An Ionically Conductive, Self-Powered and Stable Organogel for Pressure Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
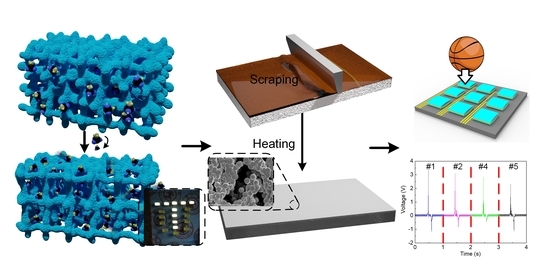
2.2. Preparation of OICs and Self-Powered OICs
2.3. Temperature Tolerance Characterization
2.4. Impedance Analysis
2.5. Mechanical Characterization
2.6. Characterization of Self-Powered OICs
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ohm, Y.; Pan, C.; Ford, M.J.; Huang, X.; Liao, J.; Majidi, C. An electrically conductive silver–polyacrylamide–alginate hydrogel composite for soft electronics. Nat. Electron. 2021, 4, 185–192. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.X.; Wang, L.; Yu, H.T.; Zhang, F.; Tang, L.; Feng, Y.Y.; Feng, W. Highly Transparent, Self-Healable, and Adhesive Organogels for Bio-Inspired Intelligent Ionic Skins. ACS Appl. Mater. Interfaces 2020, 12, 15657–15666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, F.X.; Wang, X.L.; Yu, J.Y.; Wu, D.Q. Hyaluronic Acid and Polyethylene Glycol Hybrid Hydrogel Encapsulating Nanogel with Hemostasis and Sustainable Antibacterial Property for Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 13304–13316. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Kim, J.H.; Xie, Z.Q.; Cho, S.; Han, H.; Jeon, S.W.; Park, M.; Namkoong, M.; Avila, R.; Song, Z.; et al. Battery-free, wireless soft sensors for continuous multi-site measurements of pressure and temperature from patients at risk for pressure injuries. Nat. Commun. 2021, 12, 5008. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.B.; Wong, M.C.; Chen, L.; Bai, G.X.; Hao, J.H. Environmentally Friendly Hydrogel-Based Triboelectric Nanogenerators for Versatile Energy Harvesting and Self-Powered Sensors. Adv. Energy Mater. 2017, 7, 1601529. [Google Scholar] [CrossRef]
- Sahu, M.; Vivekananthan, V.; Hajra, S.; Khatua, D.K.; Kim, S.J. Porosity modulated piezo-triboelectric hybridized nanogenerator for sensing small energy impacts. Appl. Mater. Today 2021, 22, 100900. [Google Scholar] [CrossRef]
- Gao, G.; Yang, F.; Zhou, F.; He, J.; Lu, W.; Xiao, P.; Yan, H.; Pan, C.; Chen, T.; Wang, Z.L. Bioinspired Self-Healing Human–Machine Interactive Touch Pad with Pressure-Sensitive Adhesiveness on Targeted Substrates. Adv. Mater. 2020, 32, 2004290. [Google Scholar] [CrossRef]
- Wang, Z.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mater. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Xiao, W.; Zhao, J.; Shi, M.; Wei, H.; Deng, Z.; Guo, B.; Zheng, Z.; Yu, Y. Visible-light-assisted multimechanism design for one-step engineering tough hydrogels in seconds. Nat. Commun. 2020, 11, 4694. [Google Scholar] [CrossRef]
- Chun, S.; Choi, I.Y.; Son, W.; Jung, J.; Lee, S.; Kim, H.S.; Pang, C.; Park, W.; Kim, J.K. High-Output and Bending-Tolerant Triboelectric Nanogenerator Based on an Interlocked Array of Surface-Functionalized Indium Tin Oxide Nanohelixes. ACS Energy Lett. 2019, 4, 1748–1754. [Google Scholar] [CrossRef]
- Qi, J.B.; Wang, A.C.; Yang, W.F.; Zhang, M.Y.; Hou, C.Y.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z. Hydrogel-based hierarchically wrinkled stretchable nanofibrous membrane for high performance wearable triboelectric nanogenerator. Nano Energy 2020, 67, 104206. [Google Scholar] [CrossRef]
- De Medeiros, M.S.; Chanci, D.; Martinez, R.V. Moisture-insensitive, self-powered paper-based flexible electronics. Nano Energy 2020, 78, 105301. [Google Scholar] [CrossRef]
- Rahimi, A.; Herzog-Arbeitman, A.; García, J.M. Conductive Recyclable Organogel Composites. Macromol. Mater. Eng. 2019, 304, 1800583. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, X.; Liu, W.; Huang, J.; Qiu, X. Synthesis of highly conductive hydrogel with high strength and super toughness. Polymer 2020, 202, 122643. [Google Scholar] [CrossRef]
- Chun, K.Y.; Seo, S.; Han, C.S. Self-Powered, Stretchable, and Wearable Ion Gel Mechanoreceptor Sensors. ACS Sens. 2021, 6, 1940–1948. [Google Scholar] [CrossRef]
- Ji, Z.; Yan, C.; Yu, B.; Zhang, X.; Cai, M.; Jia, X.; Wang, X.; Zhou, F. 3D Printing of Hydrogel Architectures with Complex and Controllable Shape Deformation. Adv. Mater. Technol. 2019, 4, 1800713. [Google Scholar] [CrossRef]
- Kazunari, Y.; Yuki, T.; Yuta, H.; Masaru, K.; Hidemitsu, F. 3D printing for gel robotics. In Proceedings of the SPIE Smart Structures and Materials + Nondestructive Evaluation and Health Monitoring, Denver, CO, USA, 4–8 March 2018. [Google Scholar]
- Schroeder, T.B.H.; Guha, A.; Lamoureux, A.; VanRenterghem, G.; Sept, D.; Shtein, M.; Yang, J.; Mayer, M. An electric-eel-inspired soft power source from stacked hydrogels. Nature 2017, 552, 214–218. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.P.; Li, C.Y.; Zhang, C.W.; Du, M.; Ying, Z.M.; Zheng, Q.; Wu, Z.L. Self-Shaping Soft Electronics Based on Patterned Hydrogel with Stencil-Printed Liquid Metal. Adv. Funct. Mater. 2021, 31, 2105481. [Google Scholar] [CrossRef]
- Li, G.X.; Li, L.W.; Zhang, P.P.; Chang, C.Y.; Xu, F.; Pu, X. Ultra-stretchable and healable hydrogel-based triboelectric nanogenerators for energy harvesting and self-powered sensing. RSC Adv. 2021, 11, 17437–17444. [Google Scholar] [CrossRef]
- Wang, S.H.; Wang, Z.L.; Yang, Y. A One-Structure-Based Hybridized Nanogenerator for Scavenging Mechanical and Thermal Energies by Triboelectric-Piezoelectric-Pyroelectric Effects. Adv. Mater. 2016, 28, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Niu, W.B.; Zhang, S.F. Extremely Stretchable, Stable, and Durable Strain Sensors Based on Double-Network Organogels. ACS Appl. Mater. Interfaces 2018, 10, 32640–32648. [Google Scholar] [CrossRef]
- Ahmed, K.; Naga, N.; Kawakami, M.; Furukawa, H. Extremely Soft, Conductive, and Transparent Ionic Gels by 3D Optical Printing. Macromol. Chem. Phys. 2018, 219, 1800216. [Google Scholar] [CrossRef]
- Li, C.; Cong, S.; Tian, Z.N.; Song, Y.Z.; Yu, L.H.; Lu, C.; Shao, Y.L.; Li, J.; Zou, G.F.; Rummeli, M.H.; et al. Flexible perovskite solar cell-driven photo-rechargeable lithium-ion capacitor for self-powered wearable strain sensors. Nano Energy 2019, 60, 247–256. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Ng, T.Y.; Sharma, P. The effect of water content on the elastic modulus and fracture energy of hydrogel. Extrem. Mech. Lett. 2020, 35, 100617. [Google Scholar] [CrossRef]
- Ming, Z.Z.; Pang, Y.; Liu, J.Y. Switching between Elasticity and Plasticity by Network Strength Competition. Adv. Mater. 2020, 32, 1906870. [Google Scholar] [CrossRef]
- Rahman, M.S.; Shiblee, M.D.N.I.; Ahmed, K.; Khosla, A.; Ogawa, J.; Kawakami, M.; Furukawa, H. Flexible and Conductive 3D Printable Polyvinylidene Fluoride and Poly(N,N-dimethylacrylamide) Based Gel Polymer Electrolytes. Macromol. Mater. Eng. 2020, 305, 2000262. [Google Scholar] [CrossRef]
- Alam, A.; Moussa, M. Preparation of graphene/poly(vinyl alcohol) composite hydrogel films with enhanced electrical and mechanical properties. Polym. Compos. 2020, 41, 809–816. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Park, S.; Waletzki, B.E.; Zhou, Z.; Terzic, A.; Lu, L. Functionalized Carbon Nanotube and Graphene Oxide Embedded Electrically Conductive Hydrogel Synergistically Stimulates Nerve Cell Differentiation. ACS Appl. Mater. Interfaces 2017, 9, 14677–14690. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, C.; Lin, X.; Zheng, J.; Wu, J.; Liu, C. Dual Conductive Network Hydrogel for a Highly Conductive, Self-Healing, Anti-Freezing, and Non-Drying Strain Sensor. ACS Appl. Polym. Mater. 2020, 2, 996–1005. [Google Scholar] [CrossRef]
- Wu, L.; Hu, Y.; Tang, P.; Wang, H.; Bin, Y. High stretchable, pH-sensitive and self-adhesive rGO/CMCNa/PAA composite conductive hydrogel with good strain-sensing performance. Compos. Commun. 2021, 24, 100669. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Yang, Y.T.; Cao, Y.X.; Wang, X.; Chen, Y.R.; Liu, H.Y.; Gao, Y.F.; Wang, J.F.; Liu, C.; Wang, W.J.; et al. Anti-freezing, resilient and tough hydrogels for sensitive and large-range strain and pressure sensors. Chem. Eng. J. 2021, 403, 126431. [Google Scholar] [CrossRef]
- Chen, H.; Huang, J.; Liu, J.; Gu, J.; Zhu, J.; Huang, B.; Bai, J.; Guo, J.; Yang, X.; Guan, L. High toughness multifunctional organic hydrogels for flexible strain and temperature sensor. J. Mater. Chem. A 2021, 9, 23243–23255. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.; Wang, M.L.; Zhu, C.Z.; Zhao, N.; Xu, J. Skin-Inspired Double-Hydrophobic-Coating Encapsulated Hydrogels with Enhanced Water Retention Capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- Xu, R.D.; Qu, L.J.; Tian, M.W. Touch-sensing fabric encapsulated with hydrogel for human-computer interaction. Soft Matter 2021, 17, 9014–9018. [Google Scholar] [CrossRef]
- Jian, Y.; Handschuh-Wang, S.; Zhang, J.; Lu, W.; Zhou, X.; Chen, T. Biomimetic anti-freezing polymeric hydrogels: Keeping soft-wet materials active in cold environments. Mater. Horiz. 2021, 8, 351–369. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Chen, P.; Zhu, Y.; Wen, C.; Gao, Y.; Yang, J.; Zhang, X.; Zhang, L. Zwitterionic Osmolyte-Based Hydrogels with Antifreezing Property, High Conductivity, and Stable Flexibility at Subzero Temperature. Adv. Funct. Mater. 2020, 30, 1907986. [Google Scholar] [CrossRef]
- Subraveti, S.N.; Raghavan, S.R. A Simple Way to Synthesize a Protective “Skin” around Any Hydrogel. ACS Appl. Mater. Interfaces 2021, 13, 37645–37654. [Google Scholar] [CrossRef]
- Gao, Y.; Shi, L.; Lu, S.; Zhu, T.; Da, X.; Li, Y.; Bu, H.; Gao, G.; Ding, S. Highly Stretchable Organogel Ionic Conductors with Extreme-Temperature Tolerance. Chem. Mater. 2019, 31, 3257–3264. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Jiao, S.; Wang, C.; Jia, Y.; Dai, K.; Zheng, G.; Liu, C.; Wan, P.; Shen, C. Environment Tolerant Conductive Nanocomposite Organohydrogels as Flexible Strain Sensors and Power Sources for Sustainable Electronics. Adv. Funct. Mater. 2021, 31, 2101696. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Kang, H.Y.; Gwon, S.H.; Choi, G.M.; Lim, S.M.; Sun, J.Y.; Joo, Y.C. A Strain-Insensitive Stretchable Electronic Conductor: PEDOT:PSS/Acrylamide Organogels. Adv. Mater. 2016, 28, 1636–1643. [Google Scholar] [CrossRef]
- Ueda, C.; Park, J.; Hirose, K.; Konishi, S.; Ikemoto, Y.; Osaki, M.; Yamaguchi, H.; Harada, A.; Tanaka, M.; Watanabe, G.; et al. Behavior of supramolecular cross-links formed by host-guest interactions in hydrogels responding to water contents. Supramol. Mater. 2022, 1, 100001. [Google Scholar] [CrossRef]
- Fu, W.L.; Du, P.Y.; Weng, W.J.; Han, G.Y. Preparation and structure of PVDF piezoelectric film. Chin. J. Mater. Res. 2005, 3, 21–26. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, Z.; Li, Y.; Luo, Y.; Lu, B.; Gao, Y.; Yu, W.; Gao, G.; Ding, S. An Ionically Conductive, Self-Powered and Stable Organogel for Pressure Sensing. Nanomaterials 2022, 12, 714. https://doi.org/10.3390/nano12040714
Wang L, Wang Z, Li Y, Luo Y, Lu B, Gao Y, Yu W, Gao G, Ding S. An Ionically Conductive, Self-Powered and Stable Organogel for Pressure Sensing. Nanomaterials. 2022; 12(4):714. https://doi.org/10.3390/nano12040714
Chicago/Turabian StyleWang, Li, Zhengduo Wang, Yingtao Li, Yu Luo, Bingheng Lu, Yiyang Gao, Wei Yu, Guoxin Gao, and Shujiang Ding. 2022. "An Ionically Conductive, Self-Powered and Stable Organogel for Pressure Sensing" Nanomaterials 12, no. 4: 714. https://doi.org/10.3390/nano12040714