Self-Assembly of Copper Oxide Interfaced MnO2 for Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Syntheses of CuO
2.3. Syntheses of CuO/MnO2
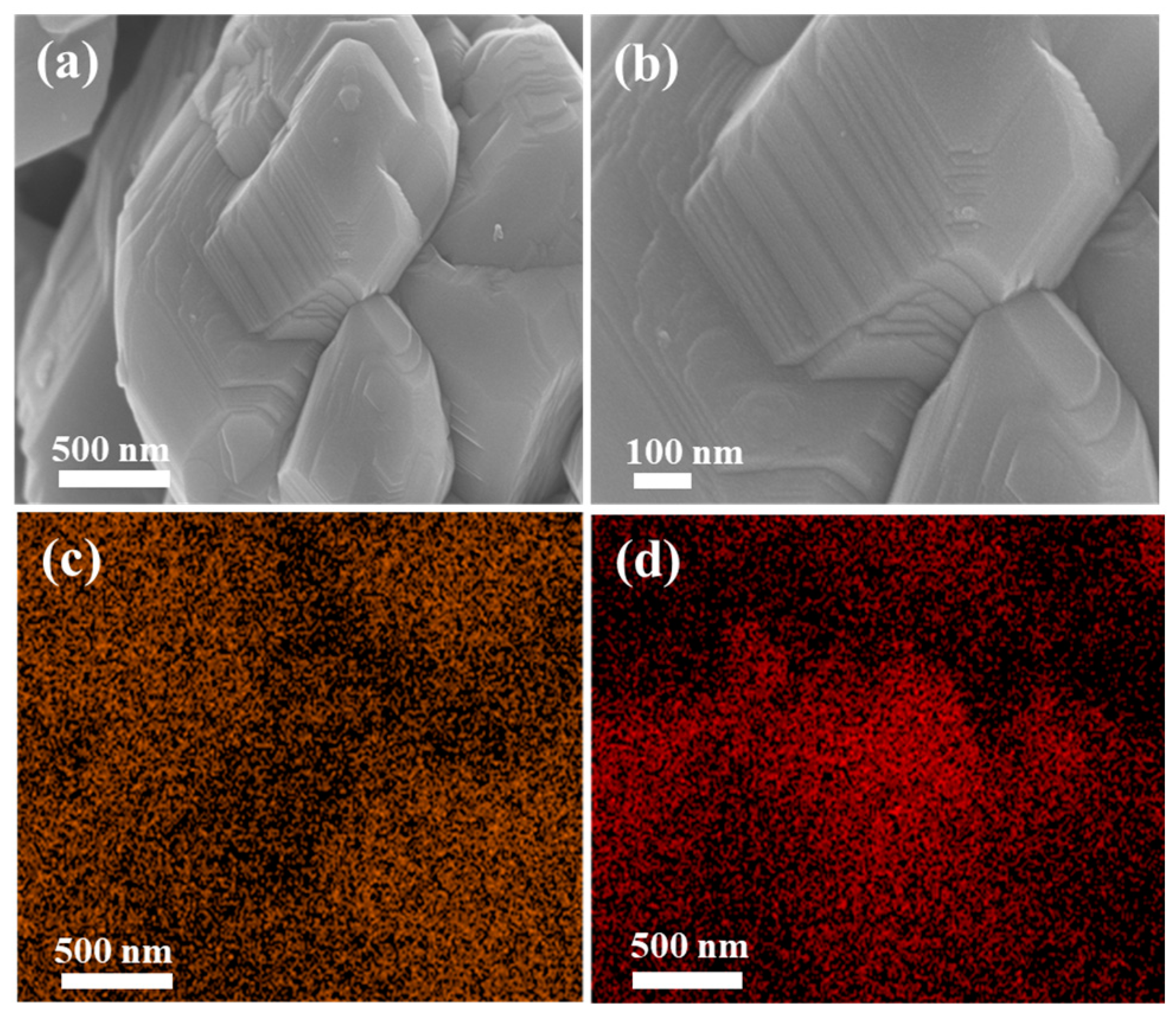
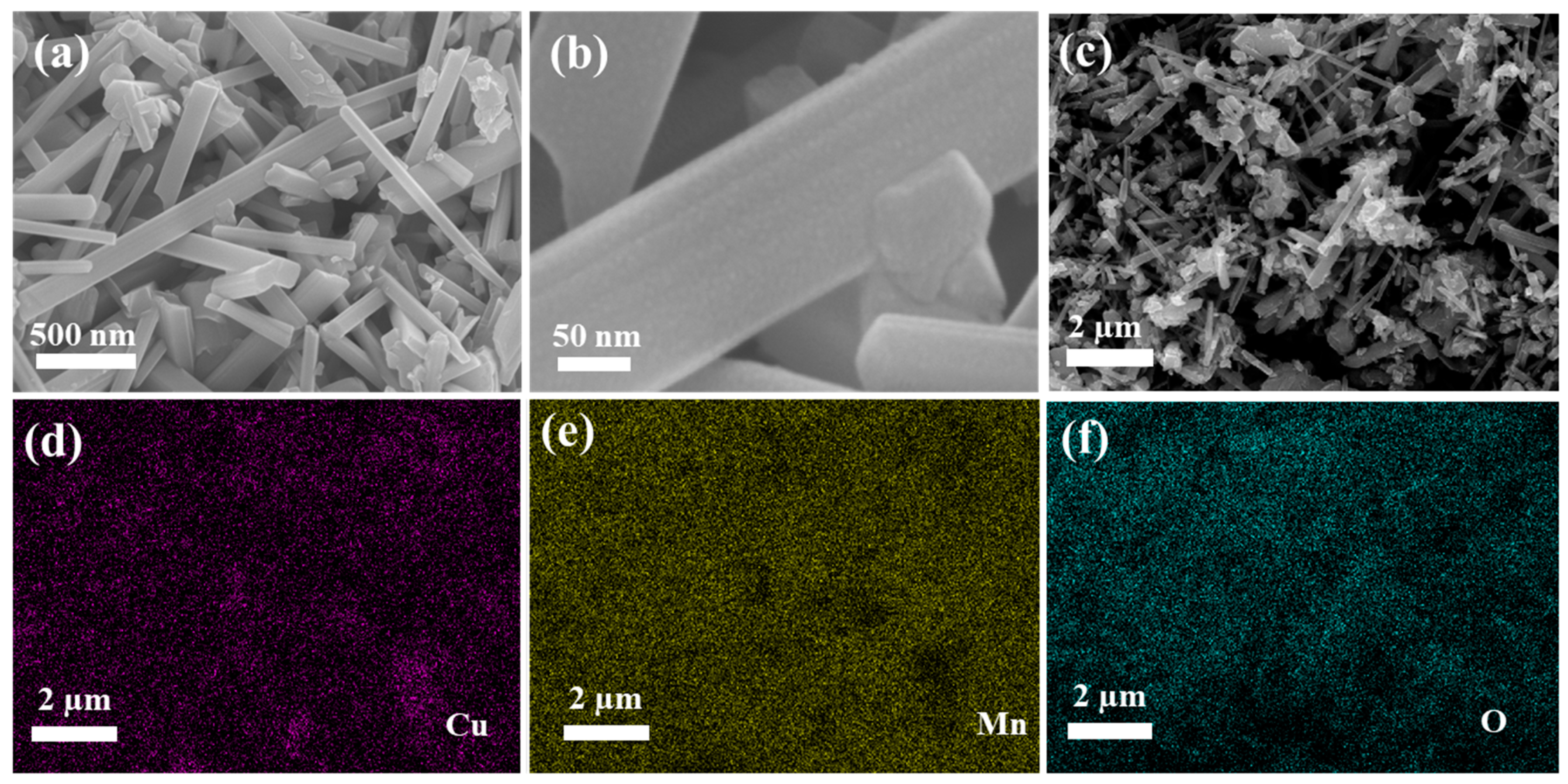
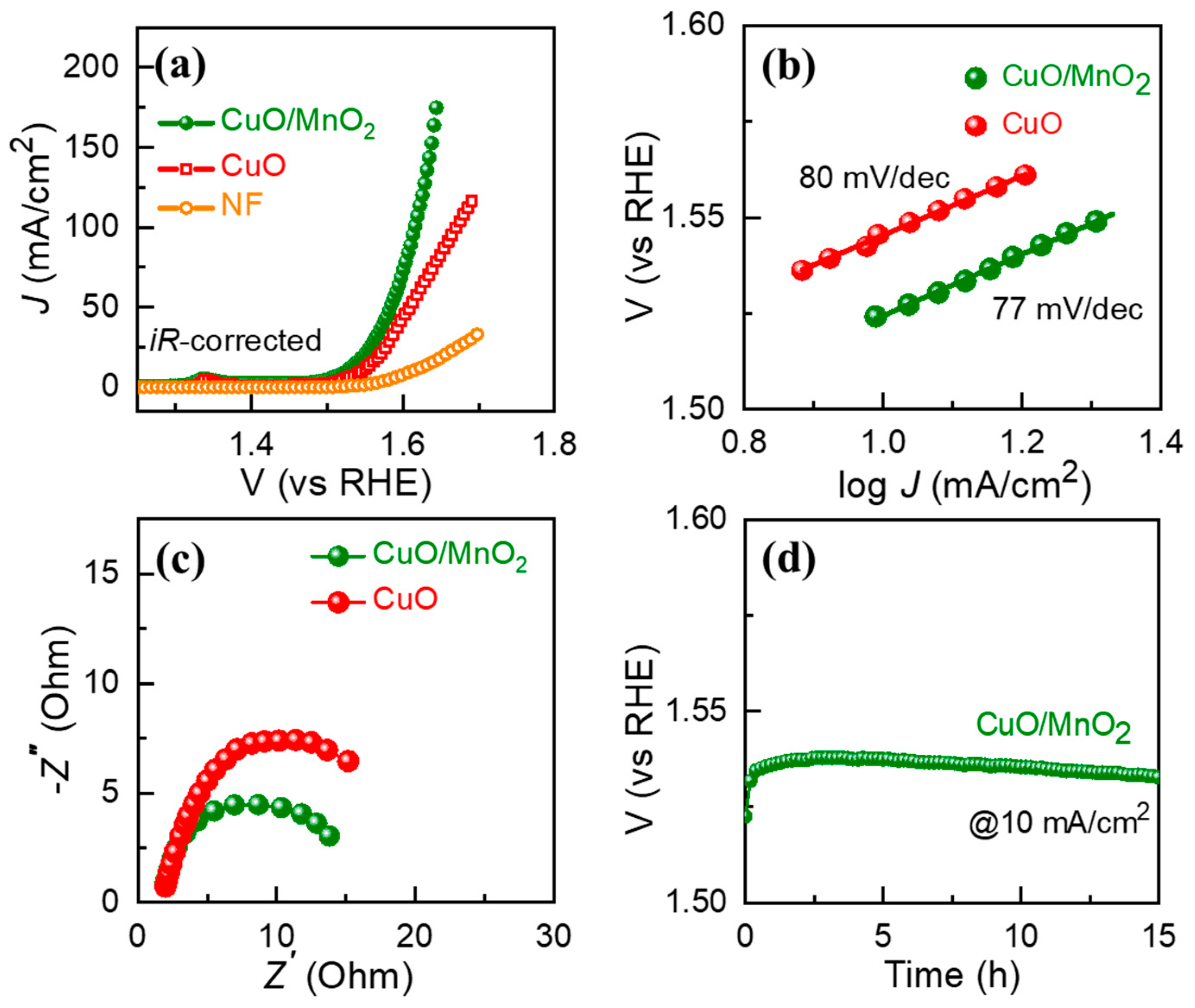
3. Results and Discussion
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.N.; Sandeep, K.; Janu, K.; Atanu, J.; Jin-Young, K.; Kwang, S.K. Interface Engineering Driven Stabilization of Halide Perovskites against Moisture, Heat, and Light for Optoelectronic Applications. Adv. Energy Mater. 2020, 10, 2000768. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Singh, A.N.; Sultan, S.; Kim, K.S. Recent Advancement of p- and d-Block Elements, Single Atoms, and Graphene-Based Photoelectrochemical Electrodes for Water Splitting. Adv. Energy Mater. 2020, 10, 2000280. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.; Eickemeyer, F.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef]
- Sultan, S.; Jitendra, N.T.; Singh, A.N.; Shynggys, Z.; Miran, H.; Chang, W.M.; Pandiarajan, T.; Kwang, S.K. Single Atoms and Clusters Based Nanomaterials for Hydrogen Evolution, Oxygen Evolution Reactions, and Full Water Splitting. Adv. Energy Mater. 2019, 1900624. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Yinlong, Z.; Wei, Z.; Yubo, C.; Jie, Y.; Meilin, L.; Zongping, S. A High-Performance Electrocatalyst for Oxygen Evolution Reaction: LiCo0.8Fe0.2O2. Adv. Mater. 2015, 27, 7150–7155. [Google Scholar]
- Zhiyi, L.; Haotian, W.; Desheng, K.; Kai, Y.; Po-Chun, H.; Guangyuan, Z.; Hongbin, Y.; Zheng, L.; Xiaoming, S.; Yi, C. Electrochemical tuning of layered lithium transition metal oxides for improvement of oxygen evolution reaction. Nat. Commun. 2014, 5, 4345. [Google Scholar]
- Kaiyue, Z.; Tao, W.; Yue, Z.; Xuning, L.; Mingrun, L.; Ruifeng, L.; Wang, J.; Xuefeng, Z.; Weishen, Y. Layered Fe-Substituted LiNiO2 Electrocatalysts for High-Efficiency Oxygen Evolution Reaction. ACS Energy Lett. 2017, 2, 1654–1660. [Google Scholar] [CrossRef]
- Yinlong, Z.; Wei, Z.; Zhi-Gang, C.; Yubo, C.; Chao, S.; Moses, O.T.; Zongping, S. SrNb0. 1Co0. 7Fe0. 2O3− δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem. 2015, 127, 3969–3973. [Google Scholar]
- Singh, A.N.; Kim, M.H.; Meena, A.; Wi, T.U.; Lee, H.W.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, 2005605. [Google Scholar] [CrossRef]
- Jamesh, M. Recent progress on earth abundant hydrogen evolution reaction and oxygen evolution reaction bifunctional electrocatalyst for overall water splitting in alkaline media. J. Power Sources 2016, 333, 213–236. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, N. Synthesis and Biomedical Applications of Copper Oxide Nanoparticles: An Expanding Horizon. ACS Biomater. Sci. Eng. 2019, 5, 1170–1188. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Copper oxide nanoflakes as highly sensitive and fast response self-sterilizing biosensors. J. Mater. Chem. 2011, 21, 12935–12940. [Google Scholar] [CrossRef]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M.S. Fabrication of anchored copper oxide nanoparticles on Graphene Oxide nanosheets via electrostatic coprecipitation and its application as supercapacitor. Electrochim. Acta 2013, 88, 347–357. [Google Scholar] [CrossRef]
- Huang, M.; Li, F.; Dong, F.; Zhang, Y.X.; Zhang, L.L. MnO2-based nanostructures for high-performance supercapacitors. J. Mater. Chem. A. 2015, 3, 21380–21423. [Google Scholar] [CrossRef]
- Husnain, S.M.; Asim, U.; Yaqub, A.; Shahzad, F.; Abbas, N. Recent trends of MnO2-derived adsorbents for water treatment: A review. New J. Chem. 2020, 44, 6096–6120. [Google Scholar] [CrossRef]
- Joya, K.; De Groot, J. Controlled Surface-Assembly of Nanoscale Leaf-Type Cu-Oxide Electrocatalyst for High Activity Water Oxidation. ACS Catal. 2016, 6, 1768–1771. [Google Scholar] [CrossRef]
- Sohal, N.; Maity, B.; Shetti, N.P.; Basu, S. Biosensors Based on MnO2 Nanostructures: A Review. ACS Appl. Nano Mater. 2021, 4, 2285–2302. [Google Scholar] [CrossRef]
- Qian, K.; Qian, Z.; Hua, Q.; Jiang, Z.; Huang, W. Structure–activity relationship of CuO/MnO2 catalysts in CO oxidation. Appl. Surf. Sci. 2013, 273, 357–363. [Google Scholar] [CrossRef]
- Angeles-Hernández, M.J.; Leeke, G.A.; Santos, R.C. Catalytic supercritical water oxidation for the destruction of quinoline over MnO2/CuO mixed catalyst. Ind. Eng. Chem. Res. 2009, 48, 1208–1214. [Google Scholar] [CrossRef]
- Martin, A.; Armbruster, U.; Schneider, M.; Radnik, J.; Pohl, M.M. Structural Transformation of an Alumina-supported MnO2–CuO oxidation catalyst by hydrothermal impact of sub- and supercritical water. J. Mater. Chem. 2002, 12, 639–645. [Google Scholar] [CrossRef]
- Pengjiao, Z.; Li, W. Microwave-assisted synthesis of CuO/MnO2 nanocomposites for supercapacitor application. Micro Nano Lett. 2020, 15, 938–942. [Google Scholar]
- Davide, S.; Ricardo, F.; Gueorgui, G.; Anelia, K. Discovering atomistic pathways for supply of metal atoms from methyl-based precursors to graphene surface. Phys. Chem. Chem. Phys. 2023, 25, 5887. [Google Scholar]
- Renato Batista, S.; Roberto, R.; Gueorgui, G.; Anelia, K. Exploring 2D structures of indium oxide of different stoichiometry. Cryst. Eng. Comm. 2021, 23, 6661–6667. [Google Scholar]
- Owidah, Z.; Aman, S.; Abdullah, M.; Manzoor, S.; Fallatah, A.; Ibrahim, M.; Seaf Elnasr, T.; Mohd, Z. Ansari. Metal oxide/carbon nanosheet arrays derivative of stacked metal organic frameworks for triggering oxygen evolution reaction. Ceram. Int. 2023, 49, 5936–5943. [Google Scholar] [CrossRef]
- Yuasa, M. Molten salt synthesis of Nb-doped TiO2 rod-like particles for use in bifunctional oxygen reduction/evolution electrodes. Ceram. Int. 2022, 48, 14726–14735. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, W.; Zhao, H.; Li, L.; Deng, P.; Wu, Y.; Luo, S.; Chen, B. Ultrathin amorphous MnO2 modified prawn shells-derived porous carbon towards robust oxygen electrocatalyst for rechargeable Zn-air battery. Ceram. Int. 2022, 48, 6506–6511. [Google Scholar] [CrossRef]
- Palem, R.; Meena, A.; Soni, R.; Meena, J.; Lee, S.; Patil, S.; Ansar, S.; Kim, H.; Im, H.; Bathula, C. Fabrication of Fe2O3 nanostructure on CNT for oxygen evolution reaction. Ceram. Int. 2022, 48, 29081–29086. [Google Scholar] [CrossRef]
- Huang, L.; Wei, M.; Qi, R.; Dong, C.; Dang, D.; Yang, C.; Xia, C.; Chen, C.; Zaman, S.; Li, F.; et al. An integrated platinum-nanocarbon electrocatalyst for efficient oxygen reduction. Nat. Commu. 2022, 13, 6703. [Google Scholar] [CrossRef]
- Ali, H.; Zaman, S.; Majeed, I.; Kanodarwala, F.; Nadeem, M.; Stride, J.; Nadeem, M. Porous Carbon/rGO Composite: An Ideal Support Material of Highly Efficient Palladium Electrocatalysts for the Formic Acid Oxidation Reaction. Chem. Electro. Chem. 2017, 4, 3126–3133. [Google Scholar] [CrossRef]
- Li, F.; Huang, L.; Zaman, S.; Guo, W.; Liu, H.; Guo, X.; Xia, B. Corrosion Chemistry of Electrocatalysts. Adv. Mater. 2022, 34, 2200840. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.Q.; Lia, M.; Li, F.; Zhu, S.J.; Liu, X.Y.; Zhang, Y.X. Morphology-controlled MnO2-Graphene Oxides-diatomaceous earth 3-dimensional (3D) composites for high-performance supercapacitors. Dalton Trans. 2016, 45, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Zahan, M.; Podder, J. Structural, optical and electrical properties of Cu:MnO2 nanostructured thin films for glucose sensitivity measurements. SN Appl. Sci. 2020, 2, 385. [Google Scholar] [CrossRef]
- Racik, K.; Manikandan, M.; Mahendiran, M.; Prabakaran, P.; Madhavan, J.; Raj, M. Fabrication of manganese oxide decorated copper oxide (MnO2/CuO) nanocomposite electrodes for energy storage supercapacitor devices. Phys. E Low. Dimens. Syst. Nanostruct. 2020, 119, 114033. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, C.; Chen, P.; Yuan, D.; Guo, K. MnO2-decorated hierarchical porous carbon composites for high performance asymmetric supercapacitors. J. Power Sources 2019, 425, 1–9. [Google Scholar] [CrossRef]
- Ramesh, S.; Kim, H.; Haldorai, Y.; Han, Y.; Kim, J. Fabrication of nanostructured MnO2/carbon nanotube composite from 3D precursor complex for high-performance supercapacitor. Mater. Lett. 2017, 196, 132–136. [Google Scholar] [CrossRef]
- Bathula, C.; Rabani, I.; Ramesh, S.; Lee, S.; Palem, R.; Ahmed, A.; Kim, H.; Seo, Y.; Kim, H. Highly efficient solid-state synthesis of Co3O4 on multiwalled carbon nanotubes for supercapacitors. J. Alloys Compd. 2021, 887, 161307. [Google Scholar] [CrossRef]
- Rabani, I.; Zafar, R.; Subalakshmi, K.; Kim, H.-S.; Bathula, C.; Seo, Y.-S. A facile mechanochemical preparation of Co3O4@ g-C3N4 for application in supercapacitors and degradation of pollutants in water. J. Hazard. Mater. 2020, 407, 124360. [Google Scholar] [CrossRef]
- Rabani, I.; Yoo, J.; Bathula, C.; Hussain, S.; Seo, Y.-S. Role of uniformly distributed ZnO nanoparticles on cellulose nanofiber for flexible solid state symmetric supercapacitors. J. Mater. Chem. A 2021, 9, 11580–11594. [Google Scholar] [CrossRef]
- Bathula, C.; Rabani, I.; Kadam, A.; Opoku, H.; Patil, S.A.; Shreshta, N.K.; Hwang, J.; Seo, Y.-S.; Kim, H.-S. Sonochemically exfoliated polymer-carbon nanotube interface for high performance supercapacitors. J. Colloid. Interface Sci. 2022, 606, 1792–1799. [Google Scholar] [CrossRef]
- Palem, R.R.; Ramesh, S.; Rabani, I.; Shimoga, G.; Bathula, C.; Kim, H.S.; Seo, Y.S.; Kim, H.S.; Lee, S.H. Microstructurally assembled transition metal oxides with cellulose nanocrystals for high-performance supercapacitors. J. Energy Storage 2022, 50, 104712. [Google Scholar] [CrossRef]
- Palem, R.R.; Ramesh, S.; Bathula, C.; Kakani, V.; Saratale, G.; Yadav, H.; Kim, J.; Kim, H.S.; Lee, S.H. Enhanced supercapacitive behavior by CuO@MnO2/carboxymethyl cellulose composites. Ceram. Int. 2021, 47, 26738–26747. [Google Scholar] [CrossRef]
- Ramesh, S.; Yadav, H.; Shinde, S.; Bathula, C.; Lee, Y.; Cheedarala, R.; Kim, H.; Kim, H.; Kim, J. Fabrication of nanostructured SnO2@Co3O4/nitrogen doped graphene oxide composite for symmetric and asymmetric storage devices. J. Mater. Res. Technol. 2020, 9, 4183–4193. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Sun, Z.; Ren, Y.; Zhang, X.; Du, P. Self-Supported Copper Oxide Electrocatalyst for Water Oxidation at Low Overpotential and Confirmation of Its Robustness by Cu K-Edge X-ray Absorption Spectroscopy. J. Phys. Chem. C 2016, 120, 831. [Google Scholar] [CrossRef]
- Chauhan, M.; Reddy, K.P.; Gopinath, C.S.; Deka, S. Copper Cobalt Sulfide Nanosheets Realizing a Promising Electrocatalytic Oxygen Evolution Reaction. ACS Catal. 2017, 7, 5871–5879. [Google Scholar] [CrossRef]
- Yang, L.; Xie, L.; Ren, X.; Wang, Z.; Liu, Z.; Du, G.; Asiri, A.M.; Yao, Y.; Sun, X. Hierarchical CuCo2S4 nanoarrays for high-efficient and durable water oxidation electrocatalysis. Chem. Commun. 2018, 54, 78–81. [Google Scholar]
- Kuang, M.; Han, P.; Wang, Q.; Li, J.; Zheng, G. CuCo Hybrid Oxides as Bifunctional Electrocatalyst for Efficient Water Splitting. Adv. Funct. Mater. 2016, 26, 8555–8561. [Google Scholar] [CrossRef]
- Ahmed, A.; Hou, B.; Chavan, H.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.; Cha, S.; Inamdar, A.; Kim, H.; et al. Self-Assembled Nanostructured CuCo2O4 for Electrochemical Energy Storage and the Oxygen Evolution Reaction via Morphology Engineering. Small 2018, 14, 1800742. [Google Scholar] [CrossRef]
- Yan, H.; Wang, X.; Linkov, V.; Ji, S.; Wang, R. Selectivity Evolution Reaction on Carbon Cloth-Supported δ-MnO2 Nanosheets in Electrolysis of Real Seawater. Molecules 2023, 28, 854. [Google Scholar] [CrossRef]
- Zheng, X.; Qin, M.; Ma, S.; Chen, Y.; Ning, H.; Yang, R.; Mao, S.; Wang, Y. Strong Oxide-Support Interaction over IrO2/V2O5 for Efficient pH-Universal Water Splitting. Adv. Sci. 2022, 9, 2104636. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, X.; Zhao, Z.L.; Wang, M.; Xiang, B.; Li, J.; Feng, Z.; Xu, H.; Gu, M. Ultrahigh-Loading of Ir Single Atoms on NiO Matrix to Dramatically Enhance Oxygen Evolution Reaction. J. Am. Chem. Soc. 2020, 142, 7425–7433. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bathula, C.; Meena, A.; Sekar, S.; Singh, A.N.; Soni, R.; El-Marghany, A.; Palem, R.R.; Kim, H.-S. Self-Assembly of Copper Oxide Interfaced MnO2 for Oxygen Evolution Reaction. Nanomaterials 2023, 13, 2329. https://doi.org/10.3390/nano13162329
Bathula C, Meena A, Sekar S, Singh AN, Soni R, El-Marghany A, Palem RR, Kim H-S. Self-Assembly of Copper Oxide Interfaced MnO2 for Oxygen Evolution Reaction. Nanomaterials. 2023; 13(16):2329. https://doi.org/10.3390/nano13162329
Chicago/Turabian StyleBathula, Chinna, Abhishek Meena, Sankar Sekar, Aditya Narayan Singh, Ritesh Soni, Adel El-Marghany, Ramasubba Reddy Palem, and Hyun-Seok Kim. 2023. "Self-Assembly of Copper Oxide Interfaced MnO2 for Oxygen Evolution Reaction" Nanomaterials 13, no. 16: 2329. https://doi.org/10.3390/nano13162329




