Microwave-Assisted Metal-Organic Frameworks Derived Synthesis of Zn2GeO4 Nanowire Bundles for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Zn-MOF (Zeolitic Imidazolate Framework-8, ZIF-8)
2.3. Synthesis of Ge-MOF
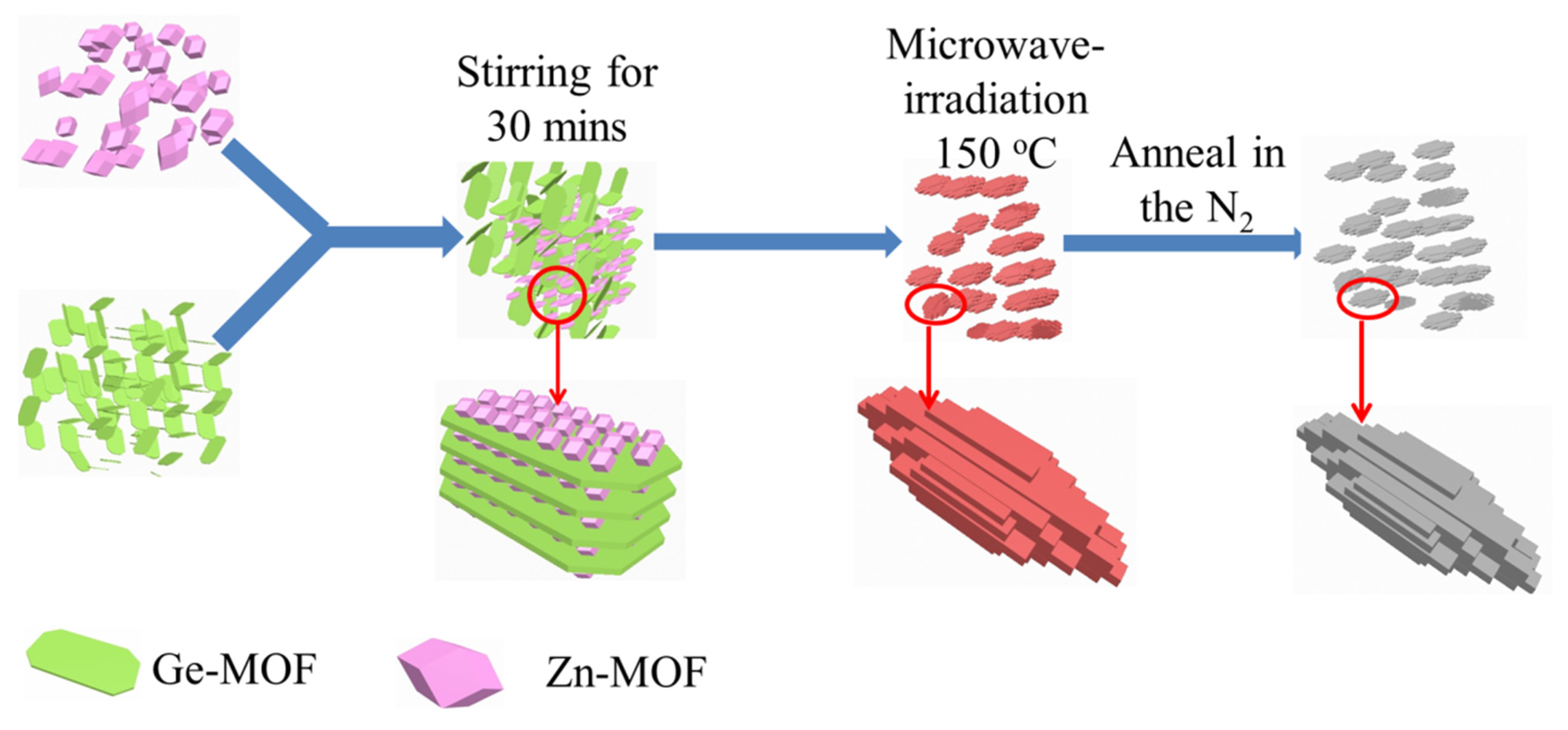
2.4. Synthesis of Zn2GeO4
2.5. Material Characterization
2.6. Electrochemical Evaluation
3. Results
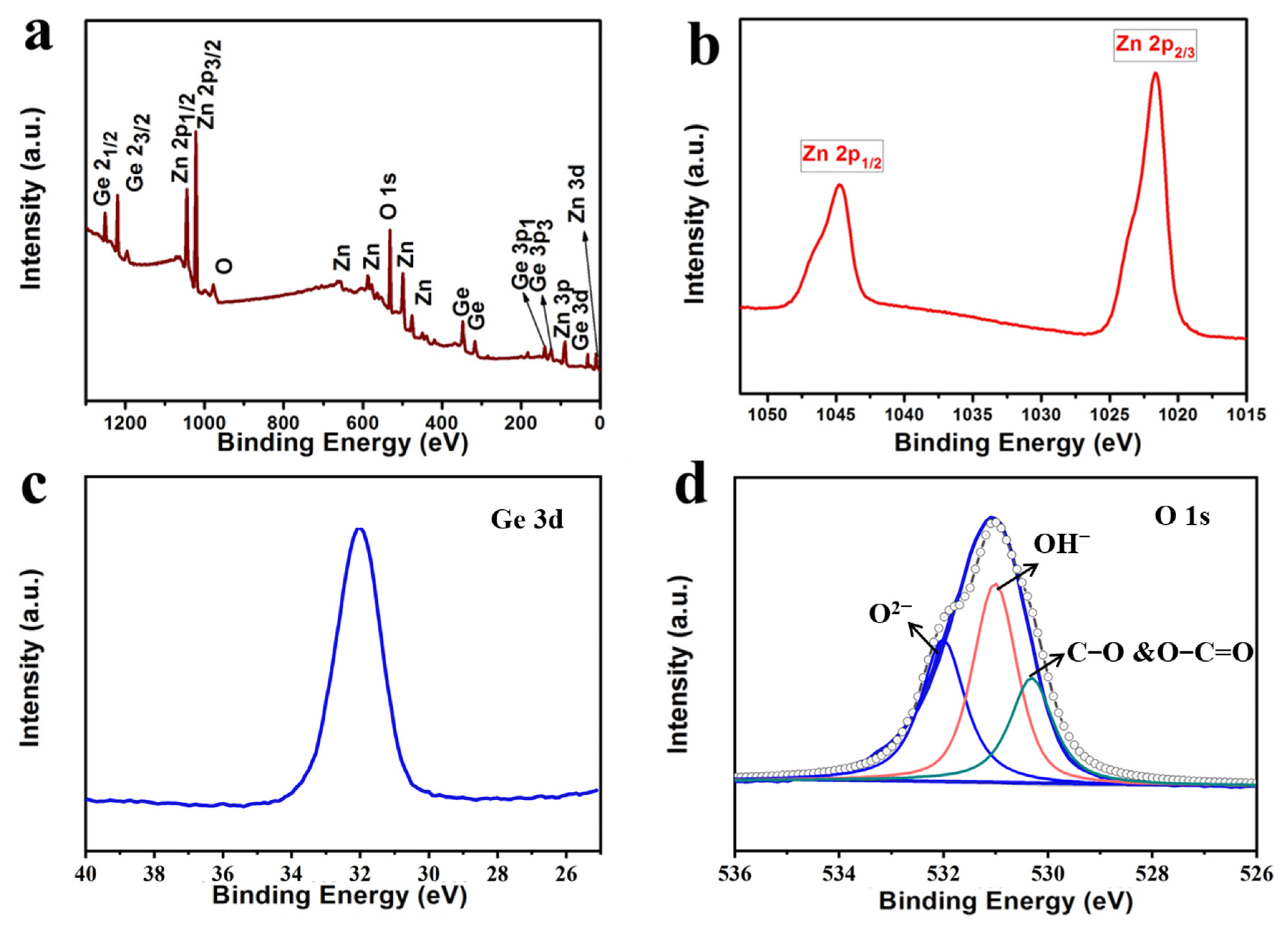
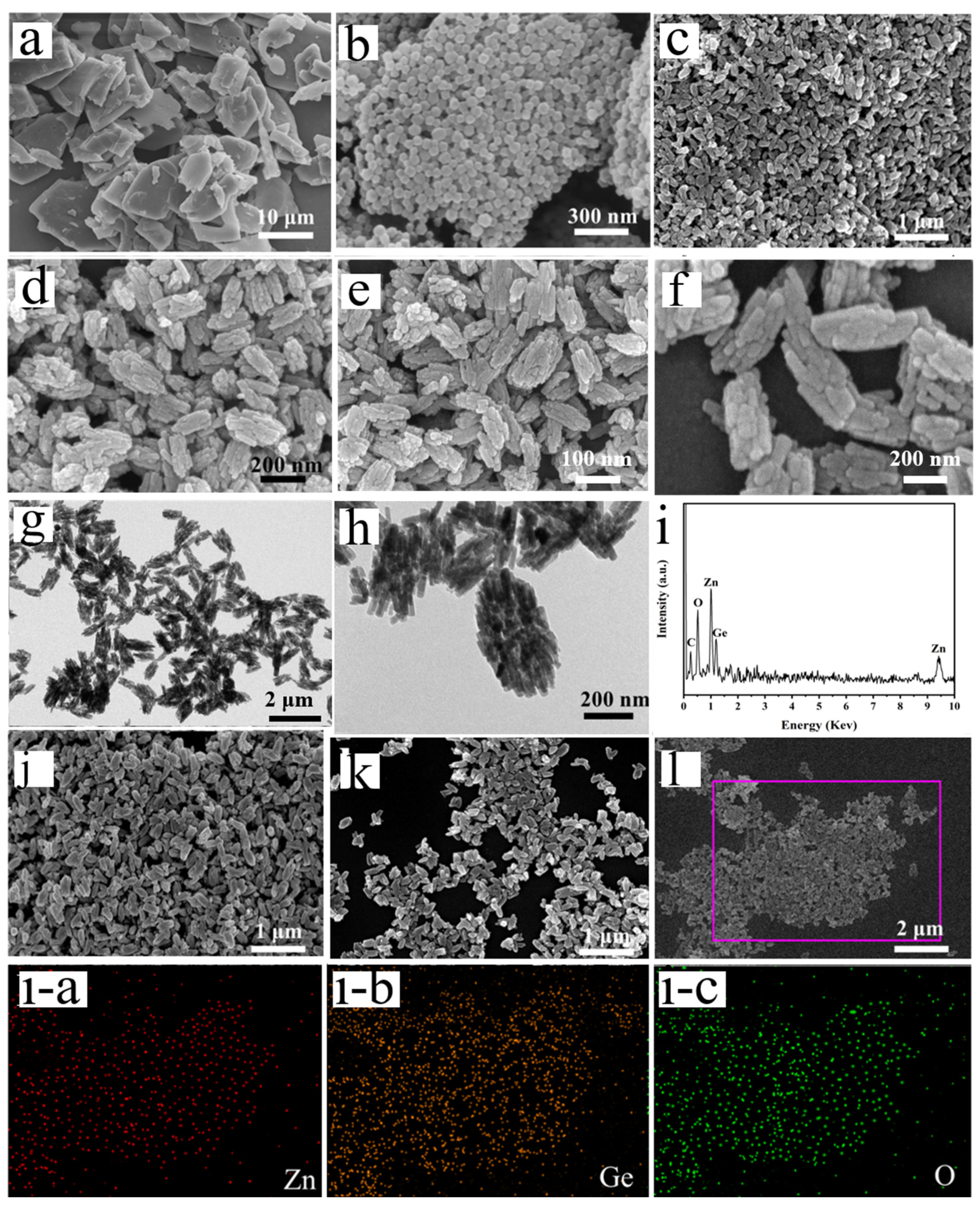
3.1. Characterization of Anode Materials
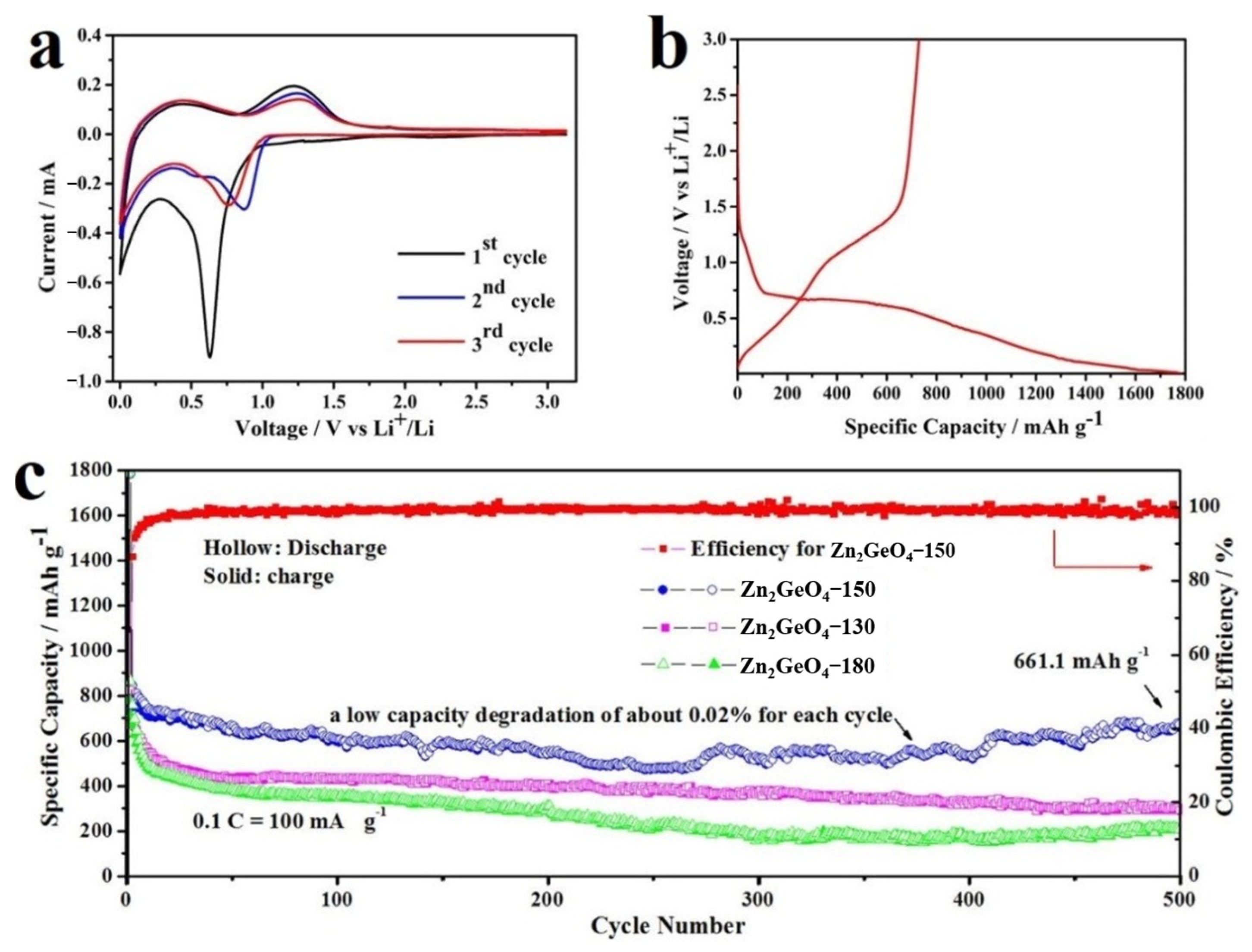
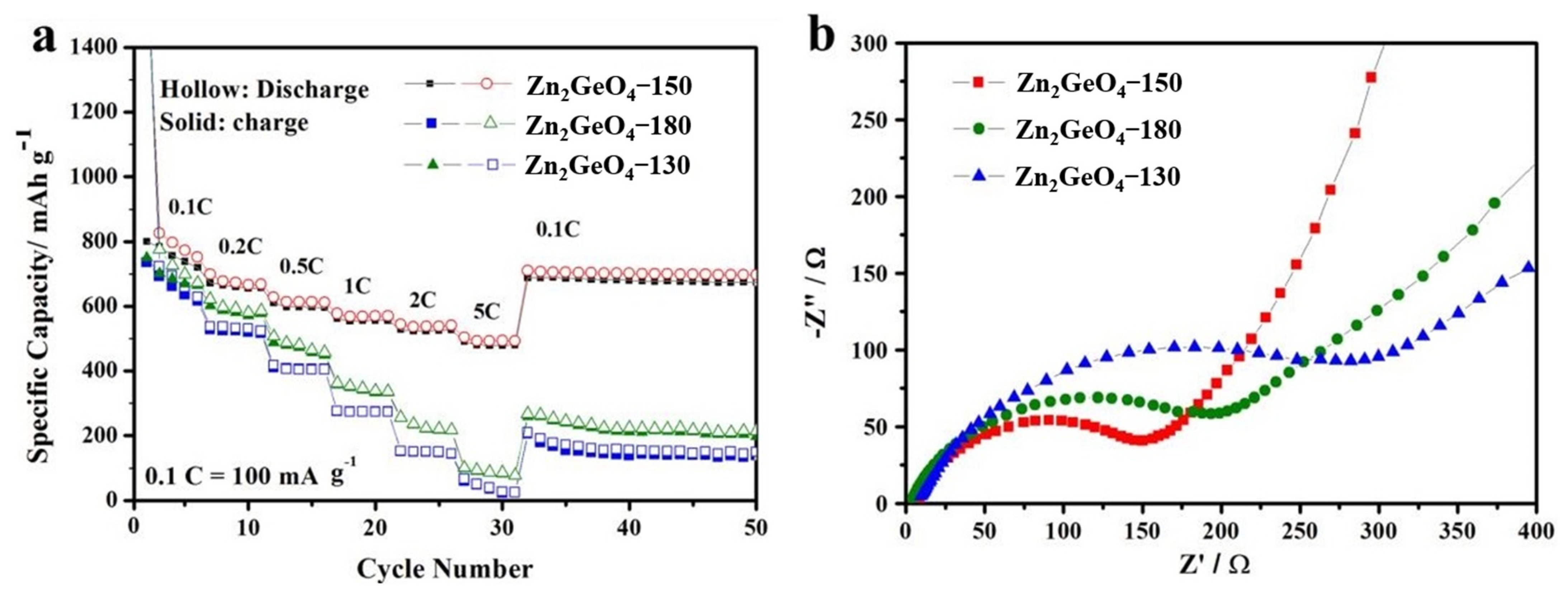
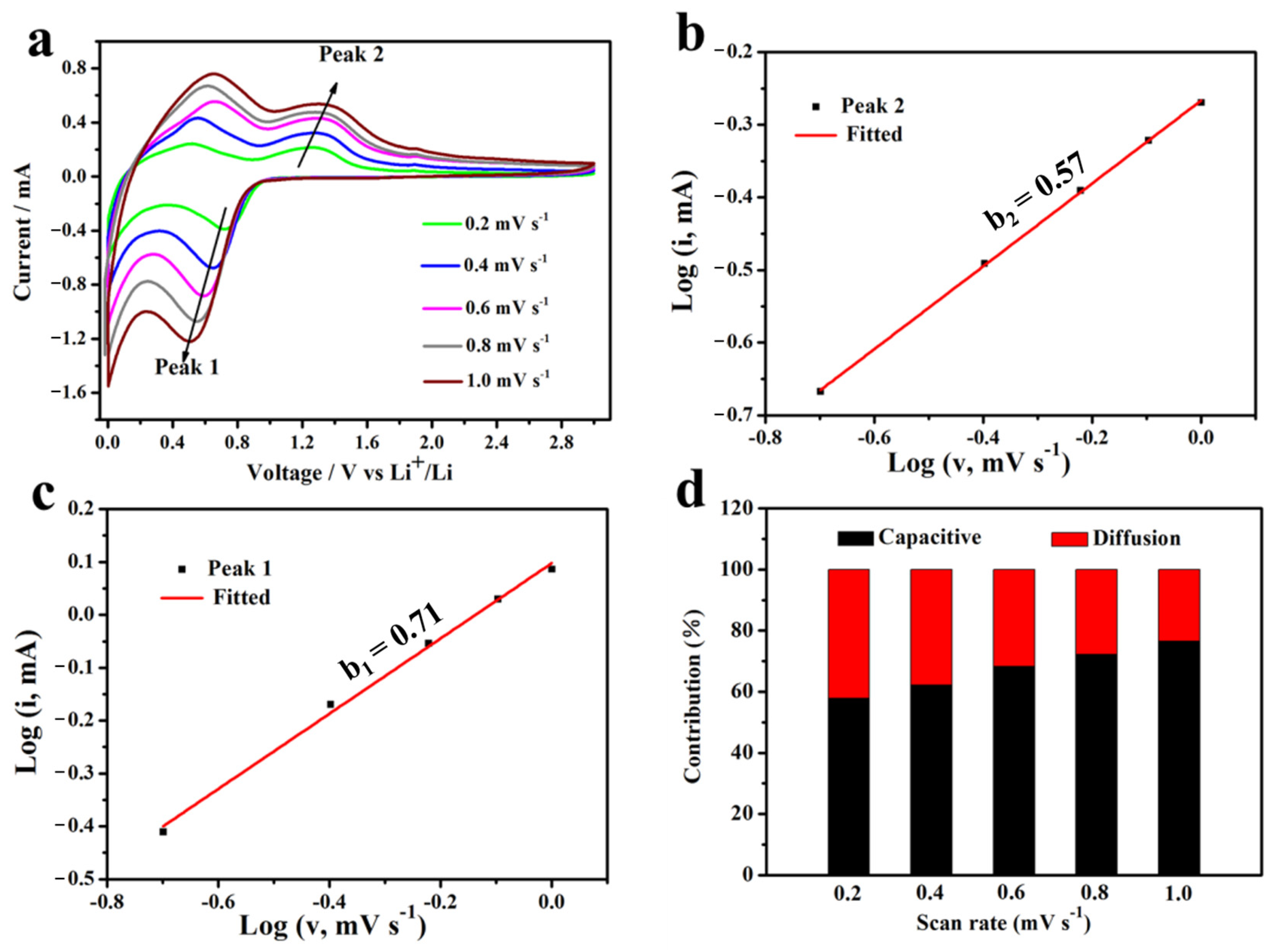
3.2. Electrochemical Performances as Anode Material for LIBs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Lev artovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Wang, H.; Zou, W.T.; Liu, C.; Sun, Y.; Xu, Y.; Sun, W.W.; Wang, Y. β-Ketoenamine-Linked Covalent Organic Framework with Co Intercalation: Improved Lithium-Storage Properties and Mechanism for High-Performance Lithium-Organic Batteries. Batter. Supercaps 2023, 6, e202200434. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Xu, D.Y.; Liang, M.X.; Qi, S.; Sun, W.W.; Lv, L.P.; Du, F.H.; Wang, B.F.; Chen, S.Q.; Wang, Y.; Yu, Y. The Progress and Prospect of Tunable Organic Molecules for Organic Lithium-Ion Batteries. ACS Nano 2021, 15, 47–80. [Google Scholar] [CrossRef]
- Hao, J.; Bai, J.; Wang, J.; Xu, L.; Guo, J.L.; Chi, C.X.; Li, H.H. Enhancing the Electrochemical Performance of a Micron-sized Ge Anode through In situ Surface Composite Flower-like Zn2GeO4 for Li-ion Batteries. Sustain. Energy Fuels 2022, 6, 4520–4527. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon Based Lithium-ion Battery Anodes: A Chronicle Perspective Review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Wang, X.; Fan, L.; Gong, D.; Zhu, J.; Zhang, Q.; Lu, B. Core-Shell Ge@Graphene@TiO2 Nanofibers as a High Cpacity and Cycle-Stable Anode for Lithium and Sodium Ion Battery. Adv. Funct. Mater. 2016, 26, 1104–1111. [Google Scholar] [CrossRef]
- Qin, J.; Liu, D.; Zhao, N.; Shi, C.; Liu, E.Z.; He, F.; Ma, L.; Li, Q.; Li, J.; He, C. Fabrication of Sn-core/CNT-shell nanocable Anchored Interconnected Carbon Networks as Anode Material for Lithium-ion Batteries. Mater. Lett. 2018, 212, 94–97. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Z.J.; Li, X.; Xie, W.; Wang, Q.; Liu, Q.; Fu, Y.; He, D. Group IVA Element (Si, Ge, Sn)-Based Alloy ing/Dealloying Anodes as Negative Electrodes for Full-Cell Lithium-Ion Batteries. Small 2017, 13, 1702000. [Google Scholar] [CrossRef]
- Sharma, P.; Sundaram, M.M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn Metal Atom Doping on the Surface Plane of One-Dimensional NiMoO4 Nanorods with Improved Redox Chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Zhang, W.B.; Pang, H.C.; Sun, W.W.; Lv, L.P.; Wang, Y. Metal-organic Frameworks Derived Germanium Oxide Nanosheets for Large Reversible Li-ion Storage. Electrochem. Commun. 2017, 84, 80–85. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, T.; Zheng, Y.; Liu, J.; Feng, C.; Shen, Y.; Huang, Y.; Guo, Z. Hierarchical Structural Evolution of Zn2GeO4 in Binary Solvent and Its Effect on Li-ion Storage Performance. ACS Appl. Mater. Interfaces 2017, 9, 9778–9784. [Google Scholar] [CrossRef] [PubMed]
- Han, L.J.; Yang, H.H.; Tang, J.; Wei, Q.H.; Wei, M.D. Conductive-free Zn2GeO4@multi-walled Carbon Nanotubes for High-performance Lithium-ion Storage. J. Alloys Compd. 2022, 918, 165720. [Google Scholar] [CrossRef]
- Wei, W.; Xu, J.L.; Xu, M.T.; Zhang, S.Y.; Guo, L. Recent progress on Ge Oxide Anode Materials for Lithium-ion Batteries. Sci. China Chem. 2018, 61, 515–525. [Google Scholar] [CrossRef]
- Breternitz, J.; Fritsch, D.; Franz, A.; Schorr, S. A thorough Investigation of the Crystal Structure of Willemite-type Zn2GeO4. Z. Anorg. Allg. Chem. 2021, 647, 2195–2200. [Google Scholar] [CrossRef]
- Bai, J.; Wang, J.; Zhao, R.R.; Hao, J.; Chi, C.X. In situ Growth Zn2GeO4 Nanorods Network on 3D Conductive Foam as Free Standing Electrode for Sodium-ion Batteries. Mater. Lett. 2022, 328, 133124. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Li, M.; Li, W.; Wei, W.H.; Song, D. Smart Hybrids of Zn2GeO4 Nanoparticles and Ultrathin g-C3N4 Layers: Synergistic Lithium Storage and Excellent Electrochemical Performance. Adv. Funct. Mater. 2015, 25, 6858–6866. [Google Scholar] [CrossRef]
- Chen, W.; Maloney, S.; Wang, W. Carbon-coated Zn2GeO4 on Ni Foam as Lithium-ion Battery Anodes with Exceptional Long Cycling Stability. Electrochim. Acta 2015, 176, 96–102. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Zou, Y.; Peng, X.; Zhang, M.; Gao, S.; Xu, J.; Tang, K.; Zhao, D. Synthesis of Ag Decoration on Carbon Coated Zn2GeO4 Nanorods and its Enhanced Properties as Anode Materials for Lithium-ion Batteries. Mater. Lett. 2016, 166, 243–246. [Google Scholar] [CrossRef]
- Sun, W.W.; Tang, X.X.; Yang, Q.S.; Xu, Y.; Wu, F.; Guo, S.Y.; Zhang, Y.F.; Wu, M.H.; Wang, Y. Coordination-Induced Interlinked Covalent- and Metal-Organic-Framework Hybrids for Enhanced Lithium Storage. Adv. Mater. 2019, 31, 1903176. [Google Scholar] [CrossRef]
- Li, H.; Liang, M.; Sun, W.; Wang, Y. Bimetal-Organic Framework: One-Step Homogenous Formation and its Derived Mesoporous Ternary Metal Oxide Nanorod for High-Capacity, High-Rate, and Long-Cycle-Life Lithium Storage. Adv. Funct. Mater. 2016, 26, 1098–1103. [Google Scholar] [CrossRef]
- Nagappan, S.; Duraivel, M.; Elayappan, V.; Muthuchamy, N.; Mohan, B.; Dhakshinamoorthy, A.; Prabakar, K.; Lee, J.M.; Park, K.H. Metal-Organic Frameworks-Based Cathode Materials for Energy Storage Applications: A Review. Energy Technol. 2023, 11, 2201200. [Google Scholar] [CrossRef]
- Mechili, M.; Vaitsis, C.; Argirusis, N.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes. Energies 2022, 15, 5460. [Google Scholar] [CrossRef]
- Shen, M.H.; Ma, H.L. Metal-organic frameworks (MOFs) and their derivative as electrode materials for lithium-ion batteries. Coord. Chem. Rev. 2022, 470, 214715. [Google Scholar] [CrossRef]
- Xu, G.Y.; Zhu, C.Y.; Gao, G. Recent Progress of Advanced Conductive Metal-Organic Frameworks: Precise Synthesis, Electrochemical Energy Storage Applications, and Future Challenges. Small 2022, 18, 2203140. [Google Scholar] [CrossRef]
- Pang, H.C.; Guan, B.Q.; Sun, W.W.; Wang, Y. Metal-Organic-Frameworks Derivation of Mesoporous NiO Nanorod for High-Performance Lithium-Ion Batteries. Electrochim. Acta. 2016, 213, 351–357. [Google Scholar] [CrossRef]
- Cui, B.B.; Fu, G.D. Process of Metal–organic Framework (MOF)/covalent–organic Framework (COF) Hybrids-Based Derivatives and their Applications on Energy Transfer and Storage. Nanoscale 2022, 14, 1679–1699. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.L.; Wang, H.Y.; Ren, X.H.; Zhan, F.Y.; He, Q.Q.; Wang, H.Y.; Chen, L.Y. Emerging Two-dimensional Nanostructured Manganese-based Materials for Electrochemical Energy Storage: Recent Advances, Mechanisms, Challenges, and Prospects. J. Mater. Chem. A 2022, 10, 21197–21250. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, A.Y.; Byeon, Y.W.; Ahn, J.P.; Byun, D.; Lee, J.K. Porous Zn2GeO4 Nanowires with Uniform Carbon-Buffer Layer for Lithium-ion Battery Anodes with Long Cycle Life. Electrochim. Acta. 2016, 195, 43–50. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Sun, Y.; Luo, W.; Xia, F.; Qie, L.; Jiang, Y.; Huang, Y. Microwave-Induced In situ Synthesis of Zn2GeO4/N-doped Graphene Nanocomposites and Their Lithium-storage Properties. Chemistry 2013, 19, 6027–6033. [Google Scholar] [CrossRef]
- Du, G.; Feng, P.; Cheng, X.; Li, J.; Luo, X. Immobilizing of ZIF-8 Derived ZnO with Controllable Morphologies on Zeolite A for Efficient Photocatalysis. J. Solid State Chem. 2017, 255, 215–218. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 Nano- and Microcrystal Formation and Reactivity through Zinc Salt Variations. CrystEngComm 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Chen, X.; Ran, X.Q.; Lu, Y.; Feng, C.Q. Synthesis and Electrochemical Properties of Zinc Germanate Nanowires as Novel Anode Material for Lithium-ion Battery. Ionics 2021, 27, 4177–4184. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, Z.Y.; Shen, X.P.; Chen, H.Y.; Qi, Y.; Yuan, A.H.; Qiu, J.X.; Li, B.L. Size-controllable Synthesis of Zn2GeO4 Hollow Rods Supported on Reduced Graphene Oxide as High-capacity Anode for Lithium-ion Batteries. J. Colloid Interface Sci. 2021, 589, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.; Shao, Z.; Wang, H. Morphology-dependent Performance of Zn2GeO4 as AH high-performance Anode Material for Rechargeable Lithium Ion Batteries. J. Mater. Chem. A 2015, 3, 15274–15279. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Qie, L.; Jiang, Y.; Xiong, X.; Qiao, Y.; Huang, Y. Facile Synthesis of Sandwiched Zn2GeO4-graphene Oxide Nanocomposite as A Stable and High-capacity Anode for Lithium-ion Batteries. Nanoscale 2014, 6, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, R.M.; Lou, Z.; Huang, T.T.; Jiang, K.; Chen, D.; Shen, G.Z. Electrospraying Preparation of Metal Germanate Nanospheres for High-performance Lithium-ion Batteries and Room-temperature Gas Sensors. Nanoscale 2019, 11, 12116–12123. [Google Scholar] [CrossRef]
- Li, H.H.; Wu, X.L.; Zhang, L.L.; Fan, C.Y.; Wang, H.F.; Li, X.Y.; Sun, H.Z.; Zhang, J.P.; Yan, Q. Carbon-Free Porous Zn2GeO4 Nanofibers as Advanced Anode Materials for High-Performance Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 31722–31728. [Google Scholar] [CrossRef]
- Chen, W.; Lu, L.; Maloney, S.; Yang, Y.; Wang, W. Coaxial Zn2GeO4@carbon Nanowires Directly Grown on Cu Foils as High-performance Anodes for Lithium ion Batteries. Phys. Chem. Chem. Phys. 2015, 17, 5109–5114. [Google Scholar] [CrossRef]
- Yi, R.; Feng, J.; Lv, D.; Gordin, M.L.; Chen, S.; Choi, D.; Wang, D. Amorphous Zn2GeO4 Nanoparticles as Anodes with High Reversible Capacity and Long Cycling Life for Li-ion Batteries. Nano Energy 2013, 2, 498–504. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, X.; Wang, L.; Xu, M.; Zhao, D.; Rui, Y.; Xu, J.; Tang, K. Fabrication of Zn2GeO4 Nanorods@TiO2 as Anodes for Lithium-ion Batteries with Enhanced Cycling Stability. Mater. Lett. 2016, 185, 307–310. [Google Scholar] [CrossRef]
- Jiang, M.; Zhou, T.; Liu, W.; Feng, C.; Liu, J.; Guo, Z. Graphene Aerogel Supported Crystalline ZnO@amorphous Zn2GeO4 Core-shell Hierarchical Structure for Lithium Storage. RSC Adv. 2017, 7, 17769–17772. [Google Scholar] [CrossRef]
- Li, H.H.; Zhang, L.L.; Fan, C.Y.; Wu, X.L.; Wang, H.F.; Li, X.Y.; Wang, K.; Sun, H.Z.; Zhang, J.P. Flexible Paper Electrodes Constructed from Zn2GeO4 Nanofibers Anchored with Amorphous Carbon for Advanced Lithium Ion Batteries. J. Mater. Chem. A 2016, 4, 2055–2059. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, T.; Yue, J.; Sheng, L.; Jiang, Z.; Yang, D.; Yuan, L.; Fan, Z. Ultra-small and Highly Crystallized ZnFe2O4 Nanoparticles within Double Graphene Networks for Super-long Life lLithium-ion Batteries. J. Mater. Chem. A 2017, 5, 11188–11196. [Google Scholar] [CrossRef]
- Feng, J.K.; Lai, M.O.; Lu, L. Zn2GeO4 Nanorods Synthesized by Low-temperature Hydrothermal Growth for High-Capacity Anode of Lithium Battery. Electrochem. Commun. 2011, 13, 287–289. [Google Scholar] [CrossRef]
- Li, Q.; Miao, X.; Wang, C.; Yin, L. Three-dimensional Mn-doped Zn2GeO4 Nanosheet Array Hierarchical Nanostructures Anchored on Porous Ni Foam as Binder-free and Carbon-free Lithium-ion Battery Anodes with Enhanced Electrochemical Performance. J. Mater. Chem. A 2015, 3, 21328–21336. [Google Scholar] [CrossRef]
- Wang, R.; Wu, S.; Lv, Y.; Lin, Z. Partially Crystalline Zn2GeO4 Nanorod/graphene Composites as Anode Materials for High Performance Lithium ion Batteries. Langmuir 2014, 30, 8215–8220. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, J.H.; Choi, Y.J.; Kang, Y.C. One-pot Aerosol Synthesis of Carbon Nanotube-Zn2GeO4 Composite Microspheres for Enhanced Lithium-ion Storage Properties. Electrochim. Acta. 2016, 190, 766–774. [Google Scholar] [CrossRef]
- Chen, X.D.; Zhang, H.; Ci, C.G.; Sun, W.W.; Wang, Y. Few-Layered Boronic Ester Based Covalent Organic Frameworks/Carbon Nanotube Composites for High-Performance K-Organic Batteries. ACS Nano 2019, 13, 3600–3607. [Google Scholar] [CrossRef]
- Lim, Y.R.; Jung, C.S.; Im, H.S.; Park, K.; Park, J.; Cho, W.I.; Cha, E.H. Zn2GeO4 and Zn2SnO4 Nanowires for High-Capacity Lithium- and Sodium-ion Batteries. J. Mater. Chem. A 2016, 4, 10691–10699. [Google Scholar] [CrossRef]
- Cui, X.; Chen, J.X.; Sun, Z.F.; Wang, L.; Peng, Q.Q.; Xiao, B.S.; Zhao, L.G.; Zheng, H.; Wang, Y.; Wang, J.B.; et al. A General Route for Encapsulating Monodispersed Transition Metal Phosphides into Carbon Multi-Chambers toward High-Efficient Lithium-Ion Storage with Underlying Mechanism Exploration. Adv. Funct. Mater. 2023, 33, 2212100. [Google Scholar] [CrossRef]

| Composite | Morphology | IRC | RRC/CN | CD | V | References |
|---|---|---|---|---|---|---|
| Zn2GeO4 Ge/Zn2GeO4NFs | rice flower | 730 1621 | 661/500 816/200 | 100 200 | 0.005–3 0.01–3 | This work [5] |
| Zn2GeO4 | nanoflower | 1143 | 1034/160 | 500 | 0.01–2.8 | [12] |
| Zn2GeO4@MWCNTs | nanorods@voids of MWCNTs | 1209 | 1397/300 | 200 | 0.01–3 | [13] |
| Zn2GeO4 | nanoparticle | ~1130 | 1175/60 | 200 | 0.01–3 | [17] |
| Zn2GeO4 | nanowire | 600 | 485/900 | 600 | 0–3 | [29] |
| Zn2GeO4 | nanowires | 2200 | 1200/150 | 100 | 0.01–3 | [33] |
| Zn2GeO4/GO | nanorod@sheets | 594 | 1150/100 | 200 | 0.001–3 | [36] |
| Zn2GeO4 | nanospheres | 1520 | 488/100 | 200 | 0.01–3 | [37] |
| Zn2GeO4 | nanofiber | 1405 | 1084/50 | 200 | 0.01–3 | [38] |
| Zn2GeO4@C/Cu | ZGO@C nanowires | 1162 | ~790/100 | 200 | 0.01–3 | [39] |
| Zn2GeO4/TiO2 | rod-like microstructure | 346 | 330/150 | 200 | 0.01–3 | [41] |
| Zn2GeO4/RGO | hollow rods@sheets | 1736 | 1005/100 | 500 | 0.01–3 | [45] |
| CNT-Zn2GeO4 | 3D CNT@microspheres | 736 | 762/300 | 150 | 0.001–3 | [48] |
| Zn2GeO4 | nanowire | 1135 | 1220/100 | 100 | 0.01–3 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Chen, S.; Aslam, J.; Li, J.; Lv, L.-P.; Sun, W.; Cao, W.; Wang, Y. Microwave-Assisted Metal-Organic Frameworks Derived Synthesis of Zn2GeO4 Nanowire Bundles for Lithium-Ion Batteries. Nanomaterials 2023, 13, 1432. https://doi.org/10.3390/nano13081432
Guo C, Chen S, Aslam J, Li J, Lv L-P, Sun W, Cao W, Wang Y. Microwave-Assisted Metal-Organic Frameworks Derived Synthesis of Zn2GeO4 Nanowire Bundles for Lithium-Ion Batteries. Nanomaterials. 2023; 13(8):1432. https://doi.org/10.3390/nano13081432
Chicago/Turabian StyleGuo, Chaofei, Shuangqiang Chen, Junaid Aslam, Jiayi Li, Li-Ping Lv, Weiwei Sun, Weimin Cao, and Yong Wang. 2023. "Microwave-Assisted Metal-Organic Frameworks Derived Synthesis of Zn2GeO4 Nanowire Bundles for Lithium-Ion Batteries" Nanomaterials 13, no. 8: 1432. https://doi.org/10.3390/nano13081432





