Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability
Abstract
:1. Introduction
2. Results and Discussion
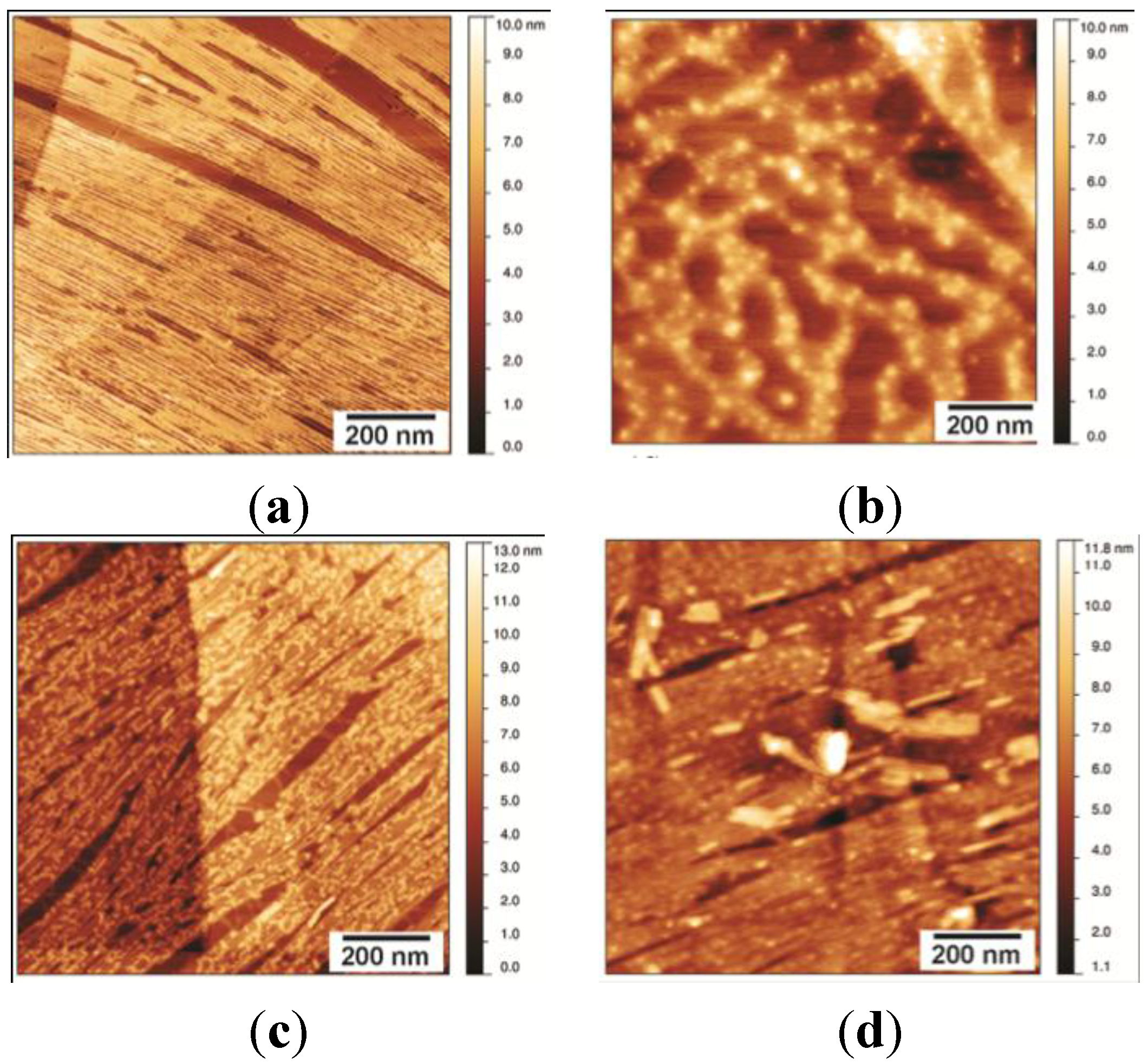
2.1. EAS∆15 and DewA Class I Hydrophobins Form Rodlets with Similar Morphology in Spite of Differences in Protein Sequence and Solution Structure
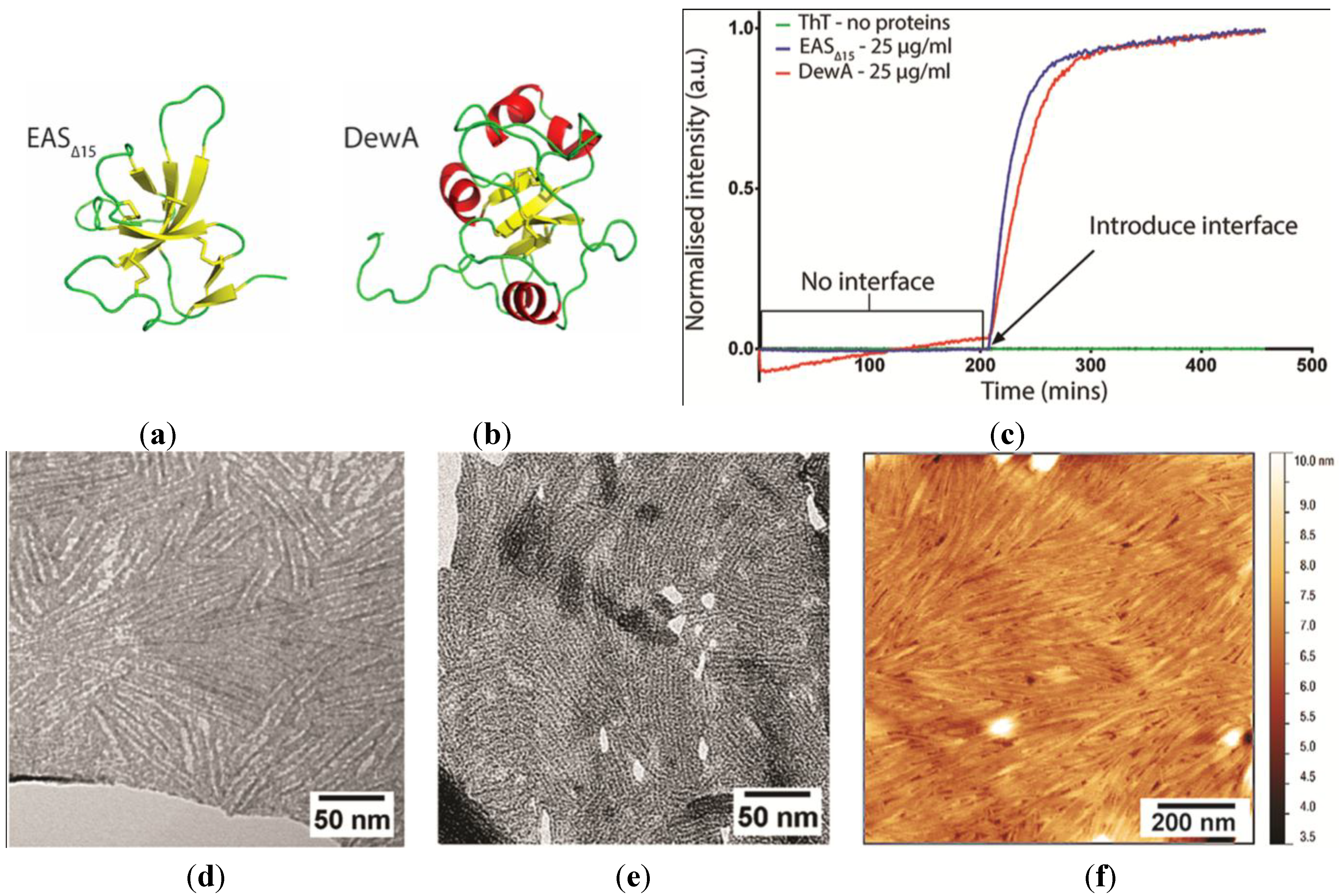
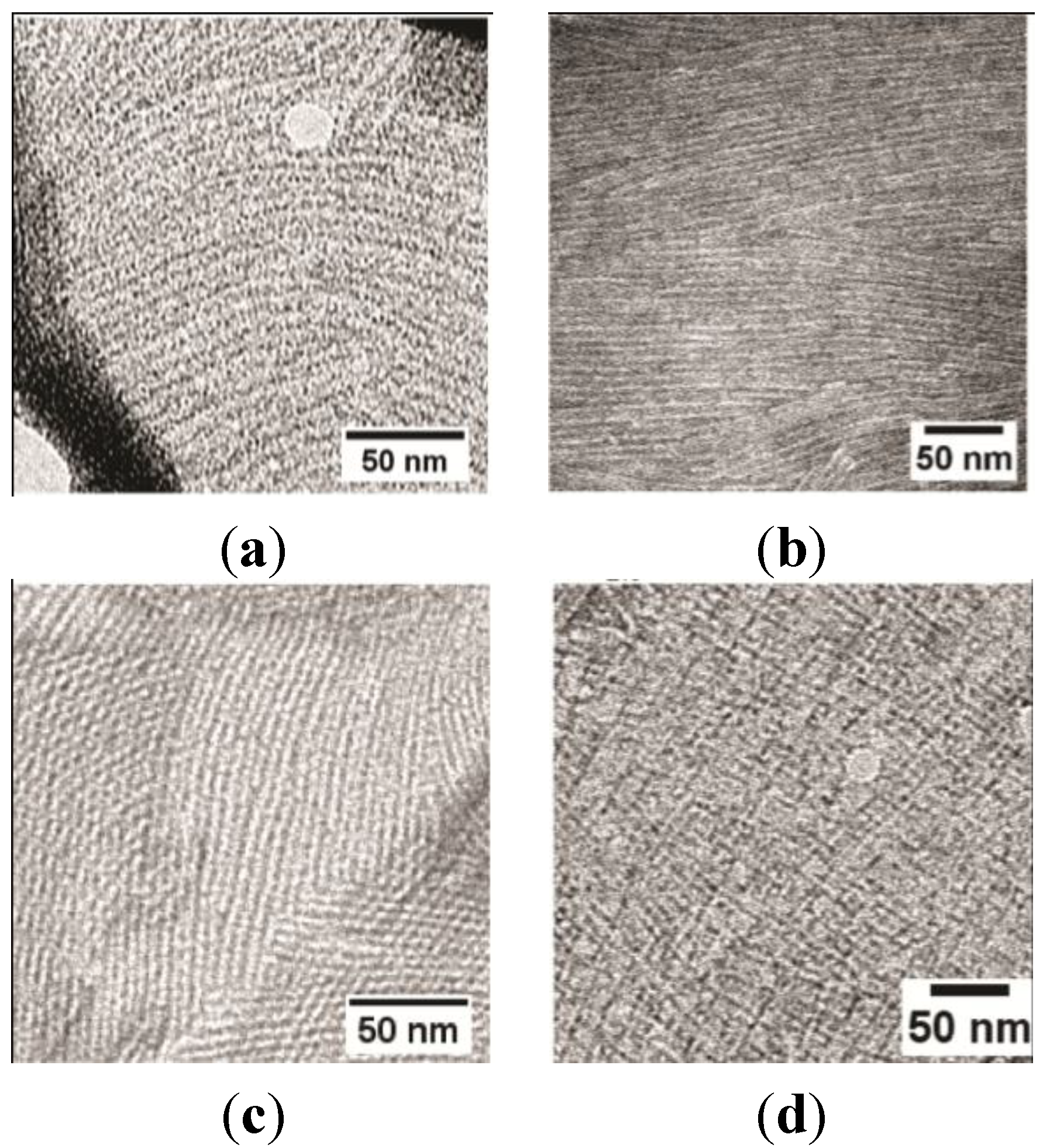
2.2. EASΔ15 Forms a Single Layer Composed of Rodlets on Highly Oriented Pyrolytic Graphite
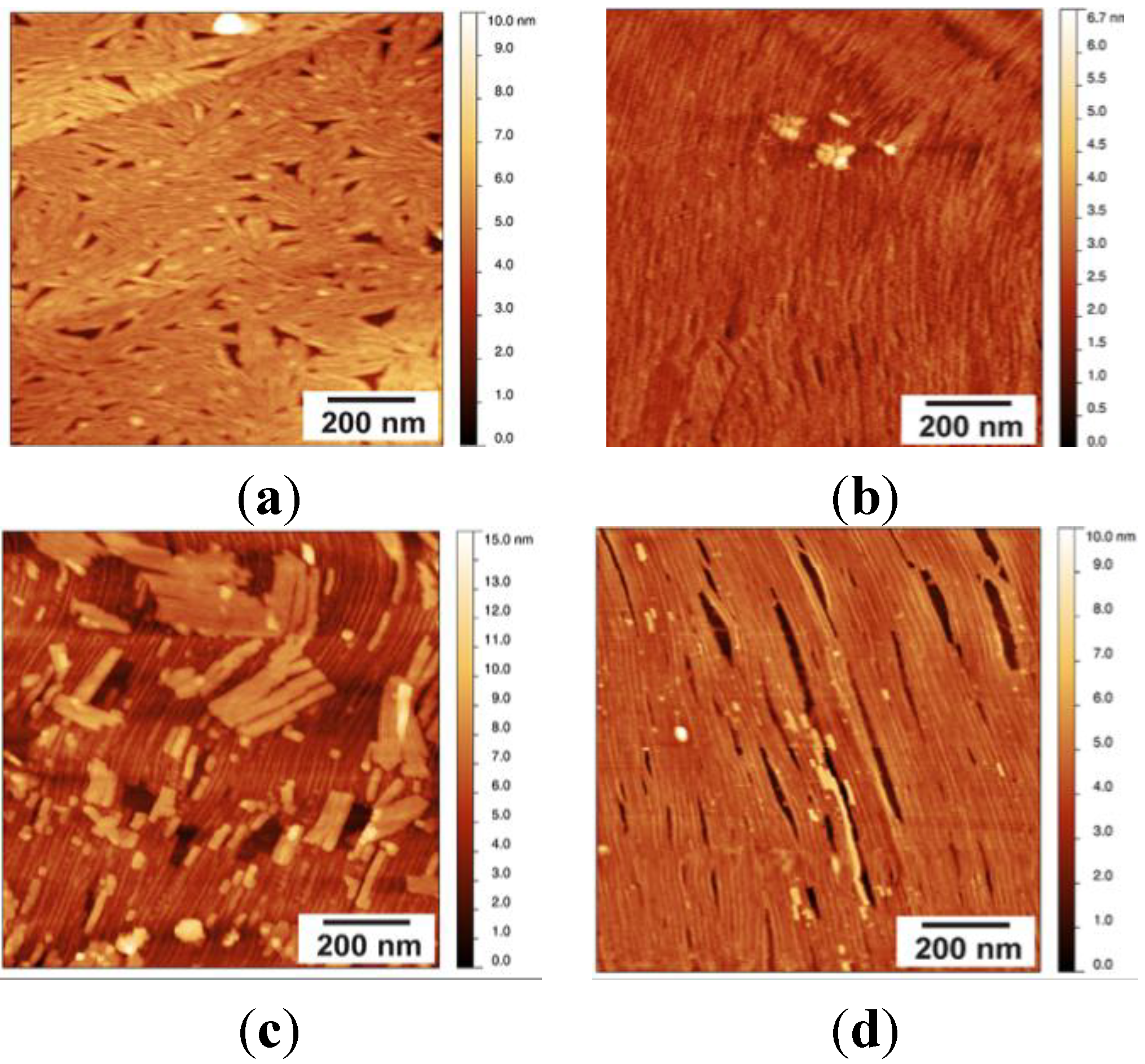
Stability of the EASΔ15 Rodlet Layer
2.3. NC2 Self-Assembles into a Protein Mesh
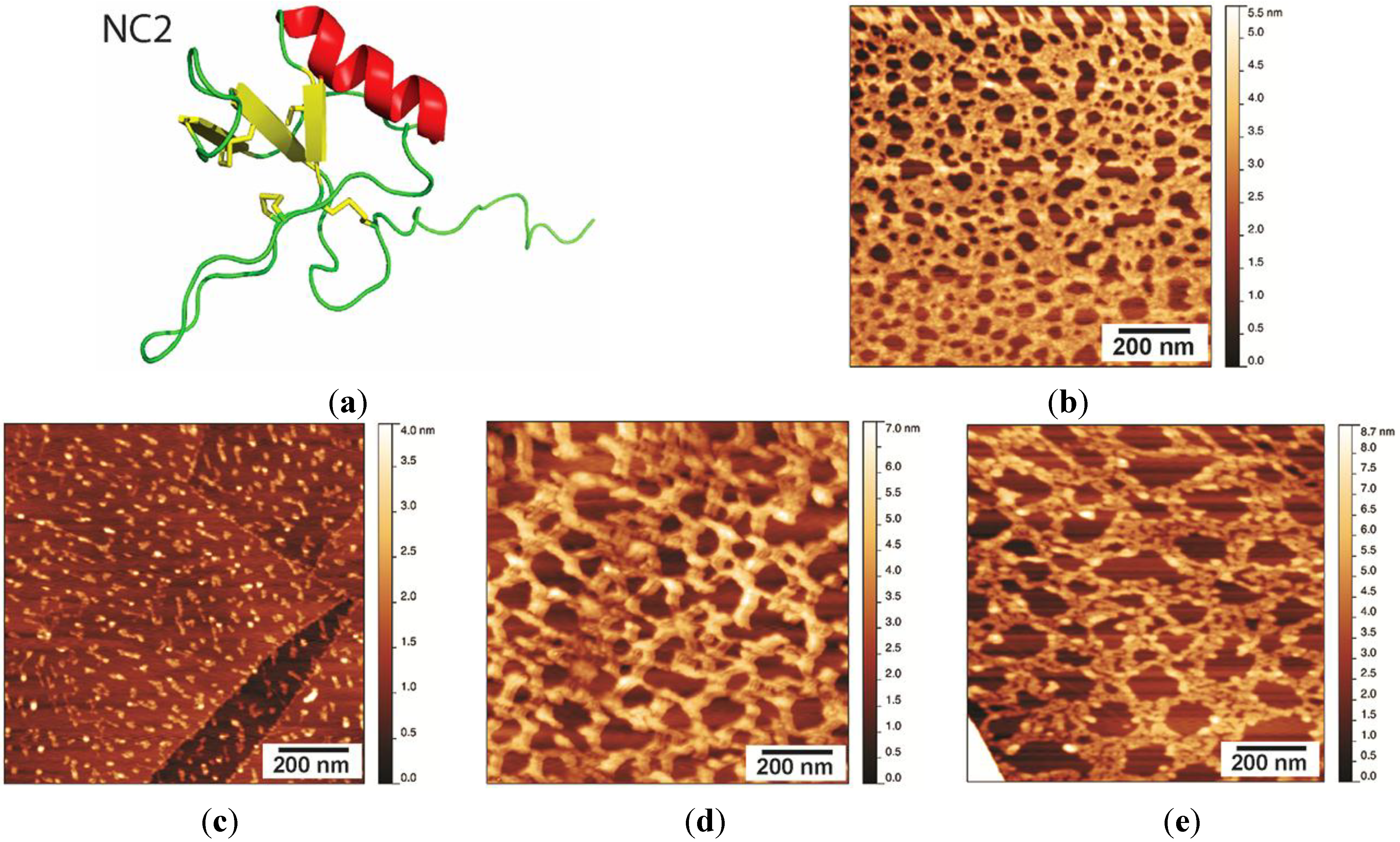
Stability of the NC2 Layer
2.4. Self-Assembly by the Chimeric Hydrophobin NChi2 on Highly Oriented Pyrolytic Graphite
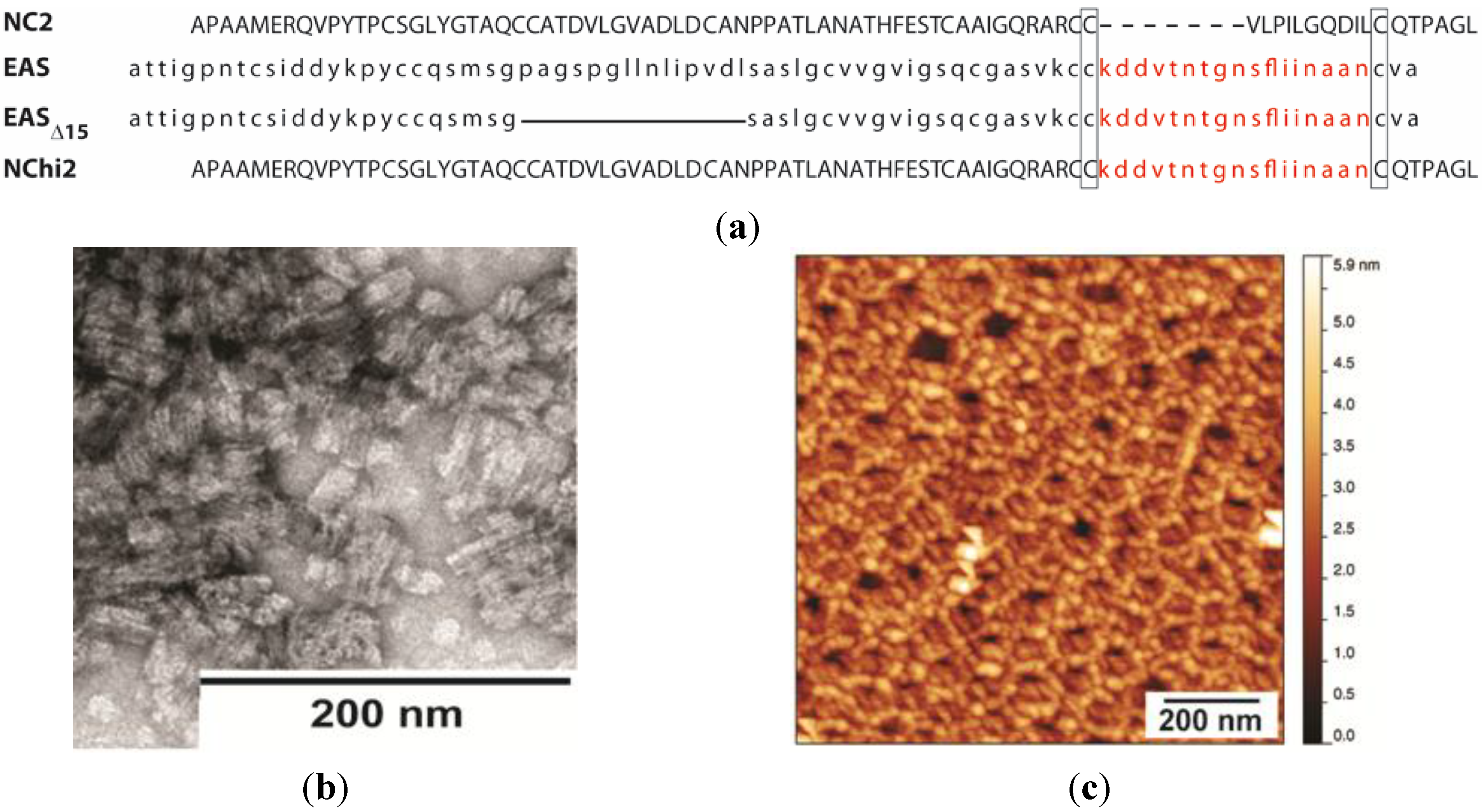
Stability of the NChi2 Layer

3. Experimental Section
3.1. Hydrophobin Production
3.2. Thioflavin T Assay
3.3. Transmission Electron Microscopy
3.4. Atomic Force Microscopy
4. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Linder, M.B.; Szilvay, G.R.; Nakari-Setala, T.; Penttila, M.E. Hydrophobins: The protein-amphiphiles of filamentous fungi. FEMS Microbiol. Rev. 2005, 29, 877–896. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latge, J.P. Hydrophobins—Unique fungal proteins. PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Zykwinska, A.; Pihet, M.; Radji, S.; Bouchara, J.P.; Cuenot, S. Self-assembly of proteins into a three-dimensional multilayer system: Investigation of the surface of the human fungal pathogen aspergillus fumigatus. Biochim. Biophys. Acta Proteins Proteomics 2014, 1844, 1137–1144. [Google Scholar] [CrossRef] [Green Version]
- Zykwinska, A.; Guillemette, T.; Bouchara, J.P.; Cuenot, S. Spontaneous self-assembly of SC3 hydrophobins into nanorods in aqueous solution. Biochim. Biophys. Acta Proteins Proteomics 2014, 1844, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Morris, V.K.; Kwan, A.H.; Sunde, M. Analysis of the structure and conformational states of DewA gives insight into the assembly of the fungal hydrophobins. J. Mol. Biol. 2013, 425, 244–256. [Google Scholar] [CrossRef]
- Houmadi, S.; Ciuchi, F.; de Santo, M.P.; de Stefano, L.; Rea, I.; Giardina, P.; Armenante, A.; Lacaze, E.; Giocondo, M. Langmuir-blodgett film of hydrophobin protein from pleurotus ostreatus at the air-water interface. Langmuir 2008, 24, 12953–12957. [Google Scholar] [CrossRef]
- Morris, V.K.; Sunde, M. Formation of amphipathic amyloid monolayers from fungal hydrophobin proteins. Methods Mol. Biol. 2013, 996, 119–129. [Google Scholar] [PubMed]
- Szilvay, G.R.; Paananen, A.; Laurikainen, K.; Vuorimaa, E.; Lemmetyinen, H.; Peltonen, J.; Linder, M.B. Self-assembled hydrophobin protein films at the air-water interface: Structural analysis and molecular engineering. Biochemistry 2007, 46, 2345–2354. [Google Scholar] [CrossRef] [PubMed]
- Bimbo, L.M.; Sarparanta, M.; Makila, E.; Laaksonen, T.; Laaksonen, P.; Salonen, J.; Linder, M.B.; Hirvonen, J.; Airaksinen, A.J.; Santos, H.A. Cellular interactions of surface modified nanoporous silicon particles. Nanoscale 2012, 4, 3184–3192. [Google Scholar] [CrossRef]
- Khalesi, M.; Deckers, S.M.; Gebruers, K.; Vissers, L.; Verachtert, H.; Derdelinckx, G. Hydrophobins: Exceptional proteins for many applications in brewery environment and other bio-industries. Cerevisia 2012, 37, 3–9. [Google Scholar] [CrossRef]
- Sarparanta, M.P.; Bimbo, L.M.; Mäkilä, E.M.; Salonen, J.J.; Laaksonen, P.H.; Helariutta, A.M.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J.; Santos, H.A.; et al. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials 2012, 33, 3353–3362. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, S.; Gravagnuolo, A.M.; Rea, I.; de Stefano, L.; Marino, G.; Giardina, P. Hydrophobin-coated plates as matrix-assisted laser desorption/ionization sample support for peptide/protein analysis. Anal. Biochem. 2014, 449, 9–16. [Google Scholar] [CrossRef]
- Yang, W.; Ren, Q.; Wu, Y.-N.; Morris, V.K.; Rey, A.A.; Braet, F.; Kwan, A.H.; Sunde, M. Surface functionalization of carbon nanomaterials by self-assembling hydrophobin proteins. Biopolymers 2013, 99, 84–94. [Google Scholar] [CrossRef]
- Ren, Q.; Kwan, A.H.; Sunde, M. Two forms and two faces, multiple states and multiple uses: Properties and applications of the self-assembling fungal hydrophobins. Biopolymers 2013, 100, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Gruner, L.J.; Ostermann, K.; Rodel, G. Layer thickness of hydrophobin films leads to oscillation in wettability. Langmuir 2012, 28, 6942–6949. [Google Scholar] [CrossRef]
- Lienemann, M.; Gandier, J.A.; Joensuu, J.J.; Iwanaga, A.; Takatsuji, Y.; Haruyama, T.; Master, E.; Tenkanen, M.; Linder, M.B. Structure-function relationships in hydrophobins: Probing the role of charged side chains. Appl. Environ. Microbiol. 2013, 79, 5533–5538. [Google Scholar] [CrossRef]
- Kwan, A.H.; Macindoe, I.; Vukasin, P.V.; Morris, V.K.; Kass, I.; Gupte, R.; Mark, A.E.; Templeton, M.D.; Mackay, J.P.; Sunde, M. The Cys3–Cys4 loop of the hydrophobin EAS is not required for rodlet formation and surface activity. J. Mol. Biol. 2008, 382, 708–720. [Google Scholar] [CrossRef]
- Kwan, A.H.; Winefield, R.D.; Sunde, M.; Matthews, J.M.; Haverkamp, R.G.; Templeton, M.D.; Mackay, J.P. Structural basis for rodlet assembly in fungal hydrophobins. Proc. Natl. Acad. Sci. USA 2006, 103, 3621–3626. [Google Scholar] [CrossRef]
- Ren, Q.; Kwan, A.H.; Sunde, M. Solution structure and interface-driven self-assembly of NC2, a new member of the Class II hydrophobin proteins. Proteins 2014, 82, 990–1003. [Google Scholar] [CrossRef]
- Macindoe, I.; Kwan, A.H.; Ren, Q.; Morris, V.K.; Yang, W.; Mackay, J.P.; Sunde, M. Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS. Proc. Natl. Acad. Sci. USA 2012, 109, E804–E811. [Google Scholar] [PubMed]
- Butko, P.; Buford, J.P.; Goodwin, J.S.; Stroud, P.A.; McCormick, C.L.; Cannon, G.C. Spectroscopic evidence for amyloid-like interfacial self-assembly of hydrophobin Sc3. Biochem. Biophys. Res. Commun. 2001, 280, 212–215. [Google Scholar] [CrossRef]
- Longobardi, S.; Picone, D.; Ercole, C.; Spadaccini, R.; de Stefano, L.; Rea, I.; Giardina, P. Environmental conditions modulate the switch among different states of the hydrophobin Vmh2 from Pleurotus ostreatus. Biomacromolecules 2012, 13, 743–750. [Google Scholar] [CrossRef]
- Morris, V.K.; Ren, Q.; Macindoe, I.; Kwan, A.H.; Byrne, N.; Sunde, M. Recruitment of Class I hydrophobins to the air:water interface initiates a multi-step process of functional amyloid formation. J. Biol. Chem. 2011, 286, 15955–15963. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, Version 1.3r1; Schrödinger, L.L.C.: Portland, OR, USA, 2010.
- Yu, L.; Zhang, B.; Szilvay, G.R.; Sun, R.; Janis, J.; Wang, Z.; Feng, S.; Xu, H.; Linder, M.B.; Qiao, M. Protein HGFI from the edible mushroom Grifola frondosa is a novel 8 kda Class I hydrophobin that forms rodlets in compressed monolayers. Microbiology 2008, 154, 1677–1685. [Google Scholar]
- Wösten, H.A.B.; de Vocht, M.L. Hydrophobins, the fungal coat unravelled. Biochim. Biophys. Acta Rev. Biomembr. 2000, 1469, 79–86. [Google Scholar] [CrossRef]
- Wosten, H.A.B.; Devries, O.M.H.; Wessels, J.G.H. Interfacial self-assembly of a fungal hydrophobin into a hydrophobic rodlet layer. Plant Cell 1993, 5, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Templeton, M.D.; Greenwood, D.R.; Beever, R.E. Solubilization of Neurospora crassa rodlet proteins and identification of the predominant protein as the proteolytically processed eas (ccg-2) gene product. Exp. Mycol. 1995, 19, 166–169. [Google Scholar] [CrossRef]
- Hou, S.; Li, X.; Li, X.; Feng, X.Z.; Wang, R.; Wang, C.; Yu, L.; Qiao, M.Q. Surface modification using a novel type I hydrophobin HGFI. Anal. Bioanal. Chem. 2009, 394, 783–789. [Google Scholar] [CrossRef]
- Askolin, S.; Linder, M.; Scholtmeijer, K.; Tenkanen, M.; Penttila, M.; de Vocht, M.L.; Wosten, H.A. Interaction and comparison of a Class I hydrophobin from Schizophyllum commune and Class II hydrophobins from Trichoderma reesei. Biomacromolecules 2006, 7, 1295–1301. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Wang, H.C.; Qin, X.; Wang, X.S.; Qiao, M.Q.; Anzai, J.; Chen, Q. Self-assembled film of hydrophobins on gold surfaces and its application to electrochemical biosensing. Colloids Surf. B 2009, 71, 102–106. [Google Scholar] [CrossRef]
- Gwyddion Version 2.30. Available online: http://gwyddion.net/download/2.30/ (accessed on 29 September 2009).
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lo, V.C.; Ren, Q.; Pham, C.L.L.; Morris, V.K.; Kwan, A.H.; Sunde, M. Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability. Nanomaterials 2014, 4, 827-843. https://doi.org/10.3390/nano4030827
Lo VC, Ren Q, Pham CLL, Morris VK, Kwan AH, Sunde M. Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability. Nanomaterials. 2014; 4(3):827-843. https://doi.org/10.3390/nano4030827
Chicago/Turabian StyleLo, Victor C., Qin Ren, Chi L. L. Pham, Vanessa K. Morris, Ann H. Kwan, and Margaret Sunde. 2014. "Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability" Nanomaterials 4, no. 3: 827-843. https://doi.org/10.3390/nano4030827
APA StyleLo, V. C., Ren, Q., Pham, C. L. L., Morris, V. K., Kwan, A. H., & Sunde, M. (2014). Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability. Nanomaterials, 4(3), 827-843. https://doi.org/10.3390/nano4030827