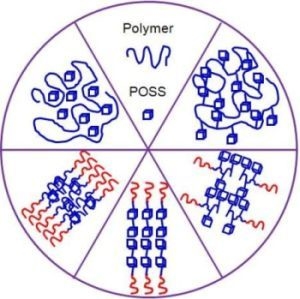
Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites
Abstract
:1. Introduction
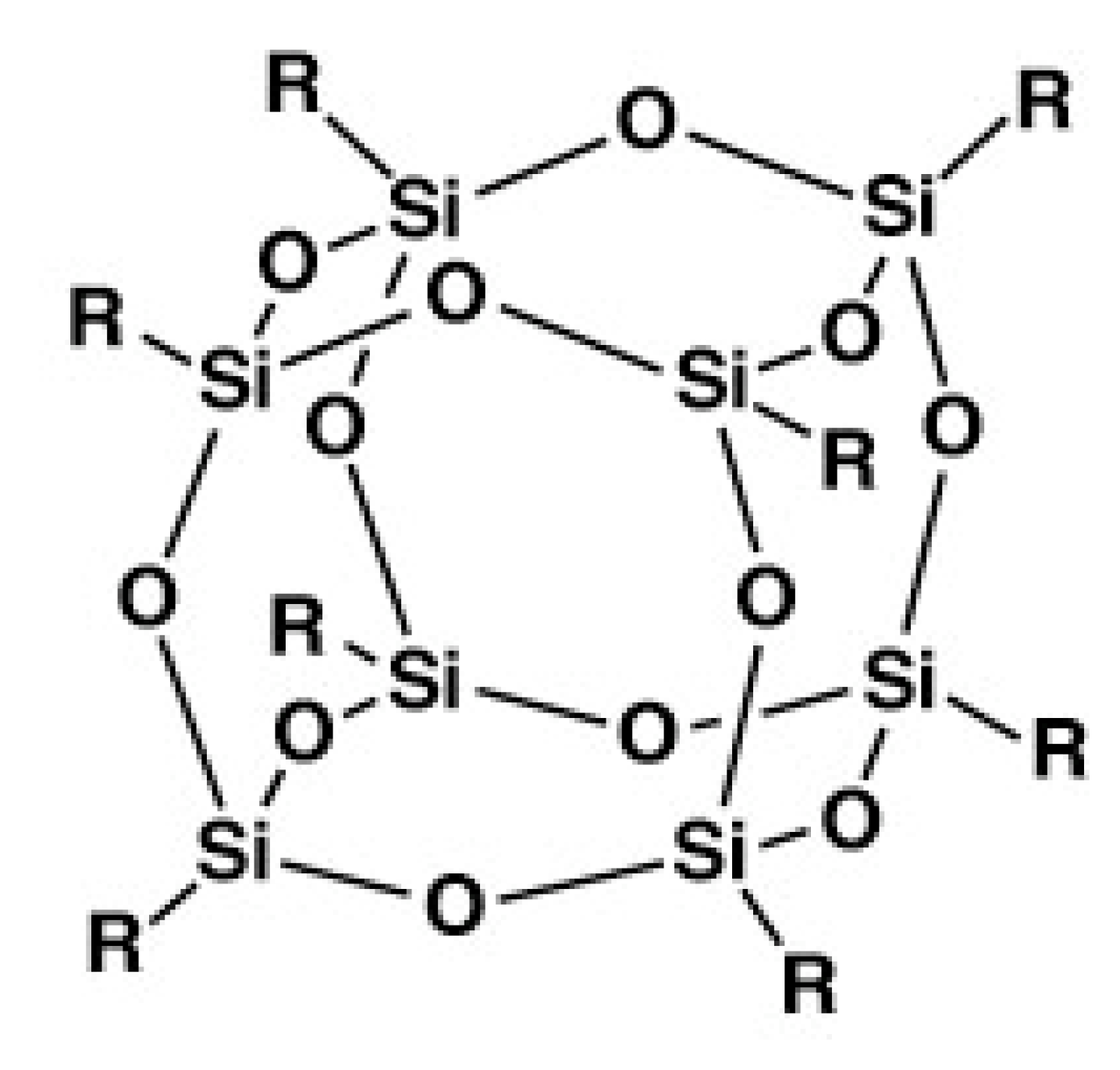
2. Chemically Cross-Linked POSS-Containing Polymer Composites
2.1. POSS-Styrene Based Nanocomposites
2.2. POSS-Methacrylate-Based Nanocomposites
2.3. POSS-Polyurethane-Based Nanocomposites
2.4. POSS-Thermosetting Polymer Based Nanocomposites
2.5. POSS-Poly(Ethylene-Oxide)-Based Nanocomposites
3. Incorporation of POSS into Polymer Matrices by Physical Blending
3.1. POSS-Polypropylene Nanocomposites
3.2. POSS-Polystyrene Nanocomposites
3.3. POSS-Polyamide Nanocomposites
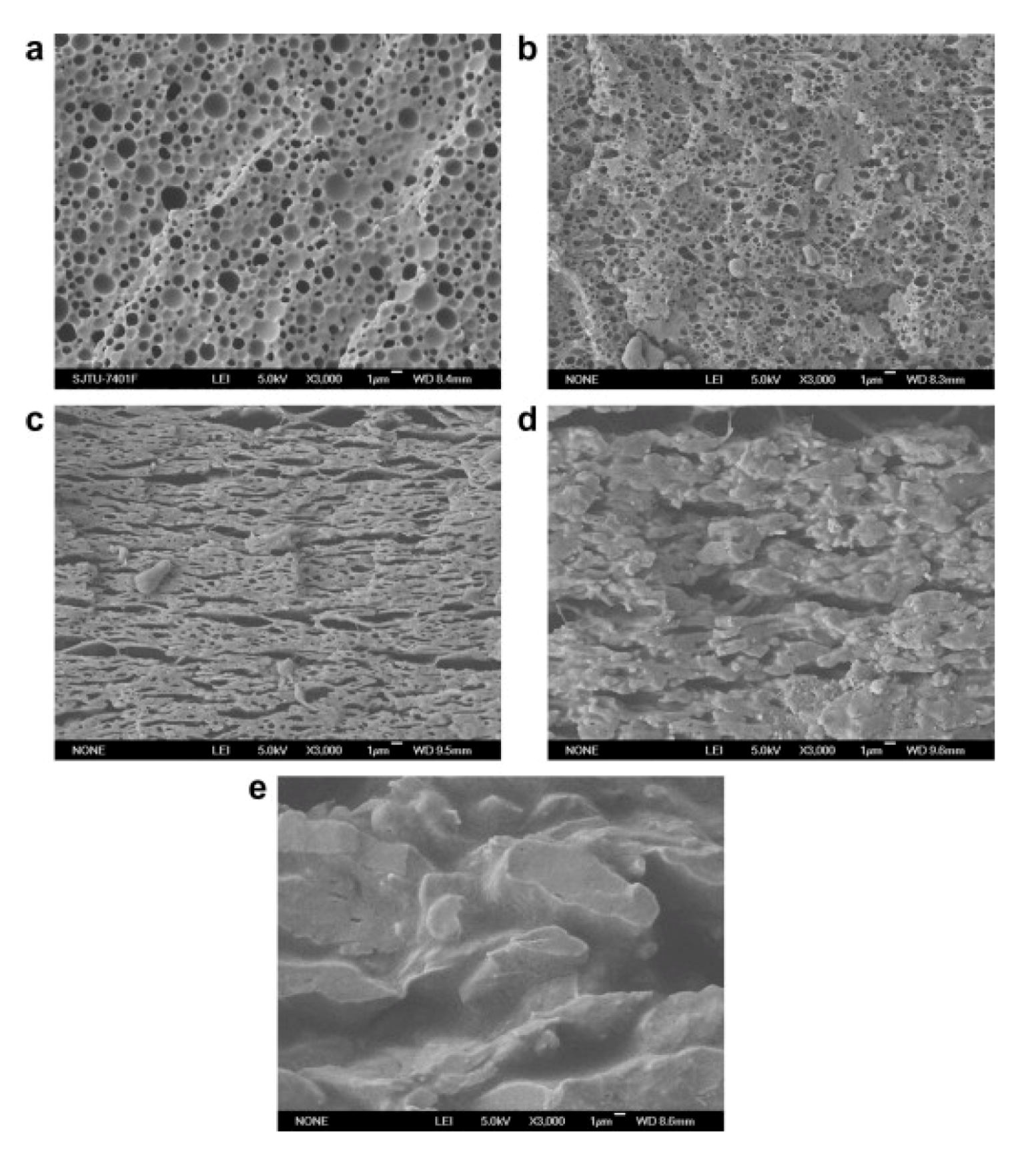
3.4. POSS-Polyoxymethylene (POM) Nanocomposites
3.5. POSS-Poly(Ethylene Oxide)-Containing Polymer/Block Copolymer Nanocomposites
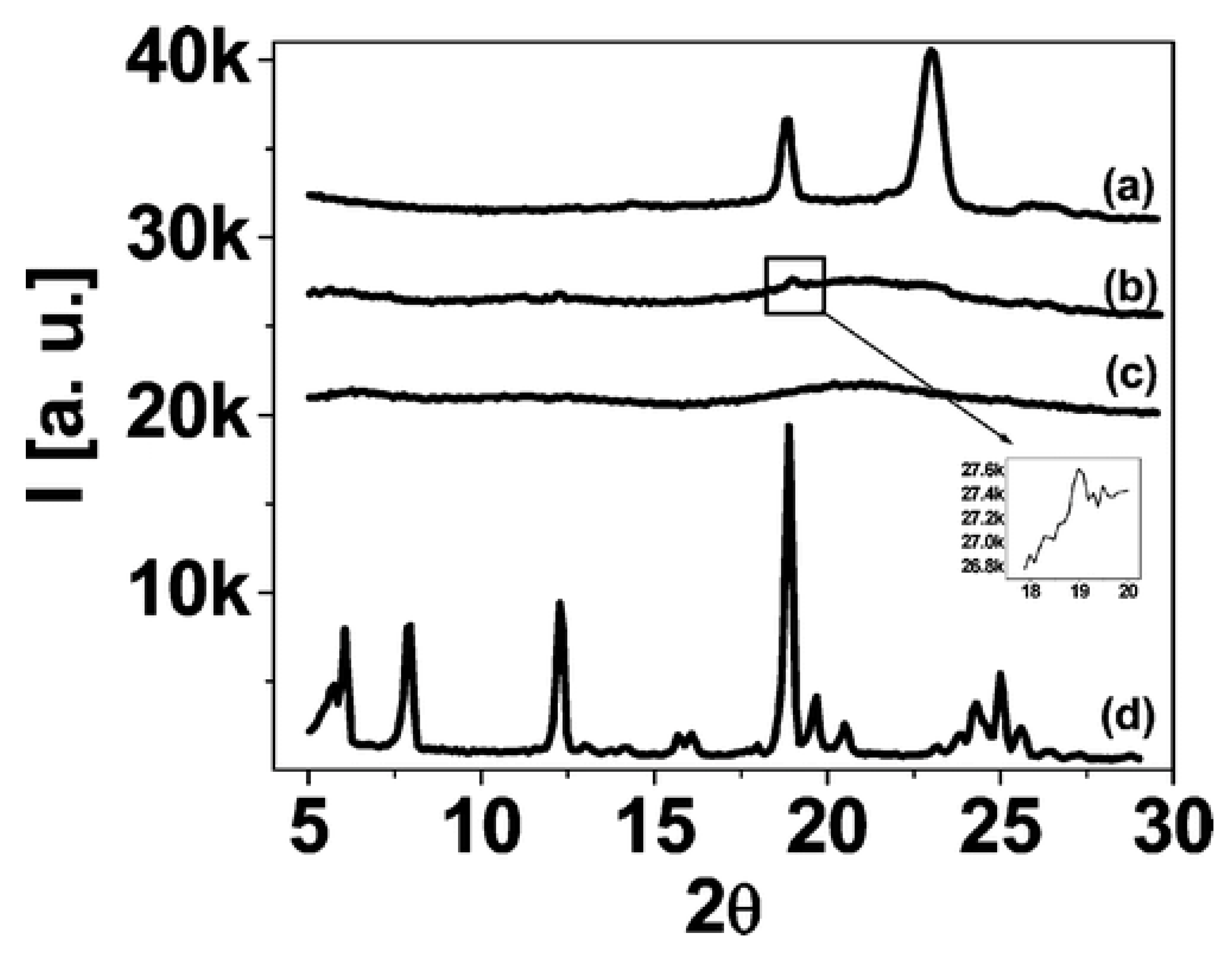
3.6. Other Physically Blended POSS-Homopolymer Nanocomposites
4. Functional Properties of POSS-Containing Polymer Composites
4.1. Mechanical Properties
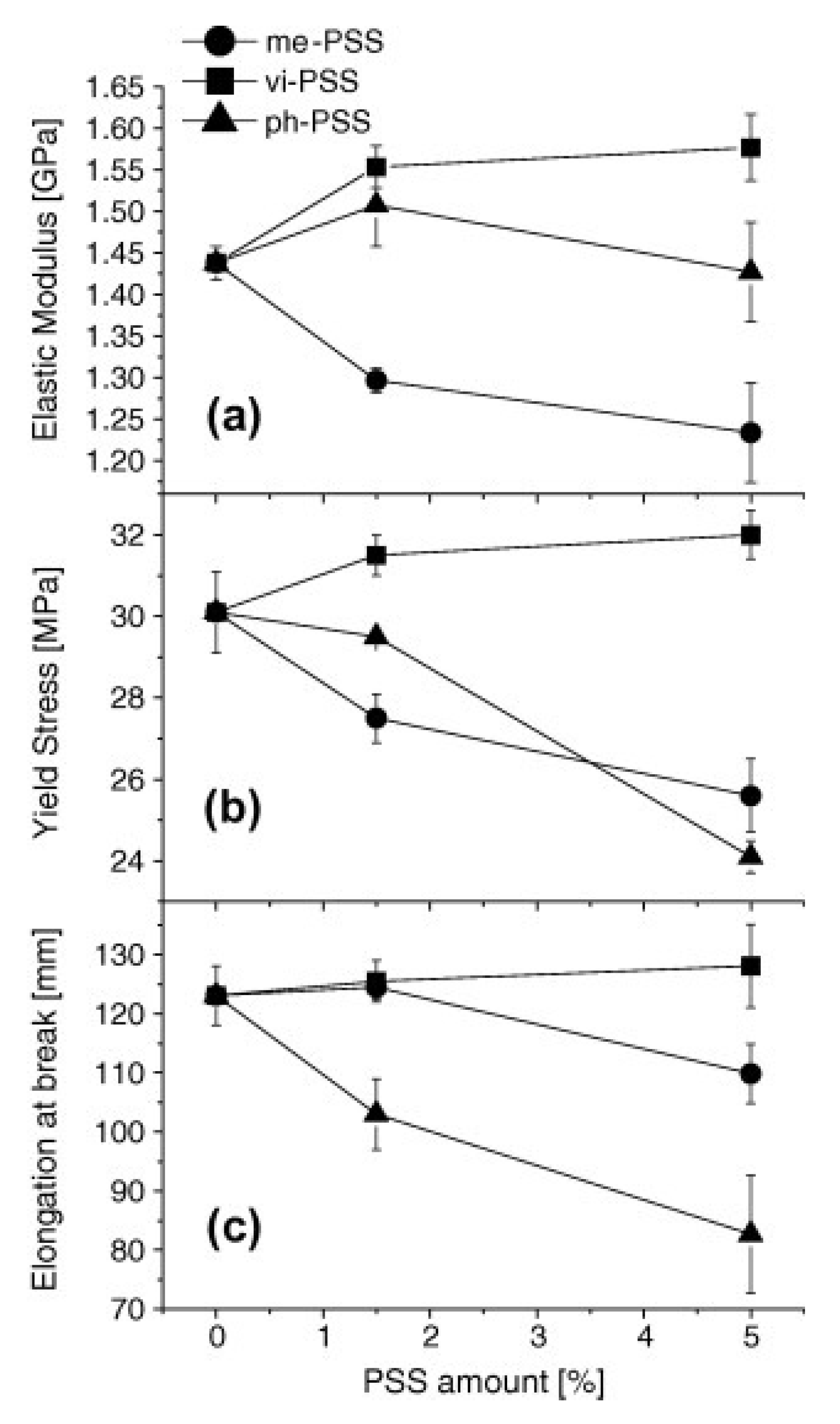
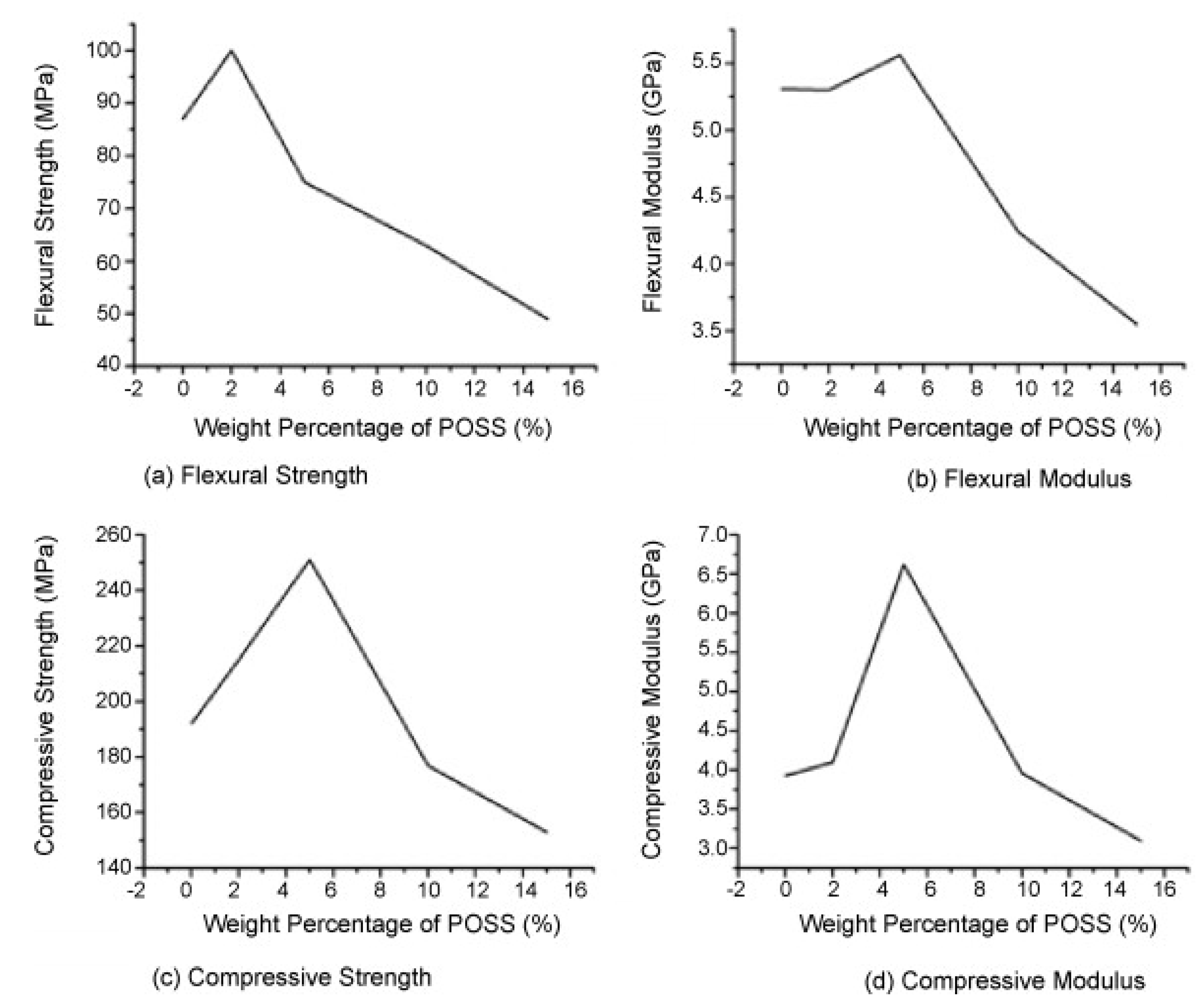
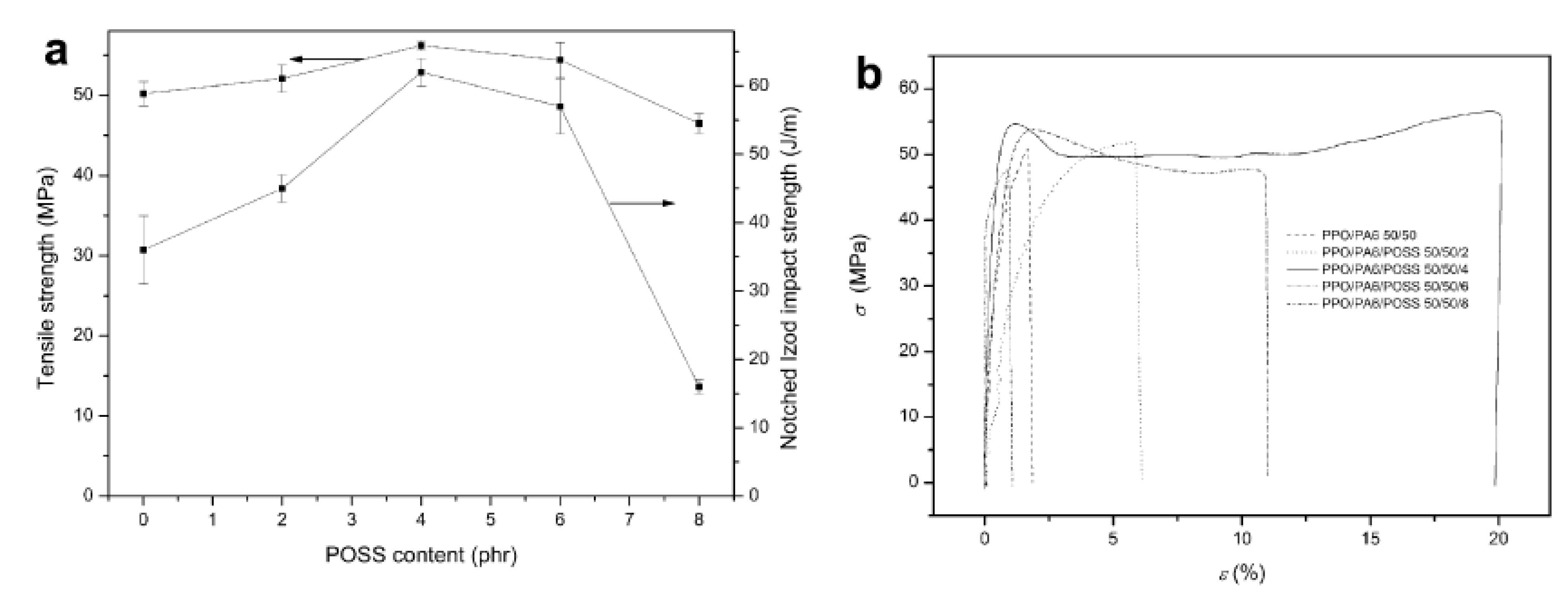
4.2. Thermal Stability
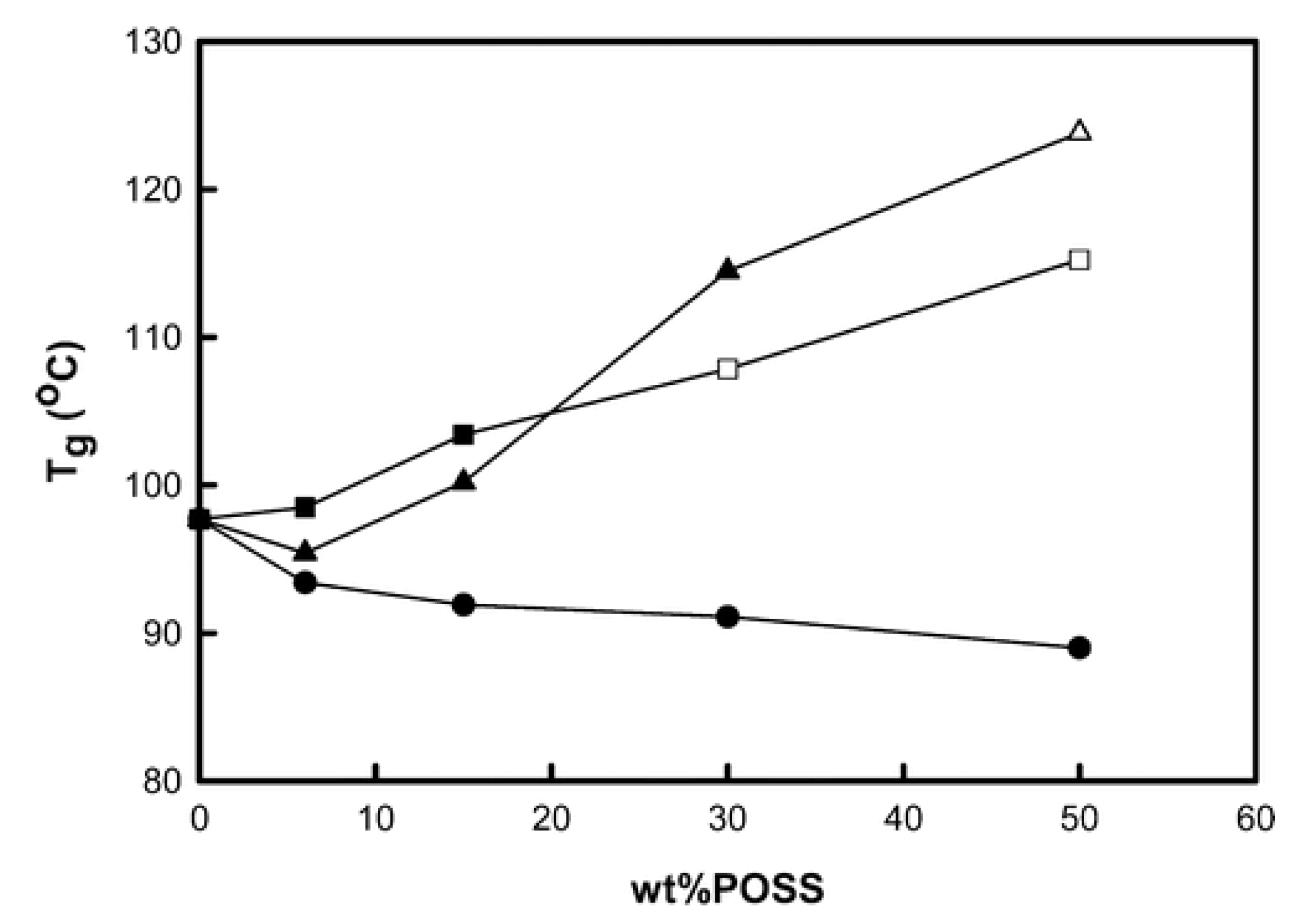
4.3. Glass Transition Temperature
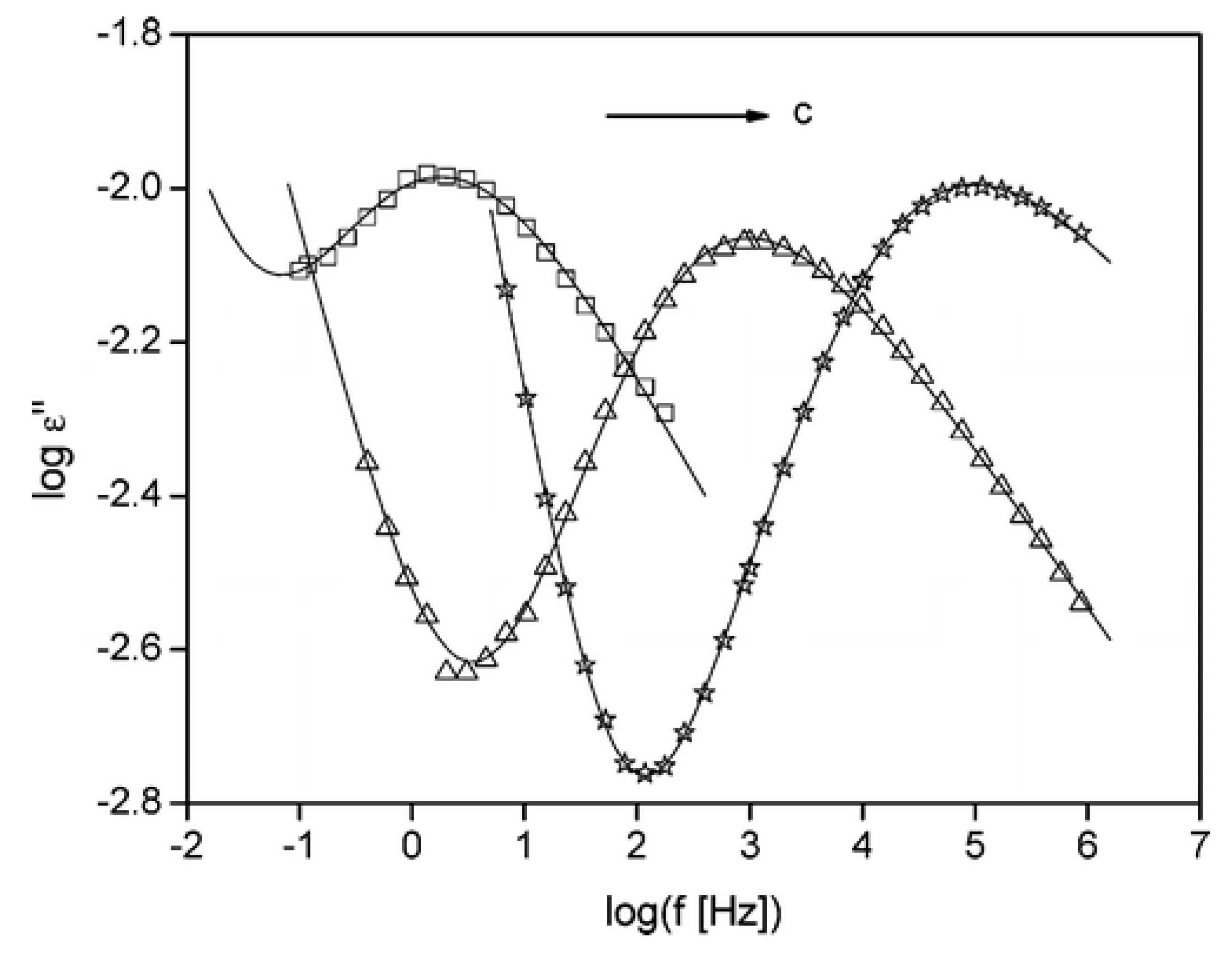
4.4. Dielectric Properties
4.5. Properties Related to Biomedical Applications
5. Summary and Perspectives
Acknowledgments
References
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Morgan, A.B.; Wilkie, C.A. Flame Retardant Polymer Nanocomposites; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lagashetty, A.; Venkataraman, A. Polymer nanocomposites. Resonance 2005, 10, 49–57. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Bockstaller, M.R.; Mickiewicz, R.A.; Thomas, E.L. Block copolymer nanocomposites: Perspectives for tailored functional materials. Adv. Mater. 2005, 17, 1331–1349. [Google Scholar] [CrossRef]
- Winey, K.I.; Vaia, R.A. Polymer nanocomposites. MRS Bull. 2007, 32, 314–319. [Google Scholar] [CrossRef]
- Iyer, P.; Iyer, G.; Coleman, M. Gas transport properties of polyimide-POSS nanocomposites. J. Membr. Sci. 2010, 358, 26–32. [Google Scholar] [CrossRef]
- Iyer, P.; Mapkar, J.A.; Coleman, M.R. A hybrid functional nanomaterial: POSS functionalized carbon nanofiber. Nanotechnology 2009, 20, 325603–325603. [Google Scholar]
- Kuo, S.W.; Chang, F.C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef]
- Shea, K.J.; Loy, D.A. Bridged polysilsesquioxanes. Molecular-engineered hybrid organic−inorganic materials. Chem. Mater. 2001, 13, 3306–3319. [Google Scholar] [CrossRef]
- Lickiss, P.D.; Rataboul, F. Fully Condensed polyhedral oligosilsesquioxanes (POSS): From synthesis to application. In Advances in Organometallic Chemistry; Hill, A.F., Fink, M.J., Eds.; Elsevier Academic Press Inc: San Diego, CA, USA, 2008; Volume 57, pp. 1–116. [Google Scholar]
- Pan, M.-J.; Gorzkowski, E.; McAlliste, K. Dielectric properties of polyhedral oligomeric silsesquioxane (POSS)-based nanocomposites at 77K. IOP Conf. Ser.: Mater. Sci. Eng. 2011, 18, 082006. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Phillips, S.H.; Haddad, T.S.; Tomczak, S.J. Developments in nanoscience: Polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr. Opin. Solid State Mater. Sci. 2004, 8, 21–29. [Google Scholar] [CrossRef]
- Hybrid Plastics Inc. POSS User's Guide. A Guide to Developong New Products with POSS. Available online: http://www.hybridplastics.com/products/catalog.htm (accessed on 15 July 2012).
- Zhou, Z.; Cui, L.; Zhang, Y.; Zhang, Y.; Yin, N. Preparation and properties of POSS grafted polypropylene by reactive blending. Eur. Polym. J. 2008, 44, 3057–3066. [Google Scholar] [CrossRef]
- Chan, E.R.; Striolo, A.; McCabe, C.; Cummings, P.T.; Glotzer, S.C. Coarse-grained force field for simulating polymer-tethered silsesquioxane self-assembly in solution. J. Chem. Phys. 2007, 127, 114102. [Google Scholar]
- Zeng, K.; Zheng, S. Nanostructures and surface dewettability of epoxy thermosets containing hepta(3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(ethylene oxide). J. Phys. Chem. B 2007, 111, 13919–13928. [Google Scholar]
- Guo, Q.Y.; Knight, P.T.; Mather, P.T. Tailored drug release from biodegradable stent coatings based on hybrid polyurethanes. J. Control. Release 2009, 137, 224–233. [Google Scholar] [CrossRef]
- Liu, Y.L. Developments of highly proton-conductive sulfonated polymers for proton exchange membrane fuel cells. Polym. Chem. 2012, 3, 1373–1383. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Lendlein, A. Shape-Memory Polymer Composites. In Shape-Memory Polymers; Advances in Polymer Science; Springer-Verlag Berlin: Berlin, Germany, 2010; Volume 226, pp. 41–95. [Google Scholar]
- Achilleos, D.S.; Vamvakaki, M. End-grafted polymer chains onto inorganic nano-objects. Materials 2010, 3, 1981–2026. [Google Scholar] [CrossRef]
- Fina, A.; Monticelli, O.; Camino, G. POSS-based hybrids by melt/reactive blending. J. Mater. Chem. 2010, 20, 9297–9305. [Google Scholar] [CrossRef]
- Zhao, J.Q.; Fu, Y.; Liu, S.M. Polyhedral oligomeric silsesquioxane (POSS)-modified thermoplastic and thermosetting nanocomposites: A review. Polym. Polym. Compos. 2008, 16, 483–500. [Google Scholar]
- Joshi, M.; Butola, B.S. Polymeric nanocomposites—Polyhedral oligomeric silsesquioxanes (POSS) as hybrid nanofiller. J. Macromol. Sci. 2004, C44, 389–410. [Google Scholar]
- Keledi, G.; Hari, J.; Pukanszky, B. Polymer nanocomposites: Structure, interaction, and functionality. Nanoscale 2012, 4, 1919–1938. [Google Scholar] [CrossRef]
- Pielichowski, K.; Njuguna, J.; Janowski, B.; Pielichowski, J. Polyhedral Oligomeric Silsesquioxanes (POSS)-Containing Nanohybrid Polymers. In Supramolecular Polymers Polymeric Betains Oligomers; Donnio, B., Guillon, D., Harada, A., Hashidzume, A., Jaeger, W., Janowski, B., Kudaibergenov, S., Laschewsky, A., Njuguna, J., Pielichowski, J., et al., Eds.; Springer-Verlag Berlin: Berlin, Germany, 2006; Volume 201, pp. 225–296. [Google Scholar]
- Madbouly, S.A.; Otaigbe, J.U. Recent advances in synthesis, characterization and rheological properties of polyurethanes and POSS/polyurethane nanocomposites dispersions and films. Prog. Polym. Sci. 2009, 34, 1283–1332. [Google Scholar] [CrossRef]
- Jones, I.K.; Zhou, Y.X.; Jeelani, S.; Mabry, J.M. Effect of polyhedral-oligomeric-sil-sesquioxanes on thermal and mechanical behavior of SC-15 epoxy. Express Polym. Lett. 2008, 2, 494–501. [Google Scholar] [CrossRef]
- Zaioncz, S.; Dahmouche, K.; Paranhos, C.M.; Gil, R.; Soares, B.G. Relationships between nanostructure and dynamic-mechanical properties of epoxy network containing PMMA-modified silsesquioxane. Express Polym. Lett. 2009, 3, 340–351. [Google Scholar] [CrossRef]
- Choi, J.; Harcup, J.; Yee, A.F.; Zhu, Q.; Laine, R.M. Organic/inorganic hybrid composites from cubic silsesquioxanes. J. Am. Chem. Soc. 2001, 123, 11420–11430. [Google Scholar]
- Striolo, A.; McCabe, C.; Cummings, P.T. Thermodynamic and transport properties of polyhedral oligomeric sislesquioxanes in poly(dimethylsiloxane). J. Phys. Chem. B 2005, 109, 14300–14307. [Google Scholar]
- Striolo, A.; McCabe, C.; Cummings, P.T. Organic-inorganic telechelic molecules: Solution properties from simulations. J. Chem. Phys. 2006, 125, 104904. [Google Scholar] [CrossRef]
- Lin, Y.; Alexandridis, P. Temperature-dependent adsorption of Pluronic F127 block copolymers onto carbon black particles dispersed in aqueous media. J. Phys. Chem. B 2002, 106, 10834–10844. [Google Scholar] [CrossRef]
- Lin, Y.N.; Smith, T.W.; Alexandridis, P. Adsorption of a rake-type siloxane surfactant onto carbon black nanoparticles dispersed in aqueous media. Langmuir 2002, 18, 6147–6158. [Google Scholar] [CrossRef]
- Anderson, J.A.; Sknepnek, R.; Travesset, A. Design of polymer nanocomposites in solution by polymer functionalization. Phys. Rev. E 2010, 82, 021803. [Google Scholar]
- Sakai, T.; Alexandridis, P. Single-step synthesis and stabilization of metal nanoparticles in aqueous Pluronic block copolymer solutions at ambient temperature. Langmuir 2004, 20, 8426–8430. [Google Scholar] [CrossRef]
- Sakai, T.; Alexandridis, P. Ag and Au monometallic and bimetallic colloids: Morphogenesis in amphiphilic block copolymer solutions. Chem. Mater. 2006, 18, 2577–2583. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Alexandridis, P.; Mallory, R.; Petrou, A.; Mountziaris, T.J. Templated synthesis of ZnSe nanostructures using lyotropic liquid crystals. Nanotechnology 2005, 16, 2372–2380. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Law, N.L.; Mallory, R.; Petrou, A.; Alexandridis, P.; Mountziaris, T.J. Water-based synthesis of ZnSe nanostructures using amphiphilic block copolymer stabilized lyotropic liquid crystals as templates. Nanotechnology 2006, 17, 3121–3128. [Google Scholar] [CrossRef]
- Sarkar, B.; Alexandridis, P. Nanoparticle incorporation in ordered phases formed by solvated block copolymers. Polym. Mater. Sci. Eng. 2010, 102, 166–167. [Google Scholar]
- Sarkar, B.; Alexandridis, P. Self-assembled block copolymer-nanoparticle hybrids: Role of nanoparticle surface interactions. Polym. Mater. Sci. Eng. 2011, 105, 805–806. [Google Scholar]
- Sarkar, B.; Alexandridis, P. Self-assembled block copolymer-nanoparticle hybrids: Interplay between enthalpy and entropy. Langmuir 2012, 28, 15975–15986. [Google Scholar] [CrossRef]
- Sarkar, B.; Venugopal, V.; Bodratti, A.; Tsianou, M.; Alexandridis, P. Nanoparticle surface modification by amphiphilic polymers in aqueous media: Role of polar organic solvents. J. Colloid Interface Sci. 2013. Article No. JCIS 12-1949.. [Google Scholar]
- Sarkar, B.; Venugopal, V.; Tsianou, M.; Alexandridis, P. Adsorption of Pluronic block copolymers on silica nanoparticles. Colloids Surf. A 2013. Article No. COLSUA-D-12-00942.. [Google Scholar]
- Zhang, W.; Müller, A.H.E. Synthesis of tadpole-shaped POSS-containing hybrid polymers via “click chemistry”. Polymer 2010, 51, 2133–2139. [Google Scholar] [CrossRef]
- Fu, B.X.; Lee, A.; Haddad, T.S. Styrene-butadiene-styrene triblock copolymers modified with polyhedral oligomeric silsesquioxanes. Macromolecules 2004, 37, 5211–5218. [Google Scholar] [CrossRef]
- Drazkowski, D.B.; Lee, A.; Haddad, T.S. Morphology and phase transitions in styrene-butadiene-styrene triblock copolymer grafted with isobutyl-substituted polyhedral oligomeric silsesquioxanes. Macromolecules 2007, 40, 2798–2805. [Google Scholar] [CrossRef]
- Drazkowski, D.B.; Lee, A.; Haddad, T.S.; Cookson, D.J. Chemical substituent effects on morphological transitions in styrene-butadiene-styrene triblock copolymer grafted with polyhedral oligomeric silsesquioxanes. Macromolecules 2006, 39, 1854–1863. [Google Scholar] [CrossRef]
- Pyun, J.; Matyjaszewski, K.; Wu, J.; Kim, G.-M.; Chun, S.B.; Mather, P.T. ABA triblock copolymers containing polyhedral oligomeric silsesquioxane pendant groups: Synthesis and unique properties. Polymer 2003, 44, 2739–2750. [Google Scholar]
- Xu, H.; Yang, B.; Wang, J.; Guang, S.; Li, C. Preparation, Tg improvement, and thermal stability enhancement mechanism of soluble poly(methyl methacrylate) nanocomposites by incorporating octavinyl polyhedral oligomeric silsesquioxanes. J. Polym. Sci. A 2007, 45, 5308–5317. [Google Scholar] [CrossRef]
- Hirai, T.; Leolukman, M.; Hayakawa, T.; Kakimoto, M.-A.; Gopalan, P. Hierarchical nanostructures of organosilicate nanosheets within self-organized block copolymer films. Macromolecules 2008, 41, 4558–4560. [Google Scholar] [CrossRef]
- Hirai, T.; Leolukman, M.; Jin, S.; Goseki, R.; Ishida, Y.; Kakimoto, M.-A.; Hayakawa, T.; Ree, M.; Gopalan, P. Hierarchical self-assembled structures from POSS-containing block copolymers synthesized by living anionic polymerization. Macromolecules 2009, 42, 8835–8843. [Google Scholar]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Nanostructured polyurethane/POSS hybrid aqueous dispersions prepared by homogeneous solution polymerization. Macromolecules 2006, 39, 7037–7043. [Google Scholar] [CrossRef]
- Ni, Y.; Zheng, S.; Nie, K. Morphology and thermal properties of inorganic–organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes. Polymer 2004, 45, 5557–5568. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.G.; Laine, R.M. Organic/inorganic hybrid epoxy nanocomposites from aminophenylsilsesquioxanes. Macromolecules 2004, 37, 99–109. [Google Scholar]
- Mu, J.; Liu, Y.; Zheng, S. Inorganic–organic interpenetrating polymer networks involving polyhedral oligomeric silsesquioxane and poly(ethylene oxide). Polymer 2007, 48, 1176–1184. [Google Scholar] [CrossRef]
- Kim, B.-S.; Mather, P.T. Morphology, microstructure, and rheology of amphiphilic telechelics incorporating polyhedral oligosilsesquioxane. Macromolecules 2006, 39, 9253–9260. [Google Scholar] [CrossRef]
- Miao, J.; Cui, L.; Lau, H.P.; Mather, P.T.; Zhu, L. Self-assembly and chain-folding in hybrid coil−coil−cube triblock oligomers of polyethylene-b-poly(ethylene oxide)-b-polyhedral oligomeric silsesquioxane. Macromolecules 2007, 40, 5460–5470. [Google Scholar]
- Zhang, L.; Lu, D.; Tao, K.; Bai, R. Synthesis, characterization and self-assembly of novel amphiphilic block copolymers with a polyhedral oligomeric silsesquioxanes moiety attached at the junction of the two blocks. Macromol. Rapid Commun. 2009, 30, 1015–1020. [Google Scholar] [CrossRef]
- Mya, K.Y.; Li, X.; Chen, L.; Ni, X.; Li, J.; He, C. Core−corona structure of cubic silsesquioxane-poly(ethylene oxide) in aqueous solution: Fluorescence, light scattering, and TEM studies. J. Phys. Chem. B 2005, 109, 9455–9462. [Google Scholar]
- Fu, B.X.; Yang, L.; Somani, R.H.; Zong, S.X.; Hsiao, B.S.; Phillips, S.; Blanski, R.; Ruth, P. Crystallization studies of isotactic polypropylene containing nanostructured polyhedral oligomeric silsesquioxane molecules under quiescent and shear conditions. J. Polym. Sci. B 2001, 39, 2727–2739. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Camino, G. Polypropylene–polysilsesquioxane blends. Eur. Polym. J. 2010, 46, 14–23. [Google Scholar] [CrossRef]
- Hao, N.; Boehning, M.; Schoenhals, A. Dielectric properties of nanocomposites based on polystyrene and polyhedral oligomeric phenethyl-silsesquioxanes. Macromolecules 2007, 40, 9672–9679. [Google Scholar]
- Carroll, J.B.; Waddon, A.J.; Nakade, H.; Rotello, V.M. “Plug and play” polymers. Thermal and X-ray characterizations of noncovalently grafted polyhedral oligomeric silsesquioxane (POSS)-Polystyrene nanocomposites. Macromolecules 2003, 36, 6289–6291. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Wang, S.; Ji, J. Effect of POSS on morphology and properties of poly(2,6-dimethyl-1,4-phenylene oxide)/polyamide 6 blends. Eur. Polym. J. 2009, 45, 2202–2210. [Google Scholar] [CrossRef]
- Sánchez-Soto, M.; Illescas, S.; Milliman, H.; Schiraldi, D.A.; Arostegui, A. Morphology and thermomechanical properties of melt-mixed polyoxymethylene/polyhedral oligomeric silsesquioxane nanocomposites. Macromol. Mater. Eng. 2010, 295, 846–858. [Google Scholar] [CrossRef]
- Illescas, S.; Sanchez-Soto, M.; Milliman, H.; Schiraldi, D.A.; Arostegui, A. The morphology and properties of melt-mixed polyoxymethylene/monosilanolisobutyl-POSS composites. High Perform. Polym. 2011, 23, 457–467. [Google Scholar] [CrossRef]
- Huang, K.-W.; Tsai, L.-W.; Kuo, S.-W. Influence of octakis-functionalized polyhedral oligomeric silsesquioxanes on the physical properties of their polymer nanocomposites. Polymer 2009, 50, 4876–4887. [Google Scholar] [CrossRef]
- Alexandridis, P.; Holzwarth, J.F.; Hatton, T.A. Micellization of poly(ethylene oxide)-poly(proplene oxide)-poly(ethlene oxide) triblock copolymers in aqueous-solutions: Thermodynamics of copolymer association. Macromolecules 1994, 27, 2414–2425. [Google Scholar] [CrossRef]
- Alexandridis, P. Structural polymorphism of poly(ethylene oxide)-poly(propylene oxide) block copolymers in nonaqueous polar solvents. Macromolecules 1998, 31, 6935–6942. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil). Langmuir 1998, 14, 2627–2638. [Google Scholar] [CrossRef]
- Alexandridis, P.; Zhou, D.L.; Khan, A. Lyotropic liquid crystallinity in amphiphilic block copolymers: Temperature effects on phase behavior and structure for poly(ethylene oxide)-b-poly(propylene oxide)-b-poly(ethylene oxide) copolymers of different composition. Langmuir 1996, 12, 2690–2700. [Google Scholar] [CrossRef]
- Svensson, B.; Alexandridis, P.; Olsson, U. Self-assembly of a poly(ethylene oxide) poly(propylene oxide) block copolymer (Pluronic P104, (EO)27(PO)61(EO)27) in the presence of water and xylene. J. Phys. Chem. B 1998, 102, 7541–7548. [Google Scholar]
- Daga, V.K.; Watkins, J.J. Hydrogen-bond-mediated phase behavior of complexes of small molecule additives with poly(ethylene oxide-b-propylene oxide-b-ethylene oxide) triblock copolymer surfactants. Macromolecules 2010, 43, 9990–9997. [Google Scholar]
- Daga, V.K.; Anderson, E.R.; Gido, S.P.; Watkins, J.J. Hydrogen bond assisted assembly of well-ordered polyhedral oligomeric silsesquioxane–block copolymer composites. Macromolecules 2011, 44, 6793–6799. [Google Scholar] [CrossRef]
- Joshi, M.; Butola, B.S.; Simon, G.; Kukaleva, N. Rheological and viscoelastic behavior of HDPE/octamethyl-POSS nanocomposites. Macromolecules 2006, 39, 1839–1849. [Google Scholar]
- Lu, C.-H.; Kuo, S.-W.; Chang, W.-T.; Chang, F.-C. The self-assembled structure of the diblock copolymer PCL-b-P4VP transforms upon competitive interactions with octaphenol polyhedral oligomeric silsesquioxane. Macromol. Rapid Commun. 2009, 30, 2121–2127. [Google Scholar] [CrossRef]
- Qiu, Z.; Pan, H. Preparation, crystallization and hydrolytic degradation of biodegradable poly(l-lactide)/polyhedral oligomeric silsesquioxanes nanocomposite. Compos. Sci. Technol. 2010, 70, 1089–1094. [Google Scholar]
- Yu, J.; Qiu, Z. Preparation and properties of biodegradable poly(l-lactide)/octamethyl-polyhedral oligomeric silsesquioxanes nanocomposites with enhanced crystallization rate via simple melt compounding. ACS Appl. Mater. Interfaces 2011, 3, 890–897. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, S.; Song, L.; Yang, H.; Lu, H.; Hu, Y. Synergistic effect of POSS on mechanical properties, flammability, and thermal degradation of intumescent flame retardant polylactide composites. J. Macromol. Sci. B 2011, 51, 255–268. [Google Scholar]
- Wu, X.; Sun, Y.; Xie, W.; Liu, Y.; Song, X. Development of novel dental nanocomposites reinforced with polyhedral oligomeric silsesquioxane (POSS). Dent. Mater. 2010, 26, 456–462. [Google Scholar] [CrossRef]
- Fong, H.; Dickens, S.H.; Flaim, G.M. Evaluation of dental restorative composites containing polyhedral oligomeric silsesquioxane methacrylate. Dent. Mater. 2005, 21, 520–529. [Google Scholar] [CrossRef]
- Gao, F.; Tong, Y.; Schricker, S.R.; Culbertson, B.M. Evaluation of neat resins based on methacrylates modified with methacryl-POSS, as potential organic–inorganic hybrids for formulating dental restoratives. Polym. Adv. Technol. 2001, 12, 355–360. [Google Scholar] [CrossRef]
- Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Lee, Y.M.; Chung, D.J.; Lee, J.I.; Hong, S.D. A biocompatibility study of a reinforced acrylic-based hybrid denture composite resin with polyhedraloligosilsesquioxane. J. Oral Rehabilit. 2007, 34, 389–395. [Google Scholar] [CrossRef]
- Zheng, L.; Farris, R.J.; Coughlin, E.B. Synthesis of polyethylene hybrid copolymers containing polyhedral oligomeric silsesquioxane prepared with ring-opening metathesis copolymerization. J. Polym. Sci. A 2001, 39, 2920–2928. [Google Scholar] [CrossRef]
- Zheng, L.; Farris, R.J.; Coughlin, E.B. Novel polyolefin nanocomposites: Synthesis and characterizations of metallocene-catalyzed polyolefin polyhedral oligomeric silsesquioxane copolymers. Macromolecules 2001, 34, 8034–8039. [Google Scholar] [CrossRef]
- Rashid, E.S.A.; Ariffin, K.; Kooi, C.C.; Akil, H.M. Preparation and properties of POSS/epoxy composites for electronic packaging applications. Mater. Des. 2009, 30, 1–8. [Google Scholar]
- Huang, J.C.; He, C.B.; Xiao, Y.; Mya, K.Y.; Dai, J.; Siow, Y.P. Polyimide/POSS nanocomposites: Interfacial interaction, thermal properties and mechanical properties. Polymer 2003, 44, 4491–4499. [Google Scholar] [CrossRef]
- Wu, S.; Hayakawa, T.; Kakimoto, M.-A.; Oikawa, H. Synthesis and characterization of organosoluble aromatic polyimides containing POSS in main chain derived from double-decker-shaped silsesquioxane. Macromolecules 2008, 41, 3481–3487. [Google Scholar]
- Wu, S.; Hayakawa, T.; Kikuchi, R.; Grunzinger, S.J.; Kakimoto, M.-A.; Oikawa, H. Synthesis and characterization of semiaromatic polyimides containing POSS in main chain derived from double-decker-shaped silsesquioxane. Macromolecules 2007, 40, 5698–5705. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Mather, P.T. Vertex group effects in entangled polystyrene-polyhedral pligosilsesquioxane (POSS) copolymers. Macromolecules 2009, 42, 1142–1152. [Google Scholar]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS particles on the mechanical, thermal, and morphological properties of PLA and plasticised PLA. J. Appl. Polym. Sci. 2011, 121, 1067–1075. [Google Scholar] [CrossRef]
- Maitra, P.; Wunder, S.L. Oligomeric poly(ethylene oxide)-functionalized silsesquioxanes: Interfacial effects on Tg, Tm, and ΔHm. Chem. Mater. 2002, 14, 4494–4497. [Google Scholar] [CrossRef]
- Hao, N.; Bohning, M.; Goering, H.; Schonhals, A. Nanocomposites of polyhedral oligomeric phenethylsilsesquioxanes and poly(bisphenol A carbonate) as investigated by dielectric spectroscopy. Macromolecules 2007, 40, 2955–2964. [Google Scholar] [CrossRef]
- Klocek, J.; Henkel, K.; Kolanek, K.; Zschech, E.; Schmeisser, D. Spectroscopic and capacitance-voltage characterization of thin aminopropylmethoxysilane films doped with copper phthalocyanine, tris(dimethylvinylsilyloxy)-POSS and fullerene cages. Appl. Surf. Sci. 2012, 258, 4213–4221. [Google Scholar]
- Guo, Y.-L.; Wang, W.; Otaigbe, J.U. Biocompatibility of synthetic poly(ester urethane)/polyhedral oligomeric silsesquioxane matrices with embryonic stem cell proliferation and differentiation. J. Tissue Eng. Regener. Med. 2010, 4, 553–564. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Y.-l.; Otaigbe, J.U. The synthesis, characterization and biocompatibility of poly(ester urethane)/polyhedral oligomeric silesquioxane nanocomposites. Polymer 2009, 50, 5749–5757. [Google Scholar] [CrossRef]
- Knight, P.T.; Kirk, J.T.; Anderson, J.M.; Mather, P.T. In vivo kinetic degradation analysis and biocompatibility of aliphatic polyester polyurethanes. J. Biomed. Mater. Res. A 2010, 94A, 333–343. [Google Scholar]
- Ghanbari, H.; Cousins, B.G.; Seifalian, A.M. A nanocage for nanomedicine: Polyhedral oligomeric silsesquioxane (POSS). Macromol. Rapid Commun. 2011, 32, 1032–1046. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. The endothelialization of polyhedral oligomeric silsesquioxane nanocomposites—An in vitro study. Cell Biochem. Biophys. 2006, 45, 129–136. [Google Scholar] [CrossRef]
- Gupta, A.; Seifalian, A.M.; Ahmad, Z.; Edirisinghe, M.J.; Winslet, M.C. Novel electrohydrodynamic printing of nanocomposite biopolymer scaffolds. J. Bioact. Compat. Polym. 2007, 22, 265–280. [Google Scholar] [CrossRef]
- Gupta, A.; Vara, D.S.; Punshon, G.; Sales, K.M.; Winslet, M.C.; Seifalian, A.M. In vitro small intestinal epithelial cell growth on a nanocomposite polycaprolactone scaffold. Biotechnol. Appl. Biochem. 2009, 54, 221–229. [Google Scholar] [CrossRef]
- Chhabra, P.; Choudhary, V. Polymer nanocomposite membranes based on sulfonated poly(ether ether ketone) and trisilanol phenyl POSS for fuel cell applications. J. Appl. Polym. Sci. 2010, 118, 3013–3023. [Google Scholar] [CrossRef]
- Devaraju, S.; Vengatesan, M.R.; Selvi, M.; Kumar, A.A.; Alagar, M. Synthesis and characterization of bisphenol-A ether diamine-based polyimide POSS nanocomposites for low K dielectric and flame-retardant applications. High Perform. Polym. 2012, 24, 85–96. [Google Scholar] [CrossRef]
- DeArmitt, C. Polyherdral Oligomeric Silesquioxane Handbook. Phanom Plastics. Available online: http://www.phantomplastics.com/attachments/File/POSS_Handbook.pdf (accessed on 5 December 2012).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445-475. https://doi.org/10.3390/nano2040445
Ayandele E, Sarkar B, Alexandridis P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials. 2012; 2(4):445-475. https://doi.org/10.3390/nano2040445
Chicago/Turabian StyleAyandele, Ebunoluwa, Biswajit Sarkar, and Paschalis Alexandridis. 2012. "Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites" Nanomaterials 2, no. 4: 445-475. https://doi.org/10.3390/nano2040445