Morphology Effects on Electro- and Photo-Catalytic Properties of Zinc Oxide Nanostructures
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Synthesis of ZnO Powders
2.3. Physical-Chemical Characterizations
2.4. Electrochemical Analysis
2.4.1. Electrode Preparation
2.4.2. CO2 Reduction Reaction Measurements
2.5. Photocatalytic Analysis
3. Results and Discussion
3.1. Morphological and Structural Characterizations of ZnO Nanostructures
3.2. Photocatalytic Activity of ZnO Nanostructures for Dye Degradation
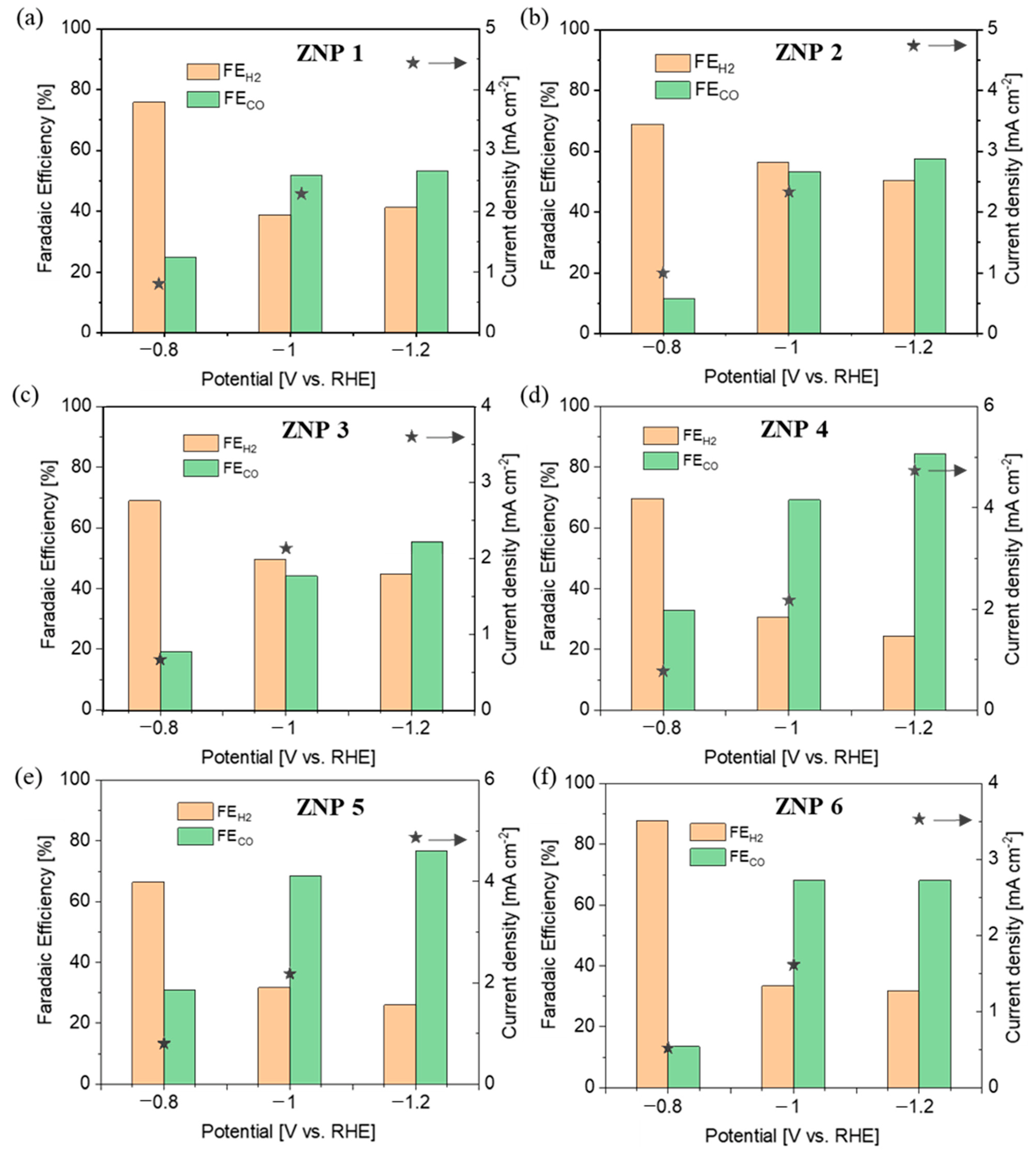
3.3. Electrocatalytic Activity of ZnO Nanostructures for CO2RR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rane, A.; Joshi, S.J. Biodecolorization and biodegradation of dyes: A review. Open Biotechnol. J. 2021, 15, 97–108. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Oyetade, J.A.; Machunda, R.L.; Hilonga, A. Photocatalytic degradation of azo dyes in textile wastewater by Polyaniline composite catalyst-a review. Sci. Afr. 2022, 17, 1305. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Javaid, R.; Qazi, U.Y.; Ikhlaq, A.; Zahid, M.; Alazmi, A. Subcritical and supercritical water oxidation for dye decomposition. J. Environ. Manag. 2021, 290, 112605. [Google Scholar] [CrossRef]
- Vaiano, V.; De Marco, I. Removal of Azo Dyes from Wastewater through Heterogeneous Photocatalysis and Supercritical Water Oxidation. Separations 2023, 10, 230. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Ashraf, A.M. Recent progress in semiconductor/graphene photocatalysts: Synthesis, photocatalytic applications, and challenges. RSC Adv. 2023, 13, 421–439. [Google Scholar] [CrossRef]
- Kulis-Kapuscinska, A.; Kwoka, M.; Borysiewicz, M.A.; Wojciechowski, T.; Licciardello, N.; Sgarzi, M.; Cuniberti, G. Photocatalytic degradation of methylene blue at nanostructured ZnO thin films. Nanotechnology 2023, 34, 155702. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, C.; Wu, Z.; Liu, Y.; Sun, S. A review on the progress of the photocatalytic removal of refractory pollutants from water by BiOBr-based nanocomposites. Chemosphere 2022, 308, 136107. [Google Scholar] [CrossRef]
- Almeida, N.A.; Martins, P.M.; Teixeira, S. TiO2/graphene oxide immobilized in P(VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- Silva, A.R.; Martins, P.; Lanceros-mendez, S.; Teixeira, S.; Carabineiro, S.A.C.; Kuehn, K.; Cuniberti, G.; Alves, M.M.; Pereira, L.R. Ciprofloxacin wastewater treated by UVA photocatalysis: Contribution of irradiated TiO2 and ZnO nanoparticles on the final toxicity as assessed by Vibrio fischeri. RSC Adv. 2016, 6, 95494–95503. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gabdullin, M.T.; Zhumagulov, S.K.; Ismailova, G.A.; Gritsenko, L.V.; Kedruk, Y.Y.; Mirzaeian, M. Stabilization of the surface of ZnO films and elimination of the aging effect. Materials 2021, 14, 6535. [Google Scholar] [CrossRef]
- Ulyankina, A.; Molodtsova, T.; Gorshenkov, M.; Leontyev, I.; Zhigunov, D.; Konstantinova, E.; Lastovina, T.; Tolasz, J.; Henych, J.; Licciardello, N.; et al. Photocatalytic degradation of ciprofloxacin in water at nano-ZnO prepared by pulse alternating current electrochemical synthesis. J. Water Process Eng. 2020, 40, 101809. [Google Scholar] [CrossRef]
- Algarni, T.S.; Naaser, A.A.Y.; Kahtani, A.A.; Aouissi, A. Photocatalytic degradation of some dyes under solar light irradiation using ZnO nanoparticles synthesized from Rosmarinus officinalis extract. Green Chem. Lett. Rev. 2022, 15, 460–473. [Google Scholar] [CrossRef]
- Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc Oxide-Based Photocatalysts: Recent Developments in Their Use for the Treatment of Dye-Polluted Wastewater. Water 2022, 14, 3899. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Wang, Y.; Guo, Y.; Zhang, S.; Song, H.; Li, X.; Gao, Q.; Shang, W.; Hu, S.; et al. MXene Ti3C2 decorated g-C3N4/ZnO photocatalysts with improved photocatalytic performance for CO2 reduction. Nano Mater. Sci. 2023, 5, 237–245. [Google Scholar] [CrossRef]
- Paltusheva, Z.U.; Alpysbaiuly, N.; Kedruk, Y.Y.; Zhaidary, A.D.; Aitzhanov, M.B.; Gritsenko, L.V.; Abdullin, K.A. Photocatalytic activity of zinc oxide–graphene oxide composites. Bulletin of the Karaganda University. Phys. Ser. 2022, 2, 102–110. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Hartomy, O.A.; Alotaibi, M.F.; Liu, J. Ionized cocatalyst to promote CO2 photoreduction activity over core–triple-shell ZnO hollow spheres. Rare Met. 2022, 41, 1077–1079. [Google Scholar] [CrossRef]
- Abdullin, K.A.; Gabdullin, M.T.; Gritsenko, L.V.; Ismailov, D.V.; Kalkozova, Z.K.; Kumekov, S.E.; Mukash, Z.O.; Sazonov, A.Y.; Terukov, E.I. Electrical, Optical, and Photoluminescence Properties of ZnO Films Subjected to Thermal Annealing and Treatment in Hydrogen Plasma. J. Semicond. 2016, 50, 1010–1014. [Google Scholar] [CrossRef]
- Hamid, N.; Suhaimi, S.; Othman, M.Z.; Ismail, W.Z.W. A Review on Thermal Evaporation Method to Synthesis Zinc Oxide as Photocatalytic Material. Nano Hybrids Compos. 2021, 31, 55–63. [Google Scholar] [CrossRef]
- Guaraldo, T.T.; Wenk, J.; Mattia, D. Photocatalytic ZnO Foams for Micropollutant Degradation. Adv. Sustain. Syst. 2021, 5, 2000208. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1993, 39, 1833–1839. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Jiao, F. Nanostructured Metallic Electrocatalysts for Carbon Dioxide Reduction. ChemCatChem 2015, 7, 38–47. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Rivera, S.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Mechanistic Insights into the Electrochemical Reduction of CO2 to CO on Nanostructured Ag Surfaces. ACS Catal. 2015, 5, 4293–4299. [Google Scholar] [CrossRef]
- Nursanto, E.B.; Jeon, H.S.; Kim, C.; Jee, M.S.; Koh, J.H.; Hwang, Y.J.; Min, B.K. Gold catalyst reactivity for CO2 electro-reduction: From nano particle to layer. Catal. Today 2016, 260, 107–111. [Google Scholar] [CrossRef]
- Lettieri, S.; Zeng, J.; Farkhondehfal, M.A.; Savino, U.; Fontana, M.; Pirri, C.F.; Sacco, A. Correlation between impedance spectroscopy and bubble-induced mass transport in the electrochemical reduction of carbon dioxide. J. Energy Chem. 2022, 67, 500–507. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Di Martino, G.; Sacco, A.; Castellino, M.; Re Fiorentin, M.; Risplendi, F.; Farkhondehfal, M.A.; Hernández, S.; Cicero, G.; et al. Microwave-Assisted Synthesis of Copper-Based Electrocatalysts for Converting Carbon Dioxide to Tunable Syngas. ChemElectroChem 2020, 7, 229. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, K.; Fan, S.; Kanan, M.W. Grain-Boundary-Dependent CO2 Electroreduction Activity. J. Am. Chem. Soc. 2015, 137, 4606–4609. [Google Scholar] [CrossRef]
- Li, C.W.; Ciston, J.; Kanan, M.W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 2014, 508, 504–507. [Google Scholar] [CrossRef]
- Monti, N.B.D.; Fontana, M.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Pirri, C.F.; Zeng, J. Facile Fabrication of Ag Electrodes for CO2-to-CO Conversion with Near-Unity Selectivity and High Mass Activity. ACS Appl. Energy Mater. 2022, 5, 14779–14788. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of Nitrogen Defects for Efficient Reduction of CO2 via Two-Electron Pathway on Three-Dimensional Graphene Foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Won, D.H.; Shin, H.; Koh, J.; Chung, J.; Lee, H.S.; Kim, H.; Woo, S.I. Highly Efficient, Selective, and Stable CO2 Electroreduction on a Hexagonal Zn Catalyst. Angew. Chem. Int. Ed. 2016, 55, 9297–9300. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, M.A.O.; Zeng, J.; Jagdale, P.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Pirri, C.F. Biochar, Zinc Oxide Composites as Effective Catalysts for Electrochemical CO2 Reduction. ACS Sustain. Chem. Eng. 2021, 9, 5445–5453. [Google Scholar] [CrossRef]
- Urbain, F.; Tang, P.; Carretero, N.M.; Andreu, T.; Gerling, L.G.; Voz, C.; Arbiol, J.; Morante, J.R. A Prototype Reactor for Highly Selective Solar-driven CO2 Reduction to Synthesis Gas Using Nanosized Earth-abundant Catalysts and Silicon Photovoltaics. Energy Environ. Sci. 2017, 10, 2256–2266. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn Dendrites with Enhanced CO Selectivity for Electrocatalytic CO2 Reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Al-Kordy, H.M.H.; Sabry, S.A.; Mabrouk, M.E.M. Statistical optimization of experimental parameters for extracellular synthesis of zinc oxide nanoparticles by a novel haloalaliphilic Alkalibacillus sp.W7. Sci. Rep. 2021, 11, 10924. [Google Scholar] [CrossRef] [PubMed]
- Maraeva, E.V.; Permiakov, N.V.; Kedruk, Y.Y.; Gritsenko, L.V.; Abdullin, K.A. Creating a virtual device for processing the results of sorption measurements in the study of zinc oxide nanorods. Chim. Techno. Acta 2020, 7, 154–158. [Google Scholar] [CrossRef]
- Zakria, M.; Huynh, T.T.; Ling, F.C.C.; Su, S.C.; Phillips, M.R.; Ton-That, C. Highly Luminescent MgZnO/ZnO Multiple Quantum Wells for Photonics Devices. ACS Appl. Nano Mater. 2019, 2, 2574–2579. [Google Scholar] [CrossRef]
- Kedruk, Y.Y.; Baigarinova, G.A.; Gritsenko, L.V.; Cicero, G.; Abdullin, K.A. Facile Low-Cost Synthesis of Highly Photocatalitycally Active Zinc Oxide Powders. Front. Mater. 2022, 9, 869493. [Google Scholar] [CrossRef]
- Zeng, J.; Fontana, M.; Sacco, A.; Sassone, D.; Pirri, C.F. A study of the effect of electrode composition on the electrochemical reduction of CO2. Catal. Today 2022, 397–399, 463–474. [Google Scholar] [CrossRef]
- Zeng, J.; Rino, T.; Bejtka, K.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Chiodoni, A.; Drago, F.; Pirri, C.F. Coupled Copper–Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide. ChemSusChem 2020, 13, 4128–4139. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Re Fiorentin, M.; Fontana, M.; Castellino, M.; Risplendi, F.; Sacco, A.; Cicero, G.; Farkhondehfal, M.A.; Drago, F.; Pirri, C.F. Novel Insights into Sb-Cu Catalysts for Electrochemical Reduction of CO2. Appl. Catal. B Environ. 2022, 306, 121089. [Google Scholar] [CrossRef]
- Zeng, J.; Castellino, M.; Bejtka, K.; Sacco, A.; Di Martino, G.; Farkhondehfal, M.; Chiodoni, A.; Hérnandez, S.; Pirri, C. Facile synthesis of cubic cuprous oxide for electrochemical reduction of carbon dioxide. J. Mater. Sci. 2021, 56, 1255–1271. [Google Scholar] [CrossRef]
- Rusdi, R.; Rahman, A.A.; Mohamed, N.S.; Kamarudin, N.; Kamarulzaman, N. Preparation and band gap energies of ZnO nanotubes, nanorods and spherical nanostructures. Powder Technol. 2011, 210, 8–22. [Google Scholar] [CrossRef]
- Di Mauro, A.; Fragalà, M.E.; Privitera, V.; Impellizzeri, G. ZnO for application in photocatalysis: From thin films to nanostructures. Mater. Sci. Semicond. Process. 2017, 69, 44–51. [Google Scholar] [CrossRef]
- Godin, R.; Hisatomi, T.; Domen, K.; Durrant, J.R. Understanding the visible-light photocatalytic activity of GaN:ZnO solid solution: The role of Rh2−yCryO3 cocatalyst and charge carrier lifetimes over tens of seconds. Chem. Sci. 2018, 9, 7546–7555. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Wang, X.; Yang, C.; Li, S.; Wang, J.; Yang, W.; Dong, F.; Hu, X.; Liu, D.; Kong, Y. Enhanced photocatalytic NO removal with the superior selectivity for NO2−/NO3− species of Bi12GeO20-based composites via a ball-milling treatment: Synergetic effect of surface oxygen vacancies and n-p heterojunctions. Compos. B Eng. 2022, 231, 109600. [Google Scholar] [CrossRef]
- Chang, F.; Zhao, S.; Lei, Y.; Wang, X.; Dong, F.; Zhu, G.; Kong, Y. Jointly augmented photocatalytic NO removal by S-scheme Bi12SiO20/Ag2MoO4 heterojunctions with surface oxygen vacancies. J. Colloid Interface Sci. 2023, 649, 713–723. [Google Scholar] [CrossRef]
- Chang, F.; Lei, Y.; Li, J.; Li, S.; Liu, D.; Kong, Y. Externally modified Bi12SiO20 with BiOI: N-p heterojunctions for effectually photocatalytic degradation of bisphenol A. Sep. Purif. Technol. 2023, 323, 124516. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Benitto, J.J.; Vijaya, J.J.; Bououdina, M. Recent Advances in ZnO-Based Nanostructures for the Photocatalytic Degradation of Hazardous, Non-Biodegradable Medicines. Crystals 2023, 13, 329. [Google Scholar] [CrossRef]
- Blazeka, D.; Car, J.; Klobucar, N.; Jurov, A.; Zavanik, J.; Jagodar, A.; Kovaevic, E.; Krstulovic, N. Photodegradation of Methylene Blue and Rhodamine B Using Laser-Synthesized ZnO Nanoparticles. Materials 2020, 13, 4357. [Google Scholar] [CrossRef]
- Aljaafari, A. Size Dependent Photocatalytic Activity of ZnO Nanosheets for Degradation of Methyl Red. Front. Mater. 2020, 7, 562693. [Google Scholar] [CrossRef]
- Dasa, A.; Nai, R.G. Effect of aspect ratio on photocatalytic performance of hexagonal ZnO nanorods. J. Alloys Compd. 2019, 817, 153277. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, Q.; Wu, A.; Jiang, M.; Liang, Z.; Jiang, B.; Fu, H. Cost-effective large-scale synthesis of ZnO photocatalyst with excellent performance for dye photodegradation. Chem. Commun. 2012, 48, 2858–2860. [Google Scholar] [CrossRef] [PubMed]
- Umar, K.; Mfarrej, M.F.B.; Rahman, Q.I.; Zuhaib, M.; Khan, A.; Zia, Q.; Banawas, S.; Nadeem, H.; Khan, M.F.; Ahmad, F. ZnO Nano-swirlings for Azo Dye AR183 photocatalytic degradation and antimycotic activity. Sci. Rep. 2022, 12, 14023. [Google Scholar] [CrossRef]
- Zeljkovic, S.; Balaban, M.; Gajic, D.; Jelić, D. Mechanochemically induced synthesis of N-ion doped ZnO: Solar photocatalytic degradation of methylene blue. Green Chem. Lett. Rev. 2022, 15, 869–880. [Google Scholar] [CrossRef]
- Blažeka, D.; Radičić, R.; Maletić, D.; Živkovi, S. Enhancement of Methylene Blue Photodegradation Rate Using ZnO Nanoparticles. Nanomaterials 2022, 12, 2677. [Google Scholar] [CrossRef]
- Etay, H.; Kumar, A.; Yadav, O.P. Kinetics of photocatalytic degradation of methylene blue dye in aqueous medium using ZnO nanoparticles under UV radiation. J. Anal. Pharm. Res. 2023, 12, 32–37. [Google Scholar] [CrossRef]
- Yusha’u, A.; Darma, M.S.; Isah, K.A. Sol-gel synthesis of ZnO nanoparticles for optmized photocatalytic degradation of Eriochrome Black T under UV irradiation. Alger. J. Eng. Technol. 2023, 8, 117–130. [Google Scholar] [CrossRef]
- Akram, R.; Fatima, A.; Almohaimeed, Z.M.; Farooq, Z.; Qadir, K.W.; Zafar, Q.; Hua, M. Photocatalytic Degradation of Methyl Green Dye Mediated by Pure and Mn-Doped Zinc Oxide Nanoparticles under Solar Light Irradiation. Adsorpt. Sci. Technol. 2023, 2023, 5069872. [Google Scholar] [CrossRef]
- Ren, D.; Ang, B.S.; Yeo, B.S. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived Cu x Zn catalysts. ACS Catal. 2016, 6, 8239–8247. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Li, C.; Gui, H. Sharp-tipped zinc nanowires as an efficient electrocatalyst for carbon dioxide reduction. Chem.–A Eur. J. 2018, 24, 15486–15490. [Google Scholar] [CrossRef]
- Lu, Y.; Han, B.; Tian, C.; Wu, J.; Geng, D.; Wang, D. Efficient electrocatalytic reduction of CO2 to CO on an electrodeposited Zn porous network. Electrochem. Commun. 2018, 97, 87–90. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Jee, M.S.; Won, D.H.; Jung, H.; Oh, H.S.; Min, B.K.; Hwang, Y.J. Selective CO2 reduction on zinc electrocatalyst: The effect of zinc oxidation state Induced by pretreatment environment. ACS Sustain. Chem. Eng. 2017, 5, 11377–11386. [Google Scholar] [CrossRef]
- Qin, B.; Li, Y.; Fu, H.; Wang, H.; Chen, S.; Liu, Z.; Peng, F. Electrochemical reduction of CO2 into tunable syngas production by regulating the crystal facets of earth-abundant Zn catalyst. ACS Appl. Mater. Interfaces 2018, 10, 20530–20539. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Shen, G.; Zhang, R.; Wu, D.; Zou, C.; Ling, T.; Liu, H.; Dong, C.; Du, X. Zn nanosheets coated with a ZnS subnanometer layer for effective and durable CO2 reduction. J. Mater. Chem. A 2019, 7, 1418–1423. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO production in electrocatalytic CO2 reduction on highly porous Zn catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef]
- Nguyen, D.L.; Lee, C.W.; Na, J.; Kim, M.; Tu, N.D.K.; Lee, S.Y.; Sa, Y.J.; Won, D.H.; Oh, H.; Kim, H.; et al. Mass transport control by surface graphene oxide for selective CO production from electrochemical CO2 reduction. ACS Catal. 2020, 10, 3222–3231. [Google Scholar] [CrossRef]
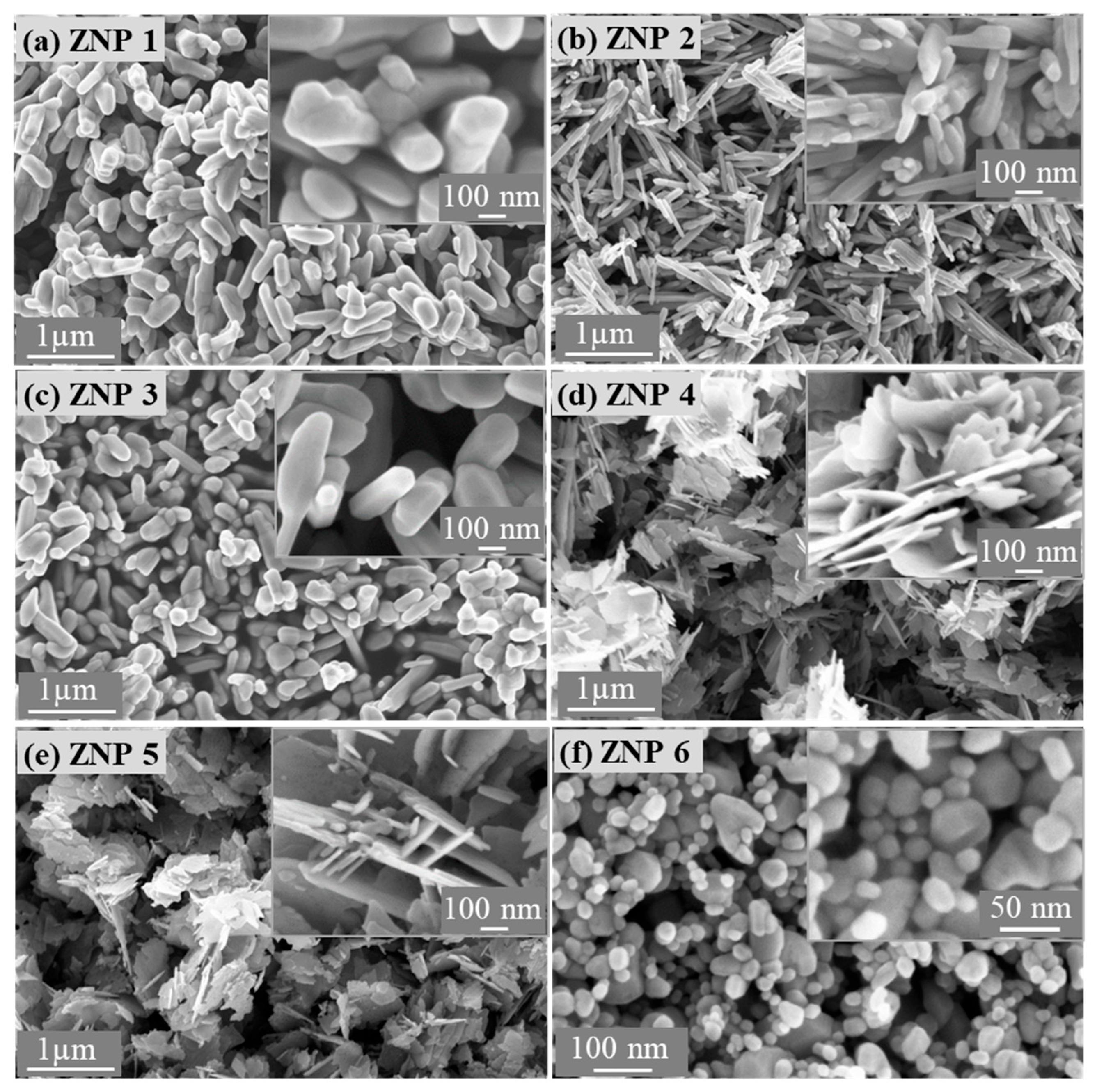

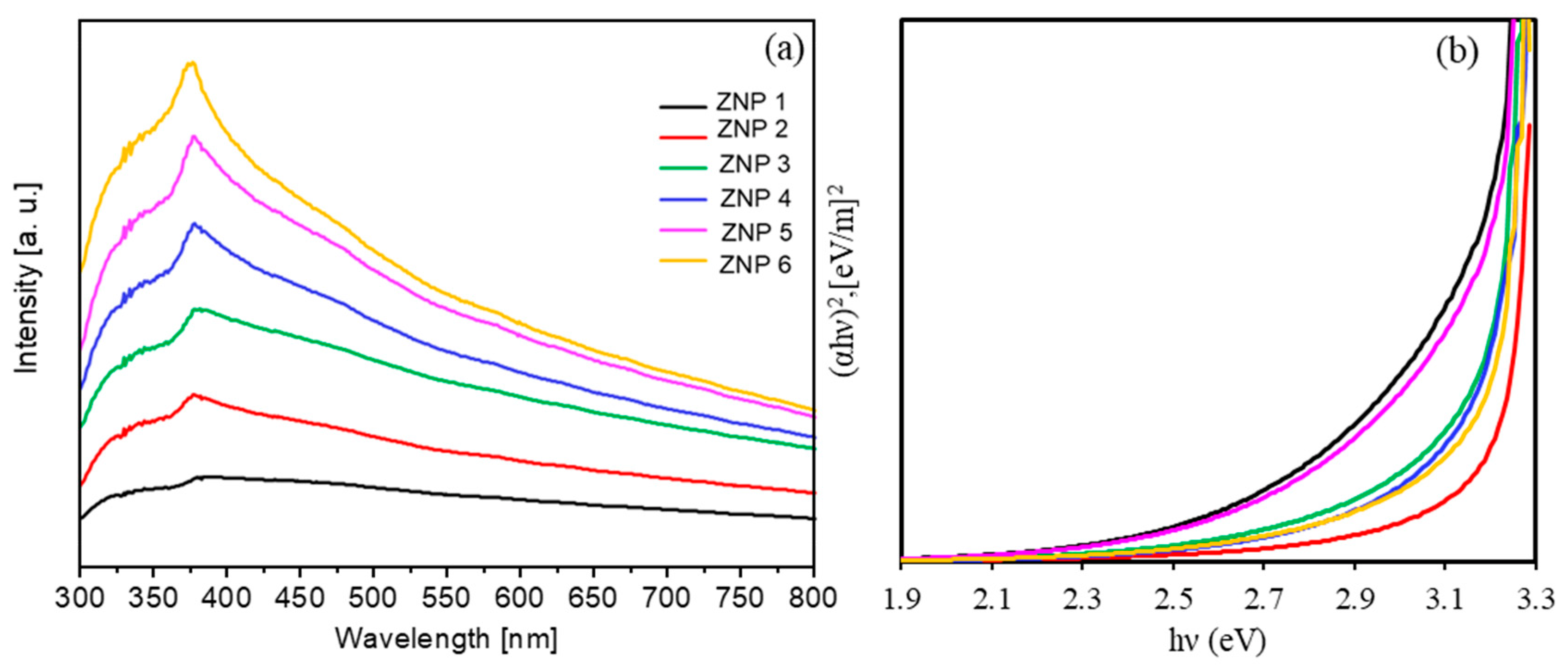
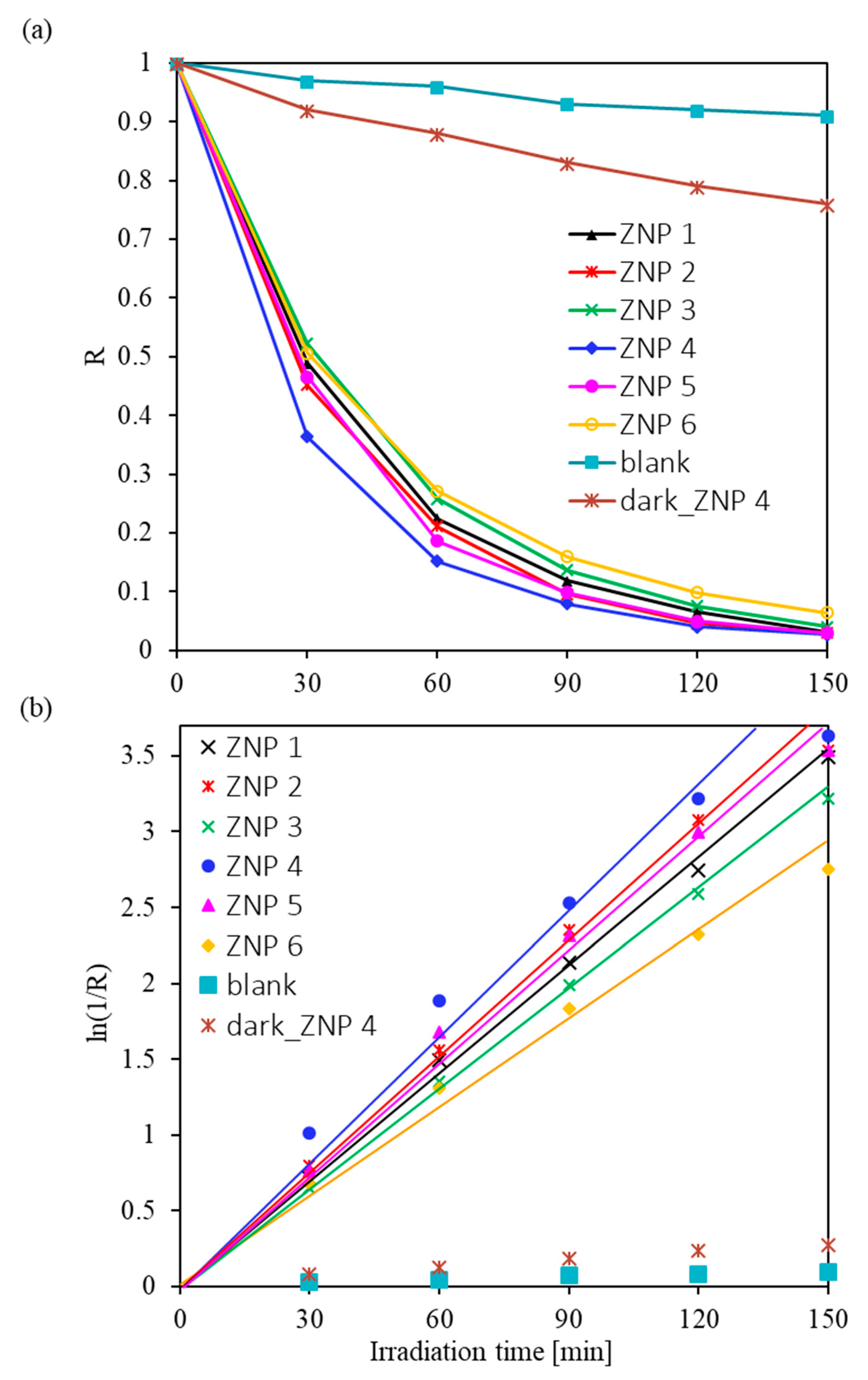
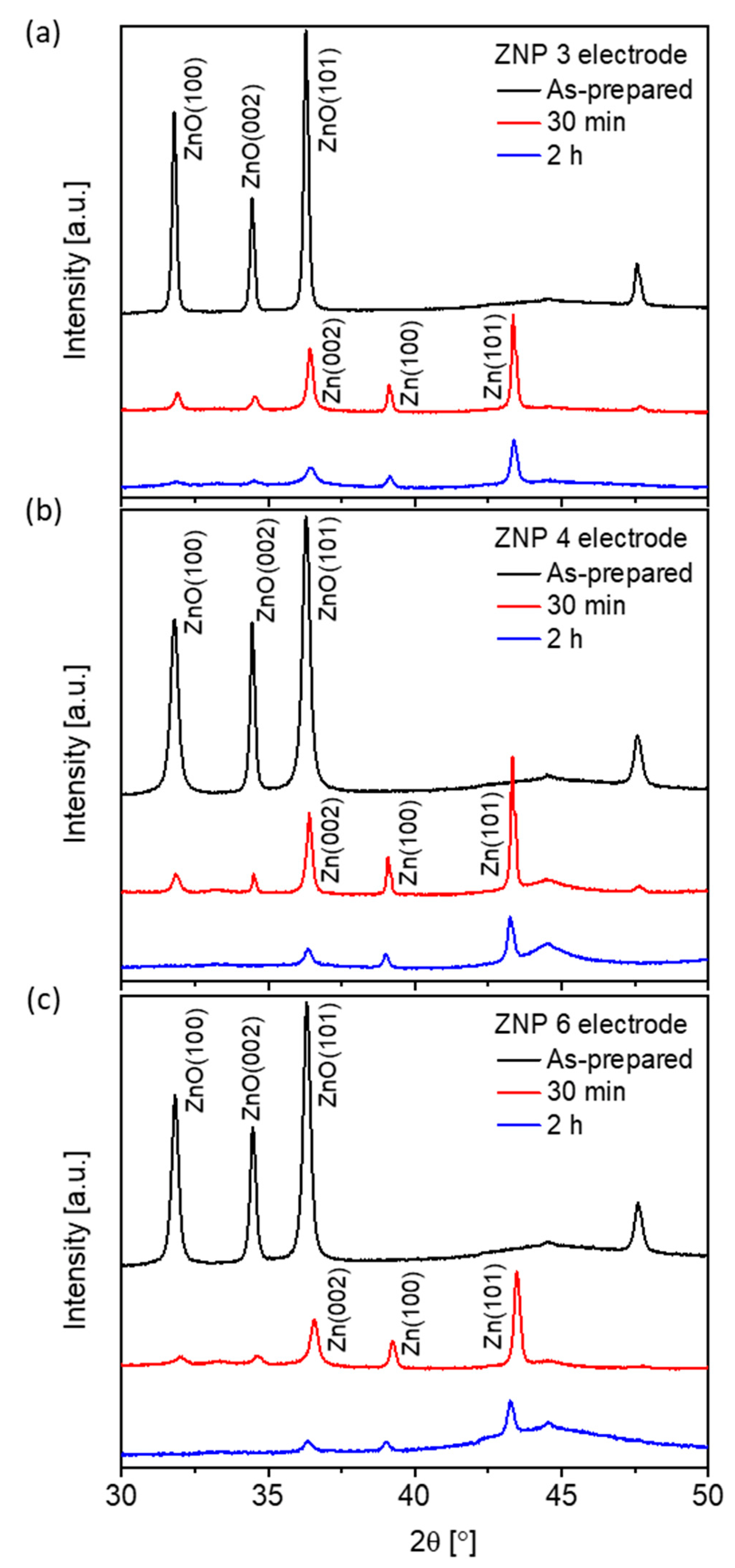

| Sample Name | Diameter * (nm) | Length (nm) | Ratio (Length/Diameter) | Cell Parameters (Å) | |
|---|---|---|---|---|---|
| a | c | ||||
| ZNP 1 | 184 | 559 | 3.0 | 3.2502 | 5.2071 |
| ZNP 2 | 60 | 550 | 9.2 | 3.2512 | 5.2088 |
| ZNP 3 | 163 | 430 | 2.6 | 3.2514 | 5.2089 |
| ZNP 4 | 23 | 879 | 38.2 | 3.2509 | 5.2079 |
| ZNP 5 | 29 | 700 | 24.1 | 3.2513 | 5.2082 |
| ZNP 6 | 40–60 | 40–60 | 1 (spherical shape) | 3.2524 | 5.2109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kedruk, Y.Y.; Contestabile, A.; Zeng, J.; Fontana, M.; Laurenti, M.; Gritsenko, L.V.; Cicero, G.; Pirri, C.F.; Abdullin, K.A. Morphology Effects on Electro- and Photo-Catalytic Properties of Zinc Oxide Nanostructures. Nanomaterials 2023, 13, 2527. https://doi.org/10.3390/nano13182527
Kedruk YY, Contestabile A, Zeng J, Fontana M, Laurenti M, Gritsenko LV, Cicero G, Pirri CF, Abdullin KA. Morphology Effects on Electro- and Photo-Catalytic Properties of Zinc Oxide Nanostructures. Nanomaterials. 2023; 13(18):2527. https://doi.org/10.3390/nano13182527
Chicago/Turabian StyleKedruk, Yevgeniya Y., Alessandra Contestabile, Juqin Zeng, Marco Fontana, Marco Laurenti, Lesya V. Gritsenko, Giancarlo Cicero, Candido F. Pirri, and Khabibulla A. Abdullin. 2023. "Morphology Effects on Electro- and Photo-Catalytic Properties of Zinc Oxide Nanostructures" Nanomaterials 13, no. 18: 2527. https://doi.org/10.3390/nano13182527






