicon polymer-coated alumina powder. J. Eur. Ceram. Soc. 2007, 27, 2385–2392. [Google Scholar] [CrossRef]
- Galusek, D.; Klement, R.; Sedláček, J.; Balog, M.; Fasel, C.; Zhang, J.; Crimp, M.A.; Riedel, R. Al2O3-SiC composites prepared by infiltration of pre-sintered alumina with a poly(allyl)carbosilane. J. Eur. Ceram. Soc. 2011, 31, 111–119. [Google Scholar] [CrossRef]
- Aguilar-Elguézabal, A.; Bocanegra-Bernal, M.H. Fracture behaviour of α-Al2O3 ceramics reinforced with a mixture of single-wall and multi-wall carbon nanotubes. Compos. B 2014, 60, 463–470. [Google Scholar] [CrossRef]
- Bi, S.; Hou, G.; Su, X.; Zhang, Y.; Guo, F. Mechanical properties and oxidation resistance of α-alumina/multi-walled carbon nanotube composite ceramics. Mater. Sci. Eng. A 2011, 528, 1596–1601. [Google Scholar] [CrossRef]
- Bakhsh, N.; Khalid, F.; Ahmad, H.; Saeed, A. Synthesis and characterization of pressureless sintered carbon nanotube reinforced alumina nanocomposites. Mater. Sci. Eng. A 2013, 578, 422–429. [Google Scholar] [CrossRef]
- Michálek, M.; Bodišová, K.; Michálková, M.; Sedláček, J.; Galusek, D. Alumina/MWCNTs composites by aqueous slip casting and pressureless sintering. Ceram. Int. 2013, 39, 6543–6550. [Google Scholar] [CrossRef]
- Hanzel, O.; Sedláček, J.; Šajgalík, P. New approach for distribution of carbon nanotubes in alumina matrix. J. Eur. Ceram. Soc. 2014, 34, 1845–1851. [Google Scholar] [CrossRef]
- Bi, S.; Su, X.J.; Hou, G.L.; Gu, G.Q.; Xiao, Z. Microstructural characterization of alumina-coated multi-walled carbon nanotubes synthesized by hydrothermal crystallization. Phys. B 2010, 405, 3312–3315. [Google Scholar] [CrossRef]
- Thaib, A.; Martin, G.A.; Pinheiro, P.; Schouler, M.C.; Gadelle, P. Formation of carbon nanotubes from the carbon monoxide disproportionation reaction over Co/Al2O3 and Co/SiO2 catalysts. Catal. Lett. 1999, 63, 135–141. [Google Scholar] [CrossRef]
- Mo, Y.H.; Kibria, A.K.M.F.; Nahm, K.S. The growth mechanism of carbon nanotubes from thermal cracking of acetylene over nickel catalyst supported on alumina. Synth. Met. 2001, 122, 443–447. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, H.B.; Lin, G.D.; Hong, Q.; Tsai, K.R. Growth of carbon nanotubes by catalytic decomposition of CH4 or CO on a Ni–MgO catalyst. Carbon 1997, 35, 1495–1501. [Google Scholar] [CrossRef]
- Beitollahi, A.; Pilehvari, S.H.; Faghihi Sani, M.A.; Moradi, H.; Akbarnejad, M. In situ growth of carbon nanotubes in alumina-zirconia nanocomposite matrix prepared by solution combustion method. Ceram. Int. 2012, 38, 3273–3280. [Google Scholar] [CrossRef]
- Merchan-Merchan, W.; Saveliev, A.V.; Kennedy, L.; Jimenez, W.C. Combustion synthesis of carbon nanotubes and related nanostructures. Prog. Energy Combust. Sci. 2010, 36, 696–727. [Google Scholar] [CrossRef]
- Çelik, Y.; Suvacı, E.; Weibel, A.; Peigney, A.; Flahaut, E. Texture development in Fe-doped alumina ceramics via templated grain growth and their application to carbon nanotube growth. J. Eur. Ceram. Soc. 2013, 33, 1093–1100. [Google Scholar] [CrossRef]
- Stearns, L.C.; Zhao, J.; Harmer, M.P. Processing and microstructure development in Al2O3-SiC nanocomposites. J. Eur. Ceram. Soc. 1992, 10, 473–477. [Google Scholar] [CrossRef]
- Jeong, Y.-K.; Nakahira, A.; Niihara, K. Effects of additives on microstructure and properties of alumina-silicon carbide nanocomposites. J. Am. Ceram. Soc. 1999, 82, 3609–3612. [Google Scholar] [CrossRef]
- Borsa, C.E.; Ferreira, H.S.; Kiminami, R.H.G.A. Liquid phase sintering of Al2O3/SiC nanocomposites. J. Eur. Ceram. Soc. 1999, 19, 615–621. [Google Scholar] [CrossRef]
- Sedláček, J.; Galusek, D.; Riedel, R.; Hoffmann, M.J. Sinter-HIP of polymer-derived Al2O3-SiC composites with high SiC contents. Mater. Lett. 2011, 65, 2462–2465. [Google Scholar] [CrossRef]
- Ahmad, I.; Unwin, M.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y.Q. Multi-walled carbon nanotubes reinforced Al2O3 nanocomposites: Mechanical properties and interfacial investigations. Comp. Sci. Technol. 2010, 70, 1199–1206. [Google Scholar] [CrossRef]
- Vasiliev, A.L.; Poyato, R.; Padture, N.P. Single-wall carbon nanotubes at ceramic grain boundaries. Scripta Mater. 2007, 56, 461–463. [Google Scholar] [CrossRef]
- Gao, L.; Jiang, L.; Sun, J. Carbon nanotube-ceramic composites. J. Electroceram. 2006, 17, 51–55. [Google Scholar]
- Zhang, S.C.; Fahrenholtz, W.G.; Hilmas, G.E.; Yadlowsky, E.J. Pressureless sintering of carbon nanotube-Al2O3 composites. J. Eur. Ceram. Soc. 2010, 30, 1373–1380. [Google Scholar] [CrossRef]
- Rice, R.W. Mechanical Properties of Ceramics and Composites: Grain and Particle Effects; CRC Press: New York, NY, USA, 2000. [Google Scholar]
- Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Wang, W.; Hashida, T. Microstructure-property relationships in pressureless-sintered carbon nanotube/alumina composites. Mater. Sci. Eng. 2014, A617, 179–186. [Google Scholar] [CrossRef]
- An, J.; You, D.; Lim, D. Tribological properties of hot-pressed alumina-CNT composites. Wear 2003, 255, 677–681. [Google Scholar] [CrossRef]
- Lim, D.-S.; An, J.-W.; Lee, H.J. Effect of carbon nanotube addition on the tribological behavior of carbon–carbon composites. Wear 2002, 252, 512–517. [Google Scholar] [CrossRef]
- Ahmad, K.; Pan, W. Hybrid nanocomposites: A new route towards tougher alumina ceramics. Comp. Sci. Technol. 2008, 68, 1321–1327. [Google Scholar] [CrossRef]
- Ahmad, K.; Pan, W. Dramatic effect of multiwalled carbon nanotubes on the electrical properties of alumina based ceramic nanocomposites. Comp. Sci. Technol. 2009, 69, 1016–1021. [Google Scholar] [CrossRef]
- Ahmad, K.; Pan, W.; Wan, C. Thermal conductivities of alumina-based multiwall carbon nanotube ceramic composites. J. Mater. Sci. 2014, 49, 6048–6055. [Google Scholar] [CrossRef]
- Carroll, L.; Sternitzke, M.; Derby, B. Silicon carbide particle size effects in alumina-based nanocomposites. Acta Mater. 1996, 44, 4543–4552. [Google Scholar] [CrossRef]
- Stearns, L.C.; Harmer, M.P. Particle-inhibited grain growth in Al2O3-SiC: I, experimental results. J. Am. Ceram. Soc. 1996, 79, 3013–3019. [Google Scholar] [CrossRef]
- Chou, I.A.; Chan, H.M.; Harmer, M.P. Machining-induced surface residual stress behavior in Al2O3-SiC. J. Am. Ceram. Soc. 1996, 79, 2403–2409. [Google Scholar] [CrossRef]
- Thompson, A.M.; Chan, H.M.; Harmer, M.P. Nanocomposites crack healing and stress relaxation in Al2O3 SiC “nanocomposites”. J. Am. Ceram. Soc. 1995, 78, 567–571. [Google Scholar] [CrossRef]
- Xu, Y.; Zangvil, A.; Kerber, A. SiC nanoparticle-reinforced Al2O3 matrix composites: Role of intra- and intergranular particles. J. Eur. Ceram. Soc. 1997, 17, 921–928. [Google Scholar] [CrossRef]
- Pezzotti, G.; Sakai, M. Effect of a silicon carbide “nano-dispersion” on the mechanical properties of silicon nitride. J. Am. Ceram. Soc. 1994, 77, 3039–3041. [Google Scholar] [CrossRef]
- Jiao, S.; Jenkins, M.L. A quantitative analysis of crack-interface interactions in alumina-based nanocomposites. Phil. Mag. A 1998, 78, 507–522. [Google Scholar] [CrossRef]
- Tiegs, T.N.; Becher, P.F. Thermal shock behavior of an Alumina-SiC whisker composite. J. Am. Ceram. Soc. 1987, 70, C109–C111. [Google Scholar] [CrossRef]
- Sedláček, J.; Galusek, D.; Švančárek, P.; Riedel, R.; Atkinson, A.; Wang, X. Abrasive wear of Al2O3-SiC and Al2O3-(SiC)-C composites with micrometer- and submicrometer-sized alumina matrix grains. J. Eur. Ceram. Soc. 2008, 28, 2983–2993. [Google Scholar] [CrossRef]
- Walker, C.N.; Borsa, C.E.; Todd, R.I.; Davidge, R.W.; Brook, R.J. Fabrication, characterisation and properties of alumina matrix nanocomposites. Br. Ceram. Proc. 1994, 53, 249–264. [Google Scholar]
- Anya, C.C. Wet erosive wear of alumina and its composites with SiC nano-particles. Ceram. Int. 1998, 24, 533–542. [Google Scholar] [CrossRef]
- Rodriguez, J.; Martin, A.; Pastor, J.Y.; Llorca, J.; Bartolome, J.F.; Moya, J.S. Sliding wear of alumina/silicon carbide nanocomposite. J. Am. Ceram. Soc. 1999, 82, 2252–2254. [Google Scholar] [CrossRef]
- Kara, H.; Roberts, S. Polishing behavior and surface quality of alumina and alumina/silicon carbide nanocomposites. J. Am. Ceram. Soc. 2000, 83, 1219–1225. [Google Scholar] [CrossRef]
- Levin, I.; Kaplan, W.D.; Brandon, D.G.; Layyous, A.A. Effect of SiC submicrometer particle size and content on fracture toughness of alumina-SiC nanocomposites. J. Am. Ceram. Soc. 1995, 78, 254–256. [Google Scholar] [CrossRef]
- Winn, A.J.; Todd, R.I. Microstructural requirements for alumina-SiC nanocomposites. Br. Ceram. Trans. 1999, 5, 219–224. [Google Scholar] [CrossRef]
- Luo, J.; Stevens, R. The role of residual stresses on the mechanical properties of Al2O3–5 vol% SiC nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 1565–1572. [Google Scholar] [CrossRef]
- Todd, R.I.; Limpichaipanit, A. Microstructure-property relationships in wear resistant alumina/SiC “nanocomposites”. Adv. Sci. Technol. 2006, 45, 555–563. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Shi, J.L.; Zhang, Y.F.; Lai, T.R.; Guo, J.K. Creep and creep behaviour in silicon carbide particle reinforced alumina. J. Am. Ceram. Soc. 1999, 82, 944–952. [Google Scholar] [CrossRef]
- Reveron, H.; Zaafrani, O.; Fantozzi, G. Microstructure development, hardness, toughness and creep behavior of pressureless sintered alumina/SiC micro/nano-composites obtained by slip-casting. J. Eur. Ceram. Soc. 2010, 30, 1351–1357. [Google Scholar] [CrossRef]
- Sternitzke, M. Review: Structural ceramic nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 1061–1082. [Google Scholar] [CrossRef]
- Ohji, T.; Hirano, T.; Nakahira, A.; Niihara, K. Particle/matrix interface and its role in creep inhibition in alumina/silicon carbide nanocomposites. J. Am. Ceram. Soc. 1996, 79, 33–45. [Google Scholar] [CrossRef]
- Ohji, T.; Nakahira, A.; Hirano, T.; Niikara, K. Tensile creep behaviour of alumina/silicon carbide nanocomposite. J. Am. Ceram. Soc. 1994, 77, 3259–3262. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Zhang, Y.F.; Shi, J.L.; Guo, J.K.; Jiang, D.Y. Pinning effect of SiC particles on mechanical properties of Al2O3/SiC ceramic matrix composites. J. Eur. Ceram. Soc. 1998, 18, 501–508. [Google Scholar] [CrossRef]
- Descamps, P.; O’Sullivan, D.; Poorteman, M.; Descamps, J.C.; Leriche, A.; Cambier, F. Creep behavior of Al2O3-SiC nanocomposites. J. Eur. Ceram. Soc. 1999, 19, 2475–2485. [Google Scholar] [CrossRef]
- Thompson, A.M.; Chan, H.M.; Harmer, M.P. Tensile creep of almina-silicon carbide nanocomposites. J. Am. Ceram. Soc. 1997, 80, 2221–2228. [Google Scholar] [CrossRef]
- Parchovianský, M.; Galusek, D.; Michálek, M.; Švančárek, P.; Kašiarová, M.; Dusza, J.; Hnatko, M. Effect of the volume fraction of SiC on the microstructure, and creep behavior of hot pressed Al2O3/SiC composites. Ceram. Int. 2014, 40, 1807–1814. [Google Scholar] [CrossRef]
- Hayashi, T.; Endo, M. Carbon nanotubes as structural material and their application in composites. Compos. B 2011, 42, 2151–2157. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H.; Echeberria, J.; Ollo, J.; Garcia-Reyes, A.; Domínguez-Rios, C.; Reyes-Rojas, A.; Aguilar-Elguezabal, A. A comparison of the effects of multi-wall and single-wall carbon nanotube additions on the properties of zirconia toughened alumina composites. Carbon 2011, 49, 1599–1607. [Google Scholar] [CrossRef]
- Li, G.H.; Hu, Z.X.; Zhang, L.D.; Zhang, Z.R. Elastic modulus of nano-alumina composite. J. Mater. Sci. Lett. 1998, 17, 1185–1186. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Inam, F.; Peijs, T.; Reece, M.J. The production of advanced fine-grained alumina by carbon nanotube addition. J. Eur. Ceram. Soc. 2011, 31, 2853–2859. [Google Scholar] [CrossRef]
- Laurent, C.; Peigney, A.; Dumortier, O.; Rousset, A. Carbon nanotubes-Fe-alumina nanocomposites. Part II: Microstructure and mechanical properties of the hot-pressed composites. J. Eur. Ceram. Soc. 1998, 18, 2005–2013. [Google Scholar]
- Michálek, M.; Sedláček, J.; Parchovianský, M.; Michálková, M.; Galusek, D. Mechanical properties and electrical conductivity of alumina/MWCNT and alumina/zirconia/MWCNT composites. Ceram. Int. 2014, 40, 1289–1295. [Google Scholar] [CrossRef]
- Zhan, D.D.; Kuntz, J.D.; Wan, J.U.; Mukherjee, A.K. Single-wall carbon nanotubes as attractive toughening agents in alumina-based nanocomposites. Nat. Mater. 2003, 2, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Zhao, D.Q.; Wu, M.S.; Xu, Z.N.; Song, J. Preparation and microstructure of multi-wall carbon nanotubes-toughened Al2O3 composite. J. Am. Ceram. Soc. 2006, 89, 750–753. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Jin, X.H. Reinforcement of alumina matrix with multi-walled carbon nanotubes. Ceram. Int. 2005, 31, 893–896. [Google Scholar] [CrossRef]
- Fan, J.P.; Zhuang, D.M.; Zhao, D.Q.; Zhang, G.; Wu, M.S.; Wei, F.; Fan, Z.-J. Toughening and reinforcing alumina matrix composite with single-wall carbon nanotubes. Appl. Phys. Lett. 2006, 89, 121910:1–121910:3. [Google Scholar]
- Duszova, A.; Dusza, J.; Tomasek, K.; Morgiel, J.; Blugan, G.; Kuebler, J. Zirconia/carbon nanofiber composite. Scripta Mater. 2008, 58, 520–523. [Google Scholar] [CrossRef]
- Cha, S.I.; Kim, K.T.; Lee, K.H.; Mo, C.B.; Hong, S.H. Strengthening and toughening of carbon nanotube reinforced alumina nanocomposite fabricated by molecular level mixing process. Scripta. Mater. 2005, 53, 793–797. [Google Scholar] [CrossRef]
- Sun, J.; Gao, L.; Li, W. Colloidal processing of carbon nanotube/alumina composites. Chem. Mater. 2002, 14, 5169–5172. [Google Scholar] [CrossRef]
- Siegel, R.W.; Chang, S.K.; Ash, B.J.; Stone, J.; Ajayan, P.M.; Doremus, R.W.; Schadler, L.S. Mechanical behavior of polymer and ceramic matrix nanocomposites. Scripta Mater. 2001, 44, 2061–2064. [Google Scholar] [CrossRef]
- Poyato, R.; Vasiliev, A.L.; Padture, N.P.; Tanaka, H.; Nishimura, T. Aqueous colloidal processing of single-wall carbon nanotubes and their composites with ceramics. Nanotechnology 2006, 17, 1770–1777. [Google Scholar] [CrossRef]
- Ahmad, I.; Cao, H.; Chen, H.; Zhao, H.; Kennedy, A.; Zhu, Y.Q. Carbon nanotube toughened aluminium oxide nanocomposite. J. Eur. Ceram. Soc. 2010, 30, 865–873. [Google Scholar] [CrossRef]
- Estili, M.; Kawasaki, A. Engineering strong intergraphene shear resistance in multi-walled carbon nanotubes and dramatic tensile improvements. Adv. Mater. 2010, 22, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Padture, N.P.; Tanaka, H. Contact-damage-resistant ceramic/single-wall carbon nanotubes and ceramic/graphite composites. Nat. Mater. 2004, 3, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.S.; Bal, S. Carbon nanotube reinforced ceramic matrix composite—A review. J. Min. Mater. Char. Eng. 2008, 7, 355–370. [Google Scholar]
- Wong, E.W.; Sheehan, P.E.; Liebert, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Yamamoto, G.; Shirasu, K.; Nozaka, Y.; Sato, Y.; Takagi, T.; Hashida, T. Structure-property relationships in thermally-annealed multi-walled carbon nanotubes. Carbon 2014, 66, 219–226. [Google Scholar] [CrossRef]
- Corral, E.L.; Garary, J.; Barrera, E.V. Engineering nanostructure for single-walled carbon nanotubes reinforced silicon nitride nanocomposites. J. Am. Ceram. Soc. 2008, 91, 3129–3137. [Google Scholar] [CrossRef]
- Fan, J.; Zhao, D.; Song, J. Preparation and microstructure of multi-walled carbon nanotubes toughened Al2O3 composite. J. Am. Ceram. Soc. 2006, 89, 750–753. [Google Scholar] [CrossRef]
- Yamamoto, G.; Omori, M.; Hashida, T.; Kimura, H. A novel structure for carbon nanotube reinforced alumina composites with improved mechanical properties. Nanotechnology 2008, 19, 315708:1–315708:7. [Google Scholar]
- Peigney, A.; Garcia, F.L.; Estournès, C.A.; Weibel, C.L. Toughening and hardening in double-walled carbon nanotube/nanostructured magnesia composites. Carbon 2010, 48, 1952–1960. [Google Scholar] [CrossRef]
- Xia, Z.; Riester, L.; Curtin, W.A.; Li, H.; Sheldon, B.W.; Liang, J.; Chang, B.; Xu, J.M. Direct observation of toughening mechanisms in carbon nanotube ceramic matrix composites. Acta Mater. 2004, 52, 931–944. [Google Scholar] [CrossRef]
- Xia, Z.; Curtin, W.A.; Sheldon, B.W. Fracture toughness of highly ordered carbon nanotube/alumina nanocomposites. J. Eng. Mater. Technol. Trans. ASME 2004, 126, 238–244. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Huang, L.W.; Zhang, J.Y.; Todd, R. Ultra-fast densification of CNTs reinforced alumina based on combustion reaction and quick pressing. Sci. China Technol. Sci. 2012, 55, 484–489. [Google Scholar] [CrossRef]
- Hirota, K.; Takaura, Y.; Kato, M.; Miyamoto, Y. Fabrication of carbon nanofibers (CNF)-dispersed Al2O3 composites by pulsed electric current pressure sintering and their mechanical and electrical properties. J. Mater. Sci. 2007, 42, 4792–4800. [Google Scholar] [CrossRef]
- Blugan, G.; Michalkova, M.; Hnatko, M.; Šajgalík, P.; Minghetti, T.; Schelle, C.; Graule, T.; Kuebler, J. Processing and properties of alumina–carbon nano fibre ceramic composites using standard ceramic technology. Ceram. Int. 2011, 37, 3371–3379. [Google Scholar] [CrossRef]
- Borrell, A.; Torrecillas, R.; Rocha, V.G.; Fernández, A.; Bonache, V.; Salvador, M.D. Effect of CNFs content on the tribological behaviour of spark plasma sintering ceramic-CNFs composites. Wear 2012, 274–275, 94–99. [Google Scholar]
- Borrell, A.; Alvarez, I.; Torrecillas, R.; Rocha, V.G.; Fernandez, A. Microstructural design form mechanical and electrical properties of spark plasma sintered Al2O3-SiC nanocomposites. Mater. Sci. Eng. 2012, A534, 693–698. [Google Scholar] [CrossRef]
- Barea, R.; Belmonte, M.; Osendi, M.I.; Miranzo, P. Thermal conductivity of Al2O3/SiC platelet composites. J. Eur. Ceram. Soc. 2003, 23, 1773–1778. [Google Scholar] [CrossRef]
- Fabbri, L.; Scafé, E.; Dinelli, G. Thermal and elastic properties of alumina-silicon carbide whiskers composites. J. Eur. Ceram. Soc. 1994, 14, 441–446. [Google Scholar] [CrossRef]
- Reddy, P.; Castelino, K.; Majumdar, A. Diffuse mismatch model of thermal boundary conductance using exact phonon dispersions. Appl. Phys. Lett. 2005, 87, 315–322. [Google Scholar] [CrossRef]
- Hasselman, D.P.H.; Johnson, L.F. Effective thermal conductivity of composites with interfacial thermal barrier resistance. J. Comp. Mater. 1987, 21, 508–515. [Google Scholar] [CrossRef]
- Parchovianský, M.; Galusek, D.; Švančárek, P.; Sedláček, J.; Šajgalík, P. Thermal behavior, electrical conductivity and microstructure of hot pressed Al2O3/SiC nanocomposites. Ceram. Int. 2014, 14, 14421–14429. [Google Scholar] [CrossRef]
- Rul, S.; Lefevre-Schlick, F.; Capria, E.; Laurent, C.; Peigney, A. Percolation of single-walled carbon nanotubes in ceramic matrix nanocomposites. Acta Mater. 2004, 52, 1061–1067. [Google Scholar] [CrossRef]
- Ahmad, K.; Pan, W.; Shi, S.L. Electrical conductivity and dielectric properties of multiwalled carbon nanotube and alumina composites. Appl. Phys. Lett. 2006, 89, 133122–133122. [Google Scholar] [CrossRef]
- Zhan, G.D.; Mukherjee, A.K. Carbon nanotube reinforced alumina-based ceramics with novel mechanical, electrical, and thermal properties. Int. J. Appl. Ceram. Technol. 2004, 1, 161–171. [Google Scholar] [CrossRef]
- Bergman, D.J. Exactly solvable microscopic geometries and rigorous bounds for the complex dielectric constant of a two-component composite material. Phys. Rev. Lett. 1980, 44, 1285–1287. [Google Scholar] [CrossRef]
- Kilbride, B.E.; Coleman, J.N.; Fraysse, J.; Fournet, P.; Cadek, M.; Drury, A.; Hutzler, S.; Roth, S.; Blau, W.J. Experimental observation of scaling laws for alternating current and direct current conductivity in polymer-carbon nanotube composite thin films. J. Appl. Phys. 2002, 92, 4024–4030. [Google Scholar] [CrossRef]
- Allaoui, A.; Bai, S.; Cheng, H.M.; Bai, J.B. Mechanical and electrical properties of a MWNT/epoxy composite. Comp. Sci. Technol. 2002, 62, 1993–1998. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Comp. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Zhou, X.W.; Zhu, Y.F.; Liang, J. Preparation and properties of powder styrene-butadiene rubber composites filled with carbon black and carbon nanotubes. Mater. Res. Bull. 2007, 42, 456–464. [Google Scholar] [CrossRef]
- Nan, C.-W. Physics of inhomogeneous inorganic materials. Prog. Mater. Sci. 1993, 37, 1–116. [Google Scholar] [CrossRef]
- Song, Y.; Noh, T.W.; Lee, S.-I.; Gaines, J.R. Experimental study of the three-dimensional AC conductivity and dielectric constant of a conductor-insulator composite near the percolation threshold. Phys. Rev. B 1986, 33, 904–908. [Google Scholar] [CrossRef]
- Kumari, L.; Zhang, T.; Du, G.H.; Li, W.Z.; Wang, Q.W.; Datye, A.; Wu, K.H. Thermal properties of CNT-Alumina nanocomposites. Compos. Sci. Technol. 2008, 68, 2178–2183. [Google Scholar] [CrossRef]
- Bakshi, S.R.; Balani, K.; Agarwal, A. Thermal conductivity of plasma-sprayed aluminum oxide-multiwalled carbon nanotube composites. J. Am. Ceram. Soc. 2008, 91, 942–947. [Google Scholar] [CrossRef]
- Zhang, H.L.; Li, J.F.; Zhang, B.P.; Yao, K.F.; Liu, W.S.; Wang, H. Electrical and thermal properties of carbon nanotube bulk materials: Experimental studies for the 328–958 K temperature range. Phys. Rev. B 2007, 75, 205407:1–205407:9. [Google Scholar]
- Dresselhaus, M.S.; Eklund, P.C. Phonons in carbon nanotubes. Adv. Phys. 2000, 49, 705–814. [Google Scholar] [CrossRef]
- Biercuk, M.J.; Llaguno, M.C.; Radosavljevic, M.; Hyun, J.K.; Johnson, A.T.; Fischer, J.E. Carbon nanotube composites for thermal management. Appl. Phys. Lett. 2002, 80, 2767–2769. [Google Scholar] [CrossRef]
- Yang, D.J.; Zhang, Q.; Chen, G.; Ahn, J.; Wang, S.G.; Zhou, Q.; Li, J.Q. Thermal conductivity of multiwalled carbon nanotubes. Phys. Rev. B 2002, 66, 165440:1–165440:6. [Google Scholar]
- Zhang, H.L.; Li, J.F.; Yao, K.F.; Chen, L.D. Spark plasma sintering and thermal conductivity of carbon nanotube bulk materials. J. Appl. Phys. 2005, 97, 114310:1–114310:5. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galusek, D.; Galusková, D. Alumina Matrix Composites with Non-Oxide Nanoparticle Addition and Enhanced Functionalities. Nanomaterials 2015, 5, 115-143. https://doi.org/10.3390/nano5010115
Galusek D, Galusková D. Alumina Matrix Composites with Non-Oxide Nanoparticle Addition and Enhanced Functionalities. Nanomaterials. 2015; 5(1):115-143. https://doi.org/10.3390/nano5010115
Chicago/Turabian StyleGalusek, Dušan, and Dagmar Galusková. 2015. "Alumina Matrix Composites with Non-Oxide Nanoparticle Addition and Enhanced Functionalities" Nanomaterials 5, no. 1: 115-143. https://doi.org/10.3390/nano5010115
APA StyleGalusek, D., & Galusková, D. (2015). Alumina Matrix Composites with Non-Oxide Nanoparticle Addition and Enhanced Functionalities. Nanomaterials, 5(1), 115-143. https://doi.org/10.3390/nano5010115




