Influence of Concentration, Surface Charge, and Natural Water Components on the Transport and Adsorption of Polystyrene Nanoplastics in Sand Columns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polystyrene Latex Nanoplastics
2.1.2. Ultrapure and Geneva Lake Water
2.1.3. Quartz Sand
2.2. Methods
2.2.1. Laboratory Column Experiments
2.2.2. Tracer Experiments
2.2.3. NPL Characterization and Concentration Determination
2.2.4. SEM Imaging
3. Results and Discussion
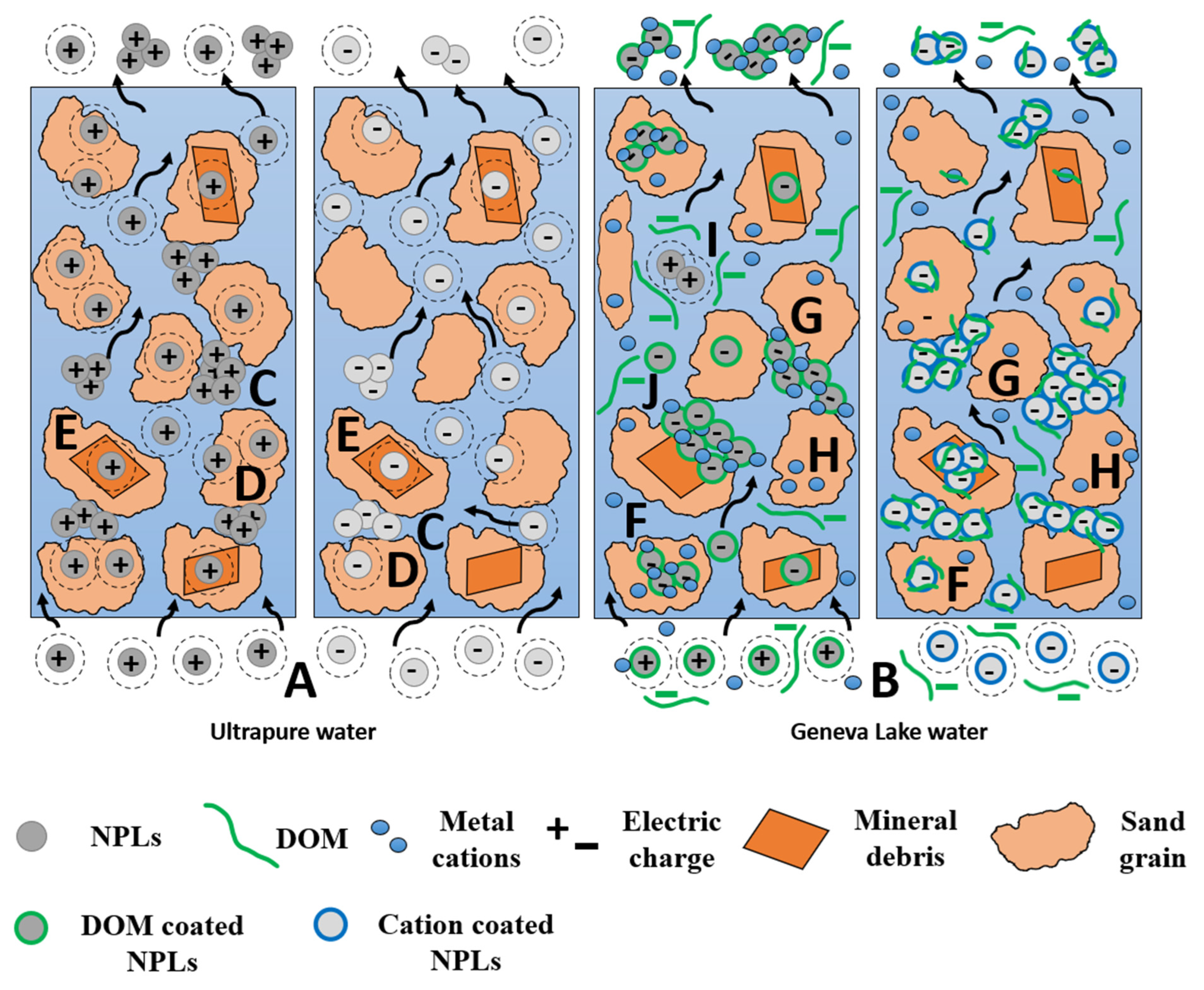
3.1. Characterization of Nanoplastics Dispersed in Ultrapure and Geneva Lake Water
3.2. Nanoplastics Transport in Saturated Quartz Sand Columns
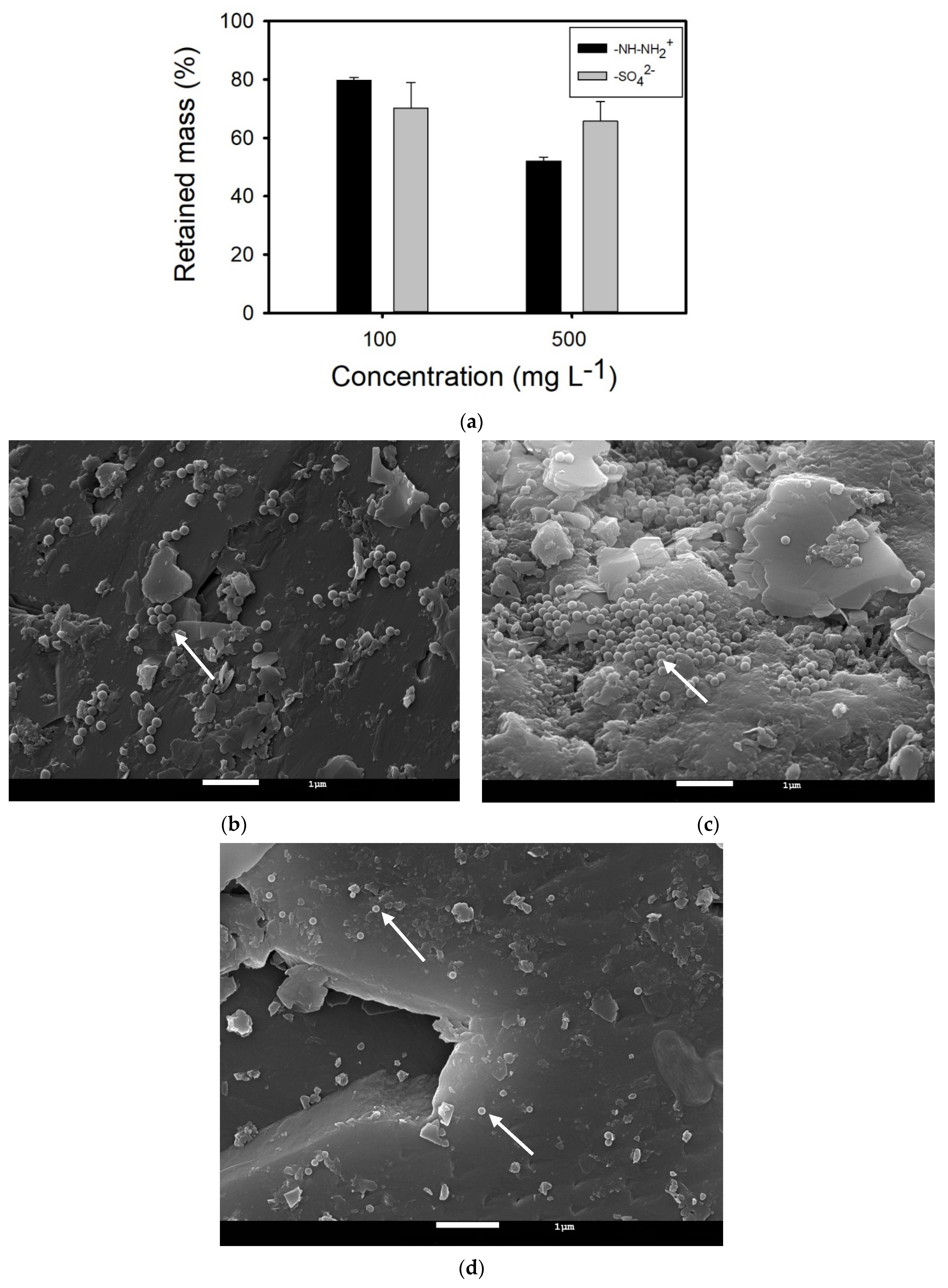
3.2.1. NPLs Transport and Retention in Ultrapure Water-Saturated Columns
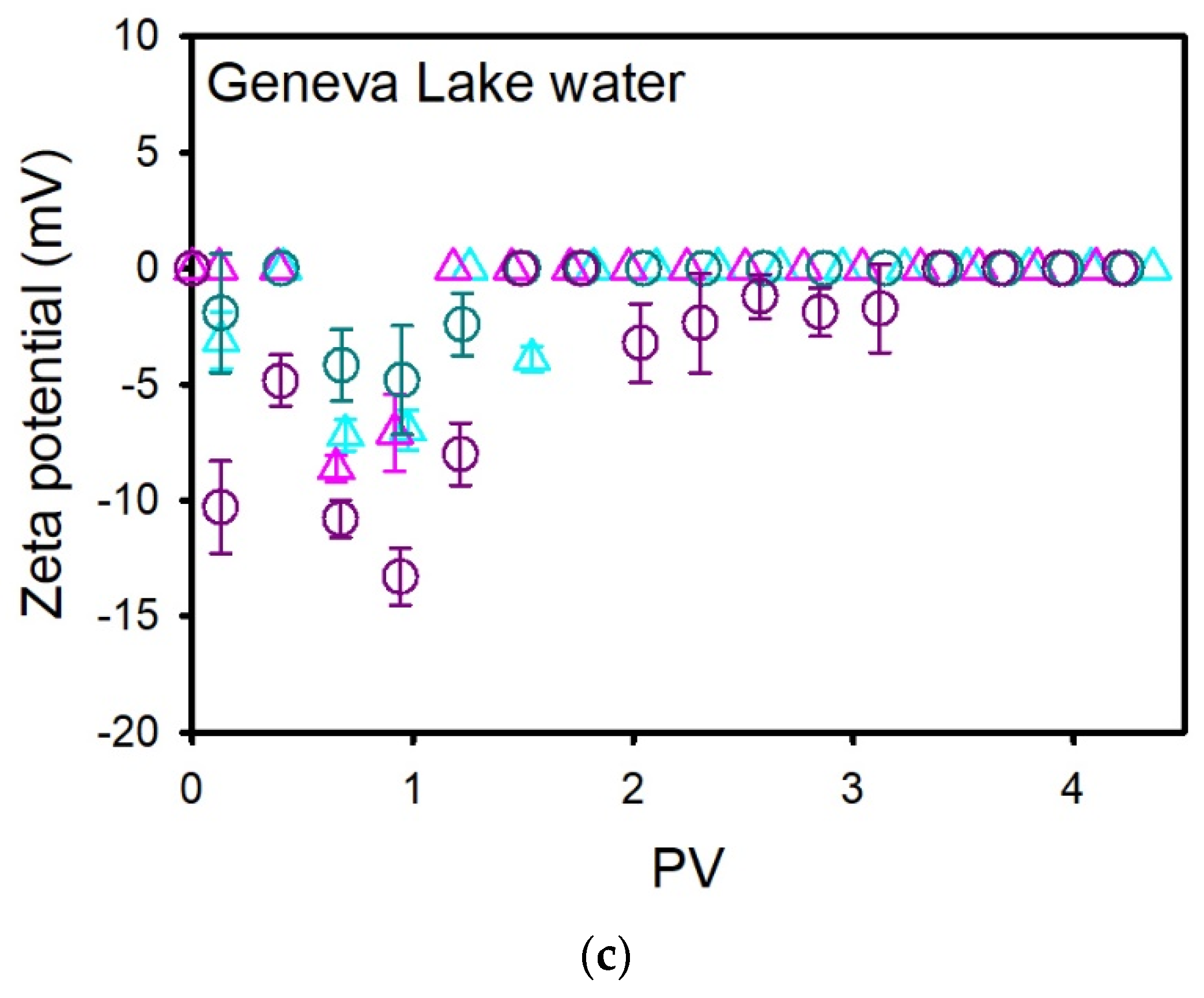
3.2.2. NPLs Transport and Retention in Geneva Lake Water Saturated Columns
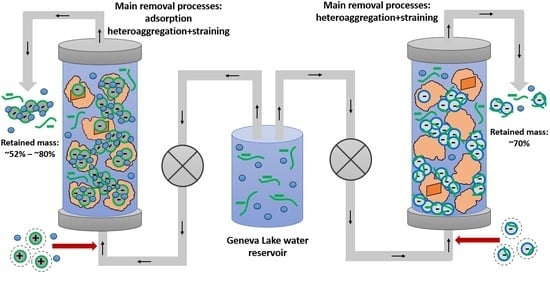
3.3. Summary of Transport and Retention Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gigault, J.; Halle, A.t.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; Song, Y.K.; Hong, S.H.; Jang, Y.C.; Jang, M.; Heo, N.W.; Han, G.M.; Lee, M.J.; Kang, D.; et al. Relationships among the abundances of plastic debris in different size classes on beaches in South Korea. Mar. Pollut. Bull. 2013, 77, 349–354. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Yousefi, N.; Tufenkji, N. Are There Nanoplastics in Your Personal Care Products? Environ. Sci. Technol. Lett. 2017, 4, 280–285. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Characterisation of nanoplastics during the degradation of polystyrene. Chemosphere 2016, 145, 265–268. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics the Facts 2022. 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 10 July 2023).
- Li, W.C.; Tse, H.F.; Fok, L. Plastic waste in the marine environment: A review of sources, occurrence and effects. Sci. Total Environ. 2016, 566–567, 333–349. [Google Scholar] [CrossRef]
- Horton, A.A.; Svendsen, C.; Williams, R.J.; Spurgeon, D.J.; Lahive, E. Large microplastic particles in sediments of tributaries of the River Thames, UK—Abundance, sources and methods for effective quantification. Mar. Pollut. Bull. 2017, 114, 218–226. [Google Scholar] [CrossRef]
- Lechner, A.; Keckeis, H.; Lumesberger-Loisl, F.; Zens, B.; Krusch, R.; Tritthart, M.; Glas, M.; Schludermann, E. The Danube so colourful: A potpourri of plastic litter outnumbers fish larvae in Europe’s second largest river. Environ. Pollut. 2014, 188, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Negrete Velasco, A.d.J.; Rard, L.; Blois, W.; Lebrun, D.; Lebrun, F.; Pothe, F.; Stoll, S. Microplastic and Fibre Contamination in a Remote Mountain Lake in Switzerland. Water 2020, 12, 2410. [Google Scholar] [CrossRef]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in marine environment: A review on sources, classification, and potential remediation by membrane technology. Environ. Sci. Water Res. Technol. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Shi, C.; Liu, Z.; Yu, B.; Zhang, Y.; Yang, H.; Han, Y.; Wang, B.; Liu, Z.; Zhang, H. Emergence of nanoplastics in the aquatic environment and possible impacts on aquatic organisms. Sci. Total Environ. 2024, 906, 167404. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino de la Serna, J. The potential impacts of micro-and-nano plastics on various organ systems in humans. eBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Gong, H.; Li, R.; Li, F.; Guo, X.; Xu, L.; Gan, L.; Yan, M.; Wang, J. Toxicity of nanoplastics to aquatic organisms: Genotoxicity, cytotoxicity, individual level and beyond individual level. J. Hazard. Mater. 2023, 443, 130266. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Sharma, V.K.; Ma, X.; Lichtfouse, E.; Robert, D. Nanoplastics are potentially more dangerous than microplastics. Environ. Chem. Lett. 2023, 21, 1933–1936. [Google Scholar] [CrossRef]
- Feng, Y.; Tu, C.; Li, R.; Wu, D.; Yang, J.; Xia, Y.; Peijnenburg, W.J.G.M.; Luo, Y. A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health. Eco-Environ. Health 2023, 2, 195–207. [Google Scholar] [CrossRef]
- Okutan, H.M.; Sağir, Ç.; Fontaine, C.; Nauleau, B.; Kurtulus, B.; Le Coustumer, P.; Razack, M. One-Dimensional Experimental Investigation of Polyethylene Microplastic Transport in a Homogeneous Saturated Medium. Front. Environ. Sci. 2022, 10, 885875. [Google Scholar] [CrossRef]
- Pradel, A.; Hadri, H.e.; Desmet, C.; Ponti, J.; Reynaud, S.; Grassl, B.; Gigault, J. Deposition of environmentally relevant nanoplastic models in sand during transport experiments. Chemosphere 2020, 255, 126912. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Bolster, C.; Johnson, W.P.; Li, K.; Pazmino, E.; Camacho, K.M.; Anselmo, A.C.; Mitragotri, S. Coupled influences of particle shape, surface property and flow hydrodynamics on rod-shaped colloid transport in porous media. J. Colloid Interface Sci. 2020, 577, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Yan, Z.; Lu, G. Vertical transport and retention behavior of polystyrene nanoplastics in simulated hyporheic zone. Water Res. 2022, 219, 118609. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, Q.; Yu, M.; Yin, J.; Sun, H.; Wang, N.; Wang, J.; Yin, X. Transport of functional group modified polystyrene nanoplastics in binary metal oxide saturated porous media. J. Hazard. Mater. 2023, 441, 129834. [Google Scholar] [CrossRef]
- Ramirez Arenas, L.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Fate and removal efficiency of polystyrene nanoplastics in a pilot drinking water treatment plant. Sci. Total Environ. 2022, 813, 152623. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Reyes, G.; Magherini, L.; Bianco, C.; Sethi, R.; von Gunten, U.; Kaegi, R.; Mitrano, D.M. Nanoplastics removal during drinking water treatment: Laboratory- and pilot-scale experiments and modeling. J. Hazard. Mater. 2022, 436, 129011. [Google Scholar] [CrossRef] [PubMed]
- Hul, G.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Towards a better understanding of CeO2 manufactured nanoparticles adsorption onto sand grains used in drinking water treatment plants. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 129000. [Google Scholar] [CrossRef]
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef]
- Gallo, F.; Fossi, C.; Weber, R.; Santillo, D.; Sousa, J.; Ingram, I.; Nadal, A.; Romano, D. Marine litter plastics and microplastics and their toxic chemicals components: The need for urgent preventive measures. Environ. Sci. Eur. 2018, 30, 13. [Google Scholar] [CrossRef] [PubMed]
- Boving, T.B.; Fetter, C.W.; Kreamer, D. Contaminant Hydrogeology, 3rd ed.; Waveland Press: Long Grove, IL, USA, 2017. [Google Scholar]
- Hul, G.; Martignier, A.; Gentile, S.R.; Zimmermann, S.; Ramaciotti, P.; Perdaems, P.; Stoll, S. Insights into polystyrene nanoplastics adsorption mechanisms onto quartz sand used in drinking water treatment plants. Sci. Total Environ. 2024, 908, 168076. [Google Scholar] [CrossRef]
- Cornelis, G.; Pang, L.; Doolette, C.; Kirby, J.K.; McLaughlin, M.J. Transport of silver nanoparticles in saturated columns of natural soils. Sci. Total Environ. 2013, 463–464, 120–130. [Google Scholar] [CrossRef]
- Chen, M.-H.; Fang, H.-W.; Huang, L. Surface charge distribution and its impact on interactions between sediment particles. Ocean. Dyn. 2013, 63, 1113–1121. [Google Scholar] [CrossRef]
- Charrière, D.; Hernández Cortázar, M.d.A.; Behra, P. Effect of the presence of pyrite traces on silver behavior in natural porous media. J. Colloid Interface Sci. 2015, 446, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, E. pH-dependent surface charging of metal oxides. Period. Polytech. Chem. Eng. 2009, 53, 77–86. [Google Scholar] [CrossRef]
- Tombácz, E.; Szekeres, M. Surface charge heterogeneity of kaolinite in aqueous suspension in comparison with montmorillonite. Appl. Clay Sci. 2006, 34, 105–124. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, Y.; Shen, C.; Bradford, S.A. Micro- and nanoplastics retention in porous media exhibits different dependence on grain surface roughness and clay coating with particle size. Water Res. 2022, 221, 118717. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Cheng, Z.; Wu, M.; Hao, Y.; Hu, B.X.; Mo, C.; Li, Q.; Xiang, L.; Zhao, H.; Wu, J.; et al. Investigating transport kinetics of polystyrene nanoplastics in saturated porous media. Ecotoxicol. Environ. Saf. 2022, 241, 113820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hou, J.; Wu, J.; Miao, L.; Zeng, Y. Effects of input concentration, media particle size, and flow rate on fate of polystyrene nanoplastics in saturated porous media. Sci. Total Environ. 2023, 881, 163237. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.A.; Torkzaban, S. Colloid Transport and Retention in Unsaturated Porous Media: A Review of Interface-, Collector-, and Pore-Scale Processes and Models. Vadose Zone J. 2008, 7, 667–681. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Yi, K.; He, L.; Han, P.; Tong, M. Transport and deposition of microplastic particles in saturated porous media: Co-effects of clay particles and natural organic matter. Environ. Pollut. 2021, 287, 117585. [Google Scholar] [CrossRef]
- Slomberg, D.L.; Ollivier, P.; Miche, H.; Angeletti, B.; Bruchet, A.; Philibert, M.; Brant, J.; Labille, J. Nanoparticle stability in lake water shaped by natural organic matter properties and presence of particulate matter. Sci. Total Environ. 2019, 656, 338–346. [Google Scholar] [CrossRef]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of polystyrene nanoplastics in natural soils: Effect of soil properties, ionic strength and cation type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef] [PubMed]
- Douch, J.; Hamdani, M.; Fessi, H.; Elaissari, A. Acid–base behavior of a colloidal clays fraction extracted from natural quartz sand: Effect of permanent surface charge. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 51–60. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, L.; Zhang, W.; Huang, R.; Lv, X.; Jing, X.; Yang, Z.; Wang, J.; Qiu, Y. Role of surface functionalities of nanoplastics on their transport in seawater-saturated sea sand. Environ. Pollut. 2019, 255, 113177. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Rong, H.; Wu, D.; Li, M.; Wang, C.; Tong, M. Influence of biofilm on the transport and deposition behaviors of nano- and micro-plastic particles in quartz sand. Water Res. 2020, 178, 115808. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hul, G.; Okutan, H.; Le Coustumer, P.; Ramseier Gentile, S.; Zimmermann, S.; Ramaciotti, P.; Perdaems, P.; Stoll, S. Influence of Concentration, Surface Charge, and Natural Water Components on the Transport and Adsorption of Polystyrene Nanoplastics in Sand Columns. Nanomaterials 2024, 14, 529. https://doi.org/10.3390/nano14060529
Hul G, Okutan H, Le Coustumer P, Ramseier Gentile S, Zimmermann S, Ramaciotti P, Perdaems P, Stoll S. Influence of Concentration, Surface Charge, and Natural Water Components on the Transport and Adsorption of Polystyrene Nanoplastics in Sand Columns. Nanomaterials. 2024; 14(6):529. https://doi.org/10.3390/nano14060529
Chicago/Turabian StyleHul, Gabriela, Hande Okutan, Philippe Le Coustumer, Stéphan Ramseier Gentile, Stéphane Zimmermann, Pascal Ramaciotti, Pauline Perdaems, and Serge Stoll. 2024. "Influence of Concentration, Surface Charge, and Natural Water Components on the Transport and Adsorption of Polystyrene Nanoplastics in Sand Columns" Nanomaterials 14, no. 6: 529. https://doi.org/10.3390/nano14060529