Molecularly Imprinted Nanomaterials for Sensor Applications
Abstract
:1. Introduction
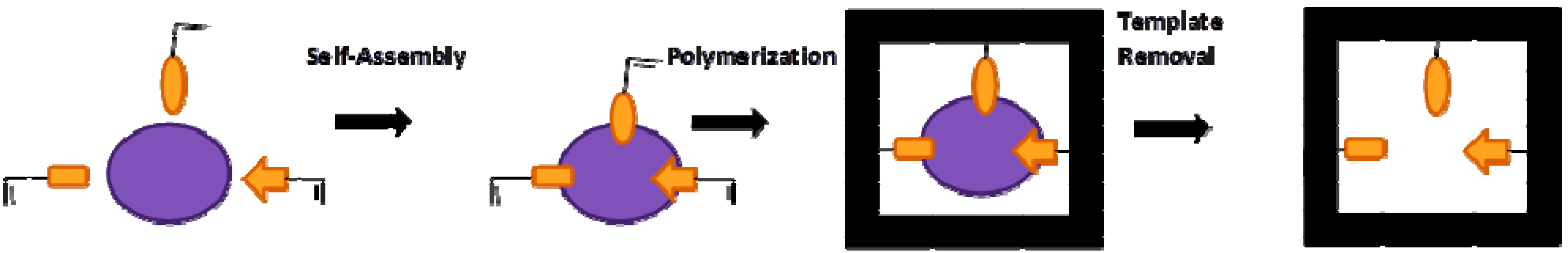
2. Molecular Imprinted Nanomaterials
2.1. Imprinted Nanoparticles (Imp-NPs)
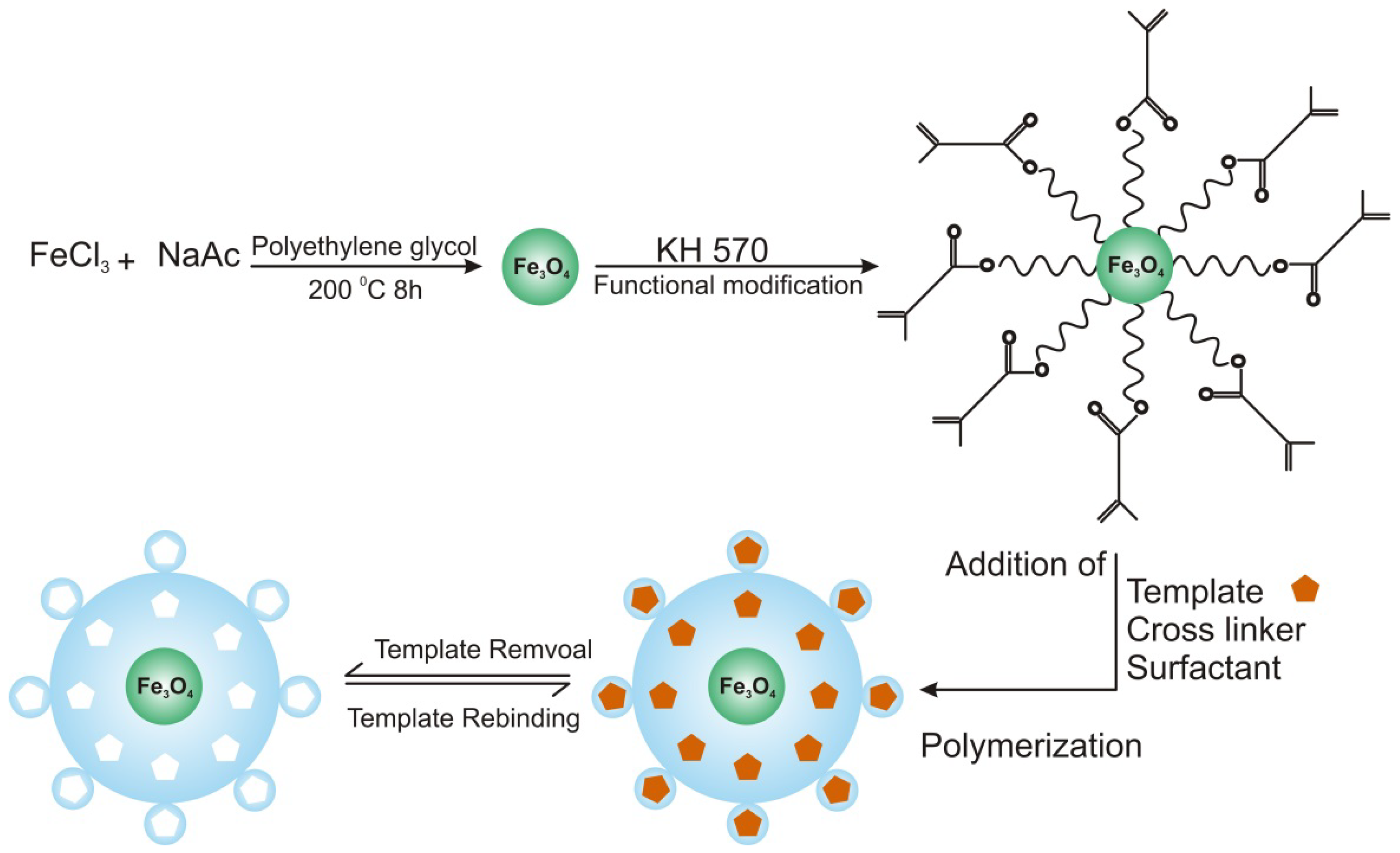
2.2. Imprinted Nanospheres
2.3. Imprinted Nanoshells
2.4. MIP Nanofibers
| Nanomaterials | Synthesis | Size | Analytes | Ref. |
|---|---|---|---|---|
| Nanoparticles | Precipitation | 100 nm–2.5 μm | (S)-propranolol | [27] |
| 50–100 nm | Levofloxacin and fluoroquinolone | [28] | ||
| 50–80 nm | d-zopiclone | [29] | ||
| 60–100 nm | Cu(II) | [30] | ||
| 50–90 nm | Cs(I) | [31] | ||
| Emulsion-suspension | 50 nm | Chlorogenic acid | [32] | |
| Emulsion | 50–200 nm | Tetracycline | [33] | |
| Miniemulsion | 180–251 nm | Carbamazepine | [34] | |
| Microemulsion | 40–100 nm | Promethazine | [35] | |
| Iniferter polymerization | 25–106 μm | Acetoguanamine | [36] | |
| Core-shell emulsion | 76 nm | Cholesterol | [38] | |
| Core-shell | – | Chrysoidine | [39] | |
| Core-shell | – | 17β-estradiol | [40] | |
| Core-shell | 480 nm | Hemoglobin | [41] | |
| Precipitation | 450 nm | di(2-ethylhexyl) phthalate | [47] | |
| Precipitation | 3μm–400 nm | 17β-estradiol | [48] | |
| Precipitation | 100–200 nm | Bensulfuron-methyl | [49] | |
| Miniemulsion | 240–255 nm | L-Boc-phenylalanine anilide and L-Boc-phenylalanine | [50] | |
| Nanoshells | Thiol ligand capping method | 45 nm | Guanosine | [52] |
| Thiol ligand capping method | 16 nm | Dipicolinic acid | [53] | |
| Thiol ligand capping method | 13 nm | Dipicolinic acid | [54] | |
| Nanofibers | Precipitation | 400 nm | dansyl-L-phenylalanine | [55] |
| Electrospinning | 150 nm | Estrone | [56] | |
| Electrospray deposition | 165–564 nm | N-α-benzyloxycarbonyl-D-glutamic acid and N-α-benzyloxycarbonyl-L-glutamic acid | [57] | |
| Electrospray deposition | 200–500 nm | N-α-benzyloxycarbonyl-D-glutamic acid and N-α-benzyloxycarbonyl-L-glutamic acid | [58] |
3. Selected Sensor Applications of Imprinted Nanomaterials
| Sensors | Transducer Type | Nanomaterial | Target Analytes | Detection Range | Ref. |
|---|---|---|---|---|---|
| Electrochemical | Square wave voltammetry | Nanoparticles | Promethazine | 1.0 × 10−8–1.0 × 10−2 M | [35] |
| Cyclic volammetry | Au- nanoparticles | Theophylline | 4 × 10−7–3.4 × 10−3 mol L−1 | [59] | |
| Cyclic volammetry | Au- nanoparticles | Bisphenol A | 8.0 × 10−6–6.0 × 10−2 mol L−1 | [60] | |
| Cyclic volammetry | Au- nanoparticles/MWCNTs | Bisphenol A | 1.13 × 10−7–8.21 × 10−3 mol L−1 | [61] | |
| Cyclic volammetry | Au- nanoparticles | Trinitrotoluene | 46 ppt | [62] | |
| Cyclic volammetry | Au-nanoparticles | Dopamine | 10−6–10−9 M | [63] | |
| Electrochemical | Molecularly imprinted polymer/MWCNTs | Dopamine | 5.0 × 10−7–2.0 × 10−4 mol L−1 | [64] | |
| Cyclic voltammetry/amperometry | Au- nanoparticles/MWCNTs | Chlortetracycline | 9.0 × 10−8–5.0 × 10−5 mol L−1 | [65] | |
| Cyclic voltammetry/differential pulse voltammetry | PbS/Au coated Fe3O4 nanoparticles/MWCNTs | Streptomycin | 1.0 × 10−6–1.0 × 10−3 mol L−1 | [66] | |
| Cyclic voltammetry | Ag-nanoparticles | Dimethoate | 1.0-1000 ng mL−1 and 1.0-50 μg mL−1 | [67] | |
| Cyclic voltammetry | Core-shell nanoparticles | Tert-Butylhydroquinone | 0.1–50.0 mg kg−1 | [68] | |
| Cyclic voltammetry | Chitosan-Pt /graphene-gold nanoparticles | Erythromycin | 7.0 × 10−8–9.0 × 10−5 mol L−1 | [69] | |
| Electrochemiluminescence | Nanoparticles | Thifensulfuron-methyl | 5.0 × 10−10–1.0 × 10−7 M | [70] | |
| Optical | Chemiluminescence | Nanoparticles | Chrysoidine | 1.0 × 10−4–2.0 × 10−6 mol L−1 | [39] |
| Reflectometric interference spectroscopy (RIfS) | Nanospheres | L-Boc-phenylalanine anilide | 0.4–1.65 mM | [51] | |
| Fluorescence | Nanocrystals | Guanosine | 50–800 µg L−1 | [52] | |
| Fluorescence | Au/Ag- nanoclusters | Dipicolinic acid | – | [53] | |
| Fluorescence | Au-nanoparticles | Dipicolinic acid | 10−7–10−4 mol L−1 | [54] | |
| Fluorescence | Nanofibers | Dansyl-L-phenylalanine | 10 μM–1 mM | [55] | |
| Fluorescence | Au/Ag nanoclusters | Dipicolinic acid | – | [71] | |
| Fluorescence | Quantum dot/imprinted polymer composite | Salivary proteins | 0.4–2.68 mg mL−1 | [72] | |
| Chemiluminescence | Fe3O4 nanoparticles | Lysozyme | 5–2000 ng mL−1 | [73] | |
| Fluorescence | Core shell nanoparticles | Naphthalene | – | [74] | |
| Mass-sensitive | QCM | Nanoparticles | 17β-estradiol | 3.67 nM–3.67 pM | [75] |
| QCM | Nanoparticles | Folic acid | 1–30 ppm | [76] | |
| QCM | Nanoparticles | Lysozyme | 0.2–1500 μg mL−1 and 460–1500 ng mL−1 | [77] | |
| QCM | Nanoparticles | Peptide | 90–900 nM | [78] |
3.1. Electrochemical Sensors
3.1.1. Bioanalysis
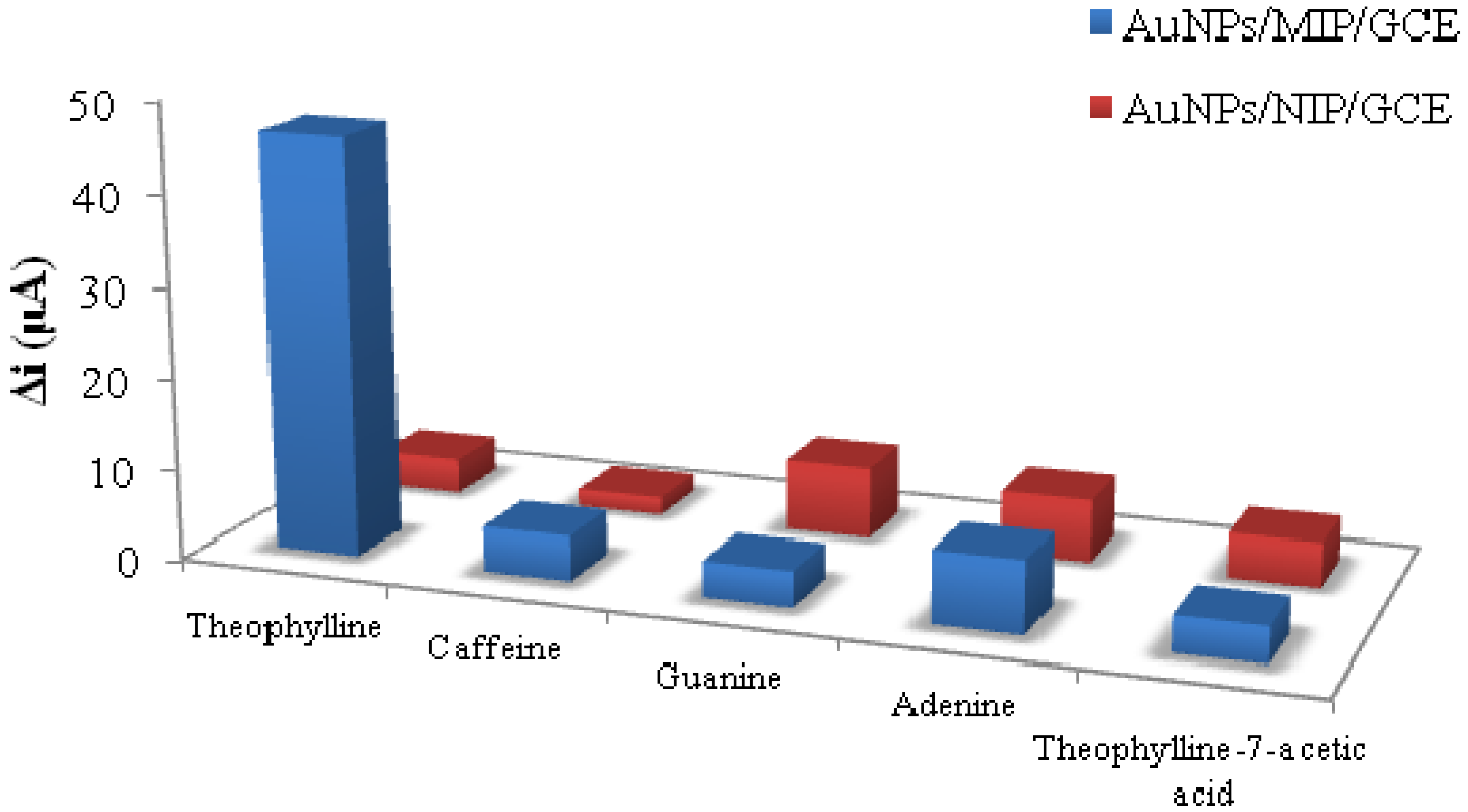
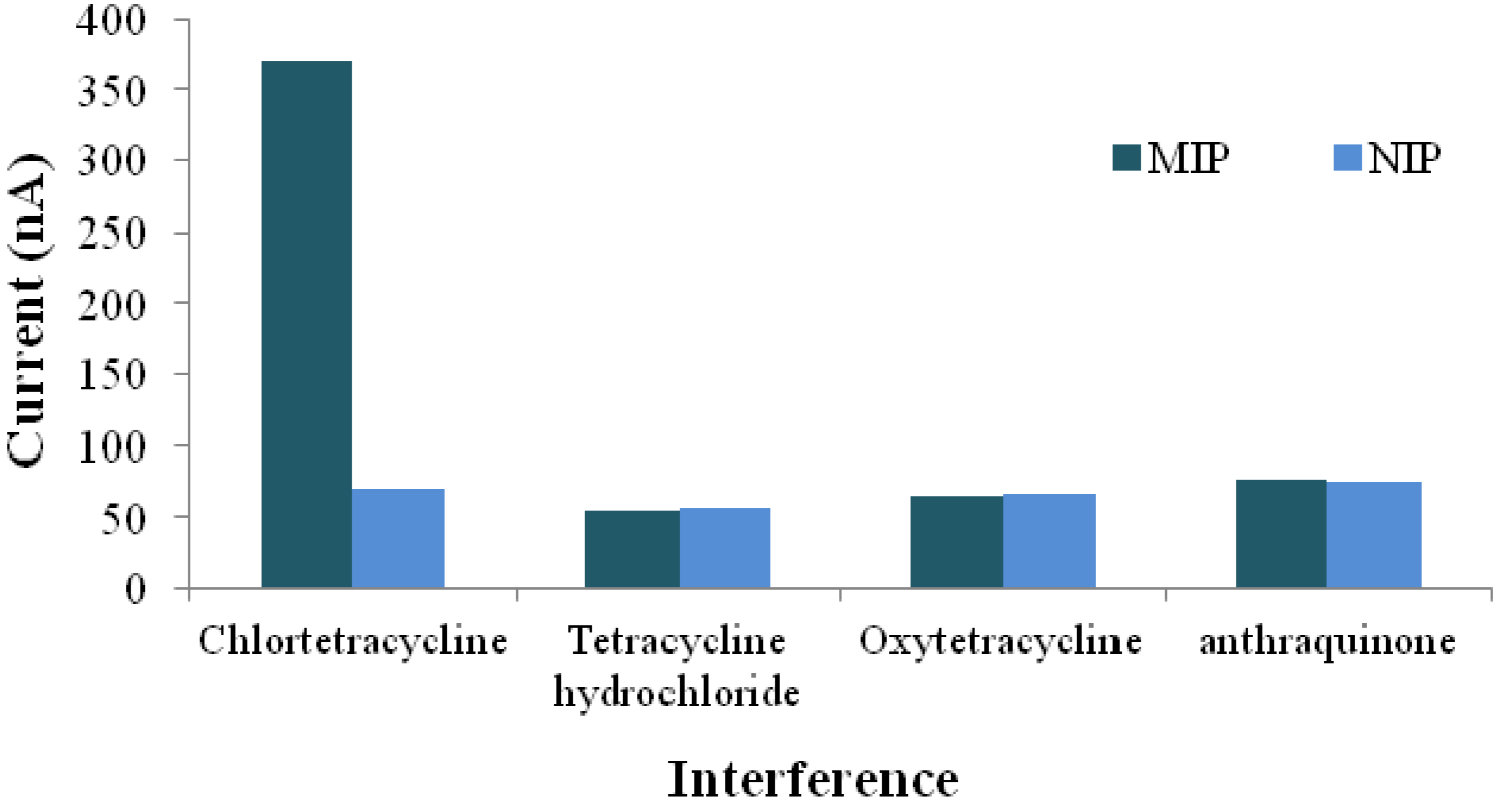
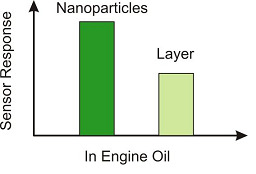
3.1.2. Environmental Analysis
3.1.3. Food Quality/Process Analytics
3.2. Optical Sensors
3.2.1. Bioanalysis
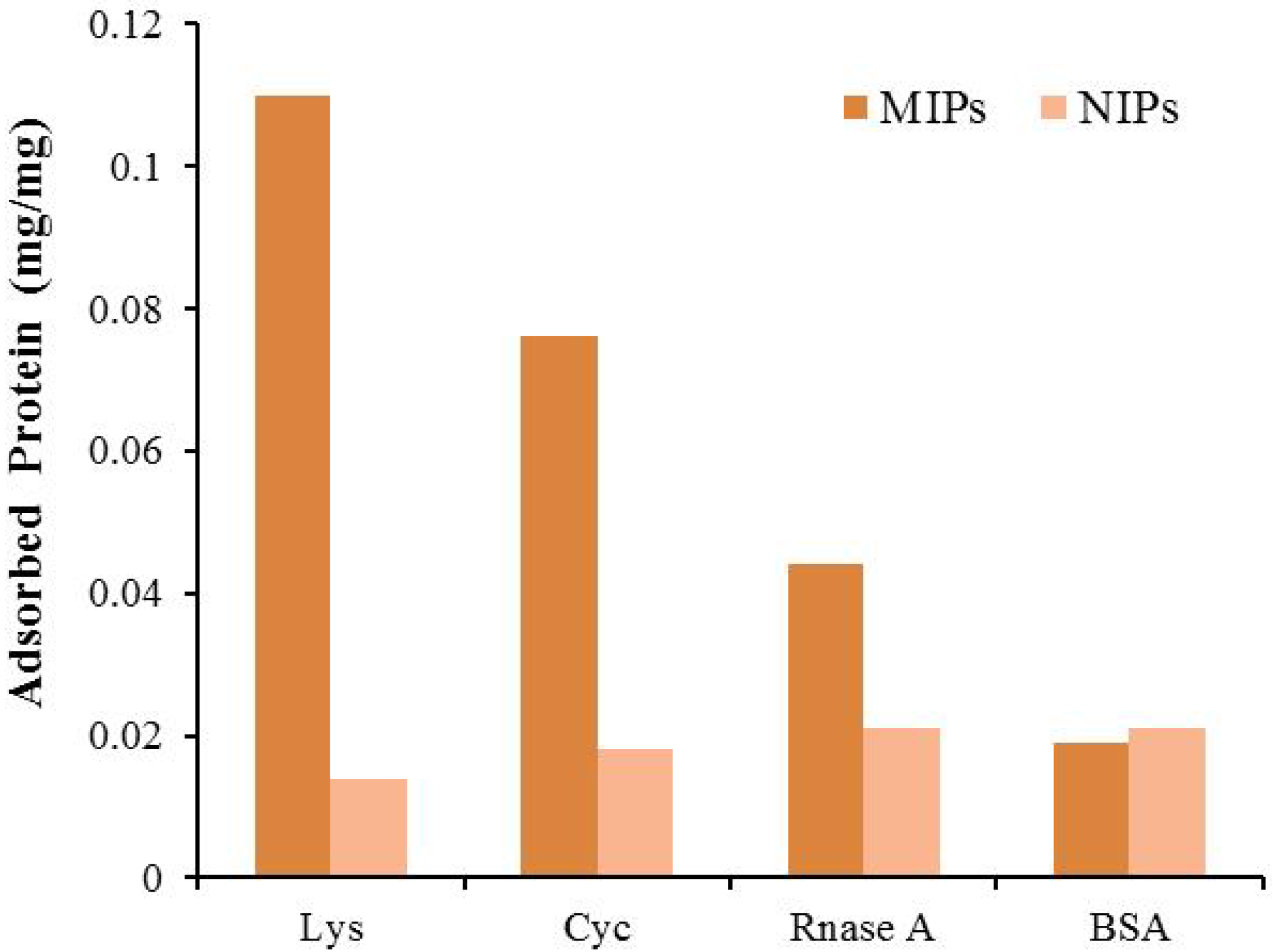
3.2.2. Environmental Analysis
3.2.3. Food Quality/Process Analytics
3.3. Mass Sensitive Devices
3.3.1. Bioanalysis
3.3.2. Environmental Analysis
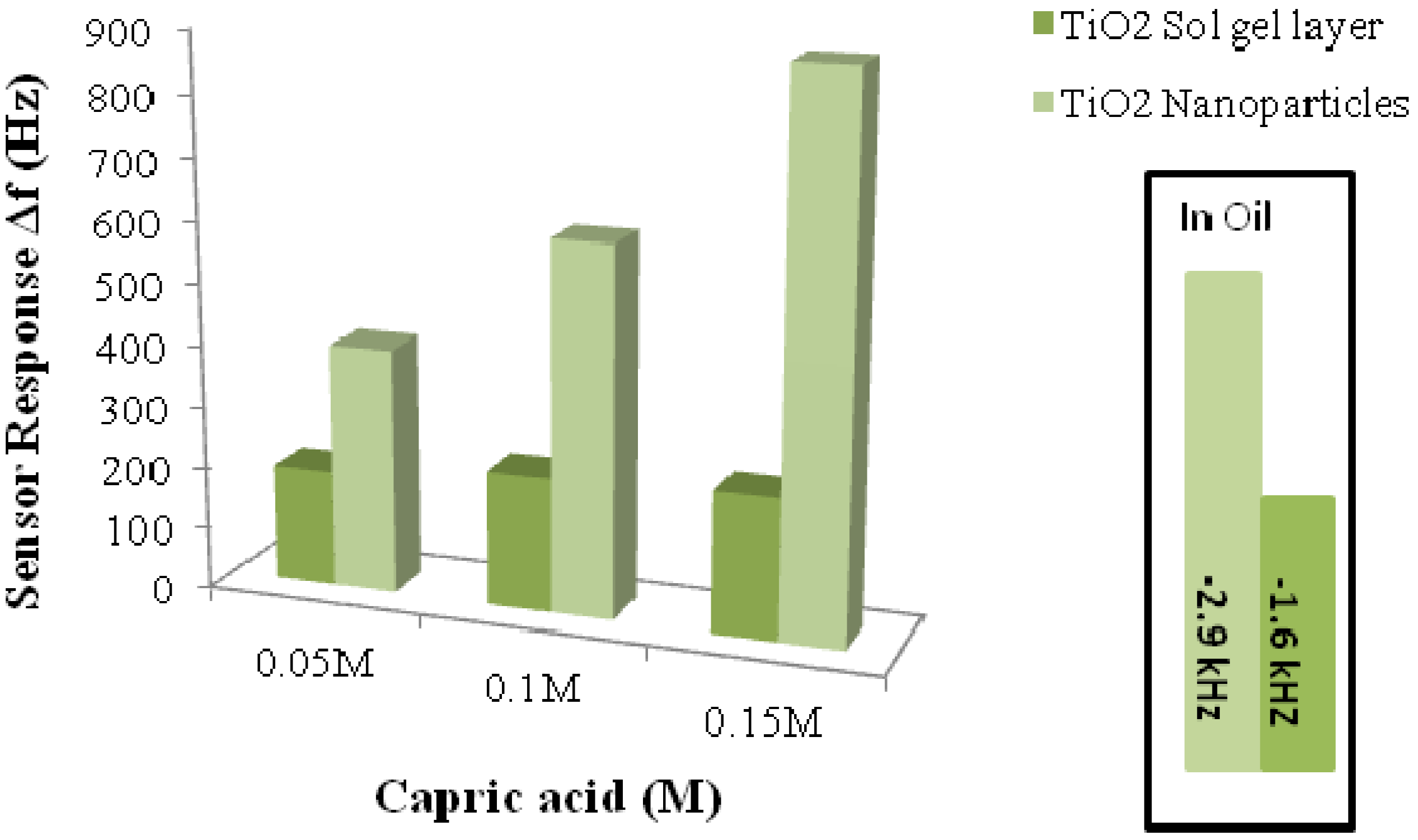
3.3.3. Food Quality/Process Analytics
4. Outlook and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Yan, H.; Row, K. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Malitesta, C.; Mazzotta, E.; Picca, R.A.; Poma, A.; Chianella, I.; Piletsky, S.A. MIP sensors—The electrochemical approach. Anal. Bioanal. Chem. 2012, 402, 1827–1846. [Google Scholar] [CrossRef]
- Poma, A.; Guerreiro, A.; Whitcombe, M.J.; Piletska, E.V.; Turner, A.P.F.; Piletsky, S.A. Solid-phase synthesis of molecularly imprinted polymer nanoparticles with a reusable template–“Plastic Antibodies”. Adv. Funct. Mater. 2013, 23, 2821–2827. [Google Scholar] [CrossRef]
- Matsui, J.; Takayose, M.; Akamatsu, K.; Nawafune, H.; Tamaki, K.; Sugimoto, N. Molecularly imprinted nanocomposites for highly sensitive SPR detection of a non-aqueous atrazine sample. Analyst 2009, 134, 80–86. [Google Scholar] [CrossRef]
- Lakshmi, D.; Bossi, A.; Whitcombe, M.J.; Chianella, I.; Fowler, S.A.; Subrahmanyam, S.; Piletska, E.V.; Piletsky, S.A. Electrochemical sensor for catechol and dopamine based on a catalytic molecularly imprinted polymer-conducting polymer hybrid recognition element. Anal. Chem. 2009, 81, 3576–3584. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Guan, G.; Liu, B.; Wang, Z.; Zhang, Z. Imprinting of molecular recognition sites on nanostructures and its applications in chemosensors. Sensors 2008, 8, 8291–8320. [Google Scholar] [CrossRef]
- Li, J.; Wei, G.; Zhang, Y. Molecularly Imprinted Polymers as Recognition Elements in Sensors. In Molecularly Imprinted Sensors; Elsevier: Amsterdam, The Netherlands, 2012; pp. 35–55. [Google Scholar]
- He, C.; Long, Y.; Pan, J.; Li, K.; Liu, F. Application of molecularly imprinted polymers to solid-phase extraction of analytes from real samples. J. Biochem. Biophys. Methods 2007, 70, 133–150. [Google Scholar] [CrossRef]
- Menaker, A.; Syritski, V.; Reut, J.; Öpik, A.; Horváth, V.; Gyurcsányi, R.E. Electrosynthesized surface-imprinted conducting polymer microrods for selective protein recognition. Adv. Mater. 2009, 21, 2271–2275. [Google Scholar] [CrossRef]
- Tokonami, S.; Shiigi, H.; Nagaoka, T. Review: Micro- and nanosized molecularly imprinted polymers for high-throughput analytical applications. Anal. Chim. Acta 2009, 641, 7–13. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Turner, N.W.; Laitenberger, P. Molecularly imprinted polymers in clinical diagnostics—Future potential and existing problems. Med. Eng. Phys. 2006, 28, 971–977. [Google Scholar] [CrossRef]
- Cai, D.; Ren, L.; Zhao, H.; Xu, C.; Zhang, L.; Yu, Y.; Wang, H.; Lan, Y.; Roberts, M.F.; Chuang, J.H.; et al. A molecular-imprint nanosensor for ultrasensitive detection of proteins. Nat. Nanotechnol. 2010, 5, 597–601. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Wang, N.; Ma, X.; Niu, D.; Jiang, B.; Liu, L.; Huang, W.; Yang, W.; Zhou, Z. Synthesis of magnetic molecularly imprinted polymer particles for selective adsorption and separation of dibenzothiophene. Microchim. Acta 2012, 179, 123–130. [Google Scholar] [CrossRef]
- Cunliffe, D.; Kirby, A.; Alexander, C. Molecularly imprinted drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 1836–1853. [Google Scholar]
- Fuchs, Y.; Soppera, O.; Haupt, K. Photopolymerization and photostructuring of molecularly imprinted polymers for sensor applications—A review. Anal. Chim. Acta 2012, 717, 7–20. [Google Scholar] [CrossRef]
- Alexander, C.; Davidson, L.; Hayes, W. Imprinted polymers: Artificial molecular recognition materials with applications in synthesis and catalysis. Tetrahedron 2003, 59, 2025–2057. [Google Scholar] [CrossRef]
- Pichon, V.; Chapuis-Hugon, F. Role of molecularly imprinted polymers for selective determination of environmental pollutants—A review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef]
- Cheong, W.J.; Ali, F.; Choi, J.H.; Lee, J.O.; Sung, K.Y. Recent applications of molecular imprinted polymers for enantio-selective recognition. Talanta 2013, 106, 45–59. [Google Scholar] [CrossRef]
- Mujahid, A.; Lieberzeit, P.A.; Dickert, F.L. Chemical sensors based on molecularly imprinted sol-gel materials. Materials 2010, 3, 2196–2217. [Google Scholar] [CrossRef]
- Abhilash, M. Potential applications of nanoparticles. Int. J. Pharma Bio Sci. 2010, V1(1). Available online: http://www.ijpbs.net/53.pdf (accessed on 21 November 2013).
- Gültekin, A.; Ersöz, A.; Denizli, A.; Say, R. Gold-silver-nanoclusters having cholic acid imprinted nanoshell. Talanta 2012, 93, 364–370. [Google Scholar] [CrossRef]
- Wang, H.T.; Wu, X.; Zhao, H.; Quan, X. Enhanced photocatalytic degradation of tetracycline hydrochloride by molecular imprinted film modified TiO2 nanotubes. Chin. Sci. Bull. 2012, 57, 601–605. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Javanbakht, M.; Atyabi, F.; Mohammadi, A.; Mohammadi, S.; Akbari-Adergani, B.; Dinarvand, R. Dipyridamole recognition and controlled release by uniformly sized molecularly imprinted nanospheres. Mater. Sci. Eng. C 2011, 31, 1692–1699. [Google Scholar] [CrossRef]
- Kryscio, D.R.; Peppas, N.A. Critical review and perspective of macromolecularly imprinted polymers. Acta Biomater. 2012, 8, 461–473. [Google Scholar] [CrossRef]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Reimhult, K.; Krozer, A.; Mosbach, K.; Sode, K.; Ye, L. Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: The control of particle size suitable for different analytical applications. Anal. Chim. Acta 2007, 584, 112–121. [Google Scholar] [CrossRef]
- Xiao, P.; Dudal, Y.; Corvini, P.F.X.; Spahr, P.; Shahgaldian, P. Synthesis and characterization of fluoroquinolone-imprinted polymeric nanoparticles. React. Funct. Polym. 2012, 72, 287–293. [Google Scholar] [CrossRef]
- Liu, X.; Wei, Z.H.; Huang, Y.P.; Yang, J.R.; Liu, Z.S. Molecularly imprinted nanoparticles with nontailing peaks in capillary electrochromatography. J. Chromatogr. A 2012, 1264, 137–142. [Google Scholar] [CrossRef]
- Shamsipur, M.; Besharati-Seidani, A.; Fasihi, J.; Sharghi, H. Synthesis and characterization of novel ion-imprinted polymeric nanoparticles for very fast and highly selective recognition of copper(II) ions. Talanta 2010, 83, 674–681. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rajabi, H. Flame photometric determination of cesium ion after its preconcentration with nanoparticles imprinted with the cesium-dibenzo-24-crown-8 complex. Microchim. Acta 2013, 180, 243–252. [Google Scholar] [CrossRef]
- Gu, X.H.; Xu, R.; Yuan, G.L.; Lu, H.; Gu, B.R.; Xie, H.P. Preparation of chlorogenic acid surface-imprinted magnetic nanoparticles and their usage in separation of traditional Chinese medicine. Anal. Chim. Acta 2010, 675, 64–70. [Google Scholar] [CrossRef]
- Dai, J.; Pan, J.; Xu, L.; Li, X.; Zhou, Z.; Zhang, R.; Yan, Y. Preparation of molecularly imprinted nanoparticles with superparamagnetic susceptibility through atom transfer radical emulsion polymerization for the selective recognition of tetracycline from aqueous medium. J. Hazard. Mater. 2012, 205–206, 179–188. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Javanbakht, M.; Dinarvand, R.; Atyabi, F. Molecularly imprinted nanoparticles prepared by miniemulsion polymerization as selective receptors and new carriers for the sustained release of carbamazepine. J. Mater. Sci. Mater. Med. 2012, 23, 963–972. [Google Scholar] [CrossRef]
- Alizadeh, T.; Ganjali, M.R.; Akhoundian, M. Synthesis and application of different nano-sized imprinted polymers for the preparation of promethazine membrane electrodes and comparison of their efficiencies. Int. J. Electrochem. Sci. 2012, 7, 7655–7674. [Google Scholar]
- Guerreiro, A.R.; Chianella, I.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. Selection of molecularly imprinted nanoparticles by affinity chromatography. Biosens. Bioelectron. 2009, 24, 2740–2743. [Google Scholar] [CrossRef] [Green Version]
- Subrahmanyam, S.; Guerreiro, A.; Poma, A.; Moczko, E.; Piletska, E.; Piletsky, S. Optimisation of experimental conditions for synthesis of high affinity MIP nanoparticles. Eur. Polym. J. 2013, 49, 100–105. [Google Scholar] [CrossRef]
- Pérez, N.; Whitcombe, M.J.; Vulfson, E.N. Molecularly imprinted nanoparticles prepared by core-shell emulsion polymerization. J. Appl. Polym. Sci. 2000, 77, 1851–1859. [Google Scholar] [CrossRef]
- Lu, F.; Sun, M.; Fan, L.; Qiu, H.; Li, X.; Luo, C. Flow injection chemiluminescence sensor based on core–shell magnetic molecularly imprinted nanoparticles for determination of chrysoidine in food samples. Sens. Actuators B 2012, 173, 591–598. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, L.; Ding, M.; Wang, S.; Ren, F.; Zhang, J.; Du, S.; Li, F.; Zhou, X. The study of core-shell molecularly imprinted polymers of 17β-estradiol on the surface of silica nanoparticles. Biosens. Bioelectron. 2011, 26, 2791–2795. [Google Scholar] [CrossRef]
- Li, L.; He, X.W.; Chen, L.X.; Zhang, Y. Preparation of novel bovine hemoglobin surface-imprinted polystyrene nanoparticles with magnetic susceptibility. Sci. China Ser. B 2009, 52, 1402–1411. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Afzal, A.; Glanzing, G.; Dickert, F.L. Molecularly imprinted sol-gel nanoparticles for mass-sensitive engine oil degradation sensing. Anal. Bioanal. Chem. 2007, 389, 441–446. [Google Scholar] [CrossRef]
- Shamsipur, M.; Rajabi, H.R.; Beyzavi, M.H.; Sharghi, H. Bulk polymer nanoparticles containing a tetrakis (3-hydroxyphenyl)porphyrin for fast and highly selective separation of mercury ions. Microchim. Acta 2013, 180, 791–799. [Google Scholar] [CrossRef]
- Tan, F.; Sun, D.; Gao, J.; Zhao, Q.; Wang, X.; Teng, F.; Quan, X.; Chen, J. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 244–245, 750–757. [Google Scholar] [CrossRef]
- Lian, W.; Liu, S.; Yu, J.; Li, J.; Cui, M.; Xu, W.; Huang, J. Electrochemical sensor using neomycin-imprinted film as recognition element based on chitosan-silver nanoparticles/graphene-multiwalled carbon nanotubes composites modified electrode. Biosens. Bioelectron. 2013, 44, 70–76. [Google Scholar] [CrossRef]
- Vlatakis, G.; Andersson, L.I.; Müller, R.; Mosbach, K. Drug assay using antibody mimics made by molecular imprinting. Nature 1993, 361, 645–647. [Google Scholar] [CrossRef]
- Lai, J.P.; Yang, M.L.; Niessner, R.; Knopp, D. Molecularly imprinted microspheres and nanospheres for di (2-ethylhexyl)phthalate prepared by precipitation polymerization. Anal. Bioanal. Chem. 2007, 389, 405–412. [Google Scholar] [CrossRef]
- Wei, S.; Molinelli, A.; Mizaikoff, B. Molecularly imprinted micro and nanospheres for the selective recognition of 17β-estradiol. Biosens. Bioelectron. 2006, 21, 1943–1951. [Google Scholar] [CrossRef]
- Yao, Q.; Zhou, Y. Synthesis of TiO2 hybrid molecular imprinted nanospheres linked by silane coupling agent. J. Inorg. Organomet Polym. Mater. 2009, 19, 466–472. [Google Scholar] [CrossRef]
- Lehmann, M.; Dettling, M.; Brunner, H.; Tovar, G.E.M. Affinity parameters of amino acid derivative binding to molecularly imprinted nanospheres consisting of poly [(ethylene glycol dimethacrylate)-co-(methacrylic acid)]. J. Chromatogr. B 2004, 808, 43–50. [Google Scholar] [CrossRef]
- Kolarov, F.; Niedergall, K.; Bach, M.; Tovar, G.M.; Gauglitz, G. Optical sensors with molecularly imprinted nanospheres: A promising approach for robust and label-free detection of small molecules. Anal. Bioanal. Chem. 2012, 402, 3245–3252. [Google Scholar] [CrossRef]
- Diltemiz, S.E.; Say, R.; Büyüktiryaki, S.; Hür, D.; Denizli, A.; Ersöz, A. Quantum dot nanocrystals having guanosine imprinted nanoshell for DNA recognition. Talanta 2008, 75, 890–896. [Google Scholar] [CrossRef]
- Gültekin, A.; Ersöz, A.; Sarıözlü, N.Y.; Denizli, A.; Say, R. Nanosensors having dipicolinic acid imprinted nanoshell for Bacillus cereus spores detection. J. Nanopart. Res. 2010, 12, 2069–2079. [Google Scholar] [CrossRef]
- Gültekin, A.; Ersöz, A.; Hür, D.; Sarıözlü, N.Y.; Denizli, A.; Say, R. Gold nanoparticles having dipicolinic acid imprinted nanoshell for Bacillus cereus spores recognition. Appl. Surf. Sci. 2009, 256, 142–148. [Google Scholar] [CrossRef]
- Piperno, S.; Tse Sum Bui, B.; Haupt, K.; Gheber, L.A. Immobilization of molecularly imprinted polymer nanoparticles in electrospun poly(vinyl alcohol) nanofibers. Langmuir 2011, 27, 1547–1550. [Google Scholar] [CrossRef]
- Kim, W.J.; Chang, J.Y. Molecularly imprinted polyimide nanofibers prepared by electrospinning. Mater. Lett. 2011, 65, 1388–1391. [Google Scholar] [CrossRef]
- Sueyoshi, Y.; Utsunomiya, A.; Yoshikawa, M.; Robertson, G.P.; Guiver, M.D. Chiral separation with molecularly imprinted polysulfone-aldehyde derivatized nanofiber membranes. J. Membr. Sci. 2012, 401–402, 89–96. [Google Scholar] [CrossRef]
- Sueyoshi, Y.; Fukushima, C.; Yoshikawa, M. Molecularly imprinted nanofiber membranes from cellulose acetate aimed for chiral separation. J. Membr. Sci. 2010, 357, 90–97. [Google Scholar] [CrossRef]
- Kan, X.; Liu, T.; Zhou, H.; Li, C.; Fang, B. Molecular imprinting polymer electrosensor based on gold nanoparticles for theophylline recognition and determination. Microchim. Acta 2010, 171, 423–429. [Google Scholar] [CrossRef]
- Huang, J.D.; Zhang, X.M.; Liu, S.; Lin, Q.; He, X.R.; Xing, X.R.; Lian, W.J. Electrochemical sensor for bisphenol A detection based on molecularly imprinted polymers and gold nanoparticles. J. Appl. Electrochem. 2011, 41, 1323–1328. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Lin, Q.; He, X.; Xing, X.; Huai, H.; Lian, W.; Zhu, H. Electrochemical sensor based on imprinted sol-gel and nanomaterials for sensitive determination of bisphenol A. Food Control 2011, 22, 786–791. [Google Scholar] [CrossRef]
- Riskin, M.; Tel-Vered, R.; Bourenko, T.; Granot, E.; Willner, I. Imprinting of molecular recognition sites through electropolymerization of functionalized Au nanoparticles: Development of an electrochemical TNT sensor based on π-Donor-acceptor interactions. J. Am. Chem. Soc. 2008, 130, 9726–9733. [Google Scholar] [CrossRef]
- Gam-Derouich, S.; Mahouche-Chergui, S.; Truong, S.; Ben Hassen-Chehimi, D.; Chehimi, M.M. Design of molecularly imprinted polymer grafts with embedded gold nanoparticles through the interfacial chemistry of aryl diazonium salts. Polymer 2011, 52, 4463–4470. [Google Scholar] [CrossRef]
- Kan, X.; Zhao, Y.; Geng, Z.; Wang, Z.; Zhu, J.J. Composites of multiwalled carbon nanotubes and molecularly imprinted polymers for dopamine recognition. J. Phys. Chem. C 2008, 112, 4849–4854. [Google Scholar] [CrossRef]
- Lian, W.J.; Huang, J.D.; Yu, J.H.; Zhang, X.M.; Lin, Q.; He, X.R.; Xing, X.R.; Liu, S. A molecularly imprinted sensor based on β-cyclodextrin incorporated multiwalled carbon nanotube and gold nanoparticles-polyamide amine dendrimer nanocomposites combining with water-soluble chitosan derivative for the detection of chlortetracycline. Food Control 2012, 26, 620–627. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Zhang, H.; Luo, L.; Yao, S. Selective and sensitive molecularly imprinted sol-gel film-based electrochemical sensor combining mercaptoacetic acid-modified PbS nanoparticles with Fe3O4@Au-multi-walled carbon nanotubes–chitosan. J. Solid State Electrochem. 2012, 16, 857–867. [Google Scholar] [CrossRef]
- Du, D.; Chen, S.; Cai, J.; Tao, Y.; Tu, H.; Zhang, A. Recognition of dimethoate carried by bi-layer electrodeposition of silver nanoparticles and imprinted poly-o-phenylenediamine. Electrochim. Acta 2008, 53, 6589–6595. [Google Scholar] [CrossRef]
- Zhao, P.; Hao, J. Tert-butylhydroquinone recognition of molecular imprinting electrochemical sensor based on core–shell nanoparticles. Food Chem. 2013, 139, 1001–1007. [Google Scholar] [CrossRef]
- Lian, W.J.; Liu, S.; Yu, J.H.; Xing, X.R.; Li, J.; Cui, M.; Huang, J.D. Electrochemical sensor based on gold nanoparticles fabricated molecularly imprinted polymer film at chitosan–platinum nanoparticles/graphene–gold nanoparticles double nanocomposites modified electrode for detection of erythromycin. Biosens. Bioelectron. 2012, 38, 163–169. [Google Scholar] [CrossRef]
- Li, H.; Xie, C.; Fu, X. Electrochemiluminescence sensor for sulfonylurea herbicide with molecular imprinting core–shell nanoparticles/chitosan composite film modified glassy carbon electrode. Sens. Actuators B 2013, 181, 858–866. [Google Scholar] [CrossRef]
- Gültekin, A.; Diltemiz, S.E.; Ersöz, A.; Sarıözlü, N.Y.; Denizli, A.; Say, R. Gold-silver nanoclusters having dipicolinic acid imprinted nanoshell for Bacillus cereus spores recognition. Talanta 2009, 78, 1332–1338. [Google Scholar] [CrossRef]
- Lee, M.H.; Chen, Y.C.; Ho, M.H.; Lin, H.Y. Optical recognition of salivary proteins by use of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles. Anal. Bioanal. Chem. 2010, 397, 1457–1466. [Google Scholar] [CrossRef]
- Jing, T.; Du, H.; Dai, Q.; Xia, H.; Niu, J.; Hao, Q.; Mei, S.; Zhou, Y. Magnetic molecularly imprinted nanoparticles for recognition of lysozyme. Biosens. Bioelectron. 2010, 26, 301–306. [Google Scholar] [CrossRef]
- Koshkin, A.V.; Sazhnikov, V.A.; Men’shikova, A.Y.; Pankova, G.A.; Evseeva, T.G.; Alfimov, M.V. Naphthalene vapor sorption by polymer nanoparticles with molecularly imprinted shells. Nanotechnol. Russia 2012, 7, 15–21. [Google Scholar] [CrossRef]
- Özgür, E.; Yılmaz, E.; Şener, G.; Uzun, L.; Say, R.; Denizli, A. A new molecular imprinting-based mass-sensitive sensor for real-time detection of 17β-estradiol from aqueous solution. Environ. Prog. Sustain. Energy 2012, 32, 1164–1169. [Google Scholar]
- Hussain, M.; Iqbal, N.; Lieberzeit, P.A. Acidic and basic polymers for molecularly imprinted folic acid sensors—QCM studies with thin films and nanoparticles. Sens. Actuators B: Chem. 2013, 176, 1090–1095. [Google Scholar] [CrossRef]
- Sener, G.; Ozgur, E.; Yılmaz, E.; Uzun, L.; Say, R.; Denizli, A. Quartz crystal microbalance based nanosensor for lysozyme detection with lysozyme molecularly imprinted nanoparticles. Biosens. Bioelectron. 2010, 26, 815–821. [Google Scholar] [CrossRef]
- Zeng, Z.; Hoshino, Y.; Rodriguez, A.; Yoo, H.; Shea, K.J. Synthetic polymer nanoparticles with antibody-like affinity for a hydrophilic peptide. ACS Nano 2010, 4, 199–204. [Google Scholar] [CrossRef]
- Hedborg, E.; Winquist, F.; Lundström, I.; Andersson, L.I.; Mosbach, K. Some studies of molecularly-imprinted polymer membranes in combination with field-effect devices. Sens. Actuators A 1993, 37–38, 796–799. [Google Scholar] [CrossRef]
- Ivanova-Mitseva, P.K.; Guerreiro, A.; Piletska, E.V.; Whitcombe, M.J.; Zhou, Z.; Mitsev, P.A.; Davis, F.; Piletsky, S.A. Cubic molecularly imprinted polymer nanoparticles with a fluorescent core. Angew. Chem. Int. Ed. 2012, 51, 5196–5199. [Google Scholar] [CrossRef]
- Ton, X.A.; Acha, V.; Haupt, K.; Tse Sum Bui, B. Direct fluorimetric sensing of UV-excited analytes in biological and environmental samples using molecularly imprinted polymer nanoparticles and fluorescence polarization. Biosens. Bioelectron. 2012, 36, 22–28. [Google Scholar] [CrossRef]
- Lieberzeit, P.A.; Afzal, A.; Rehman, A.; Dickert, F.L. Nanoparticles for detecting pollutants and degradation processes with mass-sensitive sensors. Sens. Actuators B 2007, 127, 132–136. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. Monitoring automotive oil degradation: Analytical tools and onboard sensing technologies. Anal. Bioanal. Chem. 2012, 404, 1197–1209. [Google Scholar] [CrossRef]
- Mujahid, A.; Afzal, A.; Glanzing, G.; Leidl, A.; Lieberzeit, P.A.; Dickert, F.L. Imprinted sol-gel materials for monitoring degradation products in automotive oils by shear transverse wave. Anal. Chim. Acta 2010, 675, 53–57. [Google Scholar] [CrossRef]
- Mustafa, G.; Hussain, M.; Iqbal, N.; Dickert, F.L.; Lieberzeit, P.A. Quartz crystal microbalance sensors based on affinity interactions between organic thiols and molybdenum disulfide nanoparticles. Sens. Actuators B 2012, 162, 63–67. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A. Imprinted polyurethane-gold nanoparticle composite films for rapid mass-sensitive detection of organic vapors. Sci. Adv. Mater. 2013, 5, 939–946. [Google Scholar] [CrossRef]
- Subramanian, V. Nanomaterials in Soil and Food Analysis. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec., J., Eds.; Springer: Berlin, Germany, 2011. [Google Scholar]
- Subramanian, V.; Jerzy, R. Nanomaterials in electrochemical biosensors for food analysis: A review. Pol. J. Food Nutr. Sci. 2008, 58, 157–164. [Google Scholar]
- Iqbal, N.; Mustafa, G.; Rehman, A.; Biedermann, A.; Najafi, B.; Lieberzeit, P.A.; Dickert, F.L. QCM-arrays for sensing terpenes in fresh and dried herbs via bio-mimetic MIP layers. Sensors 2010, 10, 6361–6376. [Google Scholar] [CrossRef]
- Dias, L.A.; Peres, A.M.; Vilas-Boas, M.; Rocha, M.A.; Estevinho, L.; Machado, A.A.S.C. An electronic tongue for honey classification. Microchim. Acta 2008, 163, 97–102. [Google Scholar] [CrossRef]
- Kong, L.J.; Pan, M.F.; Fang, G.Z.; He, X.L.; Yang, Y.K.; Dai, J.; Wang, S. Molecularly imprinted quartz crystal microbalance sensor based on poly (o-aminothiophenol) membrane and Au nanoparticles for ractopamine determination. Biosens. Bioelectron. 2014, 51, 286–292. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Irshad, M.; Iqbal, N.; Mujahid, A.; Afzal, A.; Hussain, T.; Sharif, A.; Ahmad, E.; Athar, M.M. Molecularly Imprinted Nanomaterials for Sensor Applications. Nanomaterials 2013, 3, 615-637. https://doi.org/10.3390/nano3040615
Irshad M, Iqbal N, Mujahid A, Afzal A, Hussain T, Sharif A, Ahmad E, Athar MM. Molecularly Imprinted Nanomaterials for Sensor Applications. Nanomaterials. 2013; 3(4):615-637. https://doi.org/10.3390/nano3040615
Chicago/Turabian StyleIrshad, Muhammad, Naseer Iqbal, Adnan Mujahid, Adeel Afzal, Tajamal Hussain, Ahsan Sharif, Ejaz Ahmad, and Muhammad Makshoof Athar. 2013. "Molecularly Imprinted Nanomaterials for Sensor Applications" Nanomaterials 3, no. 4: 615-637. https://doi.org/10.3390/nano3040615