Constructing Co3O4/La2Ti2O7 p-n Heterojunction for the Enhancement of Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
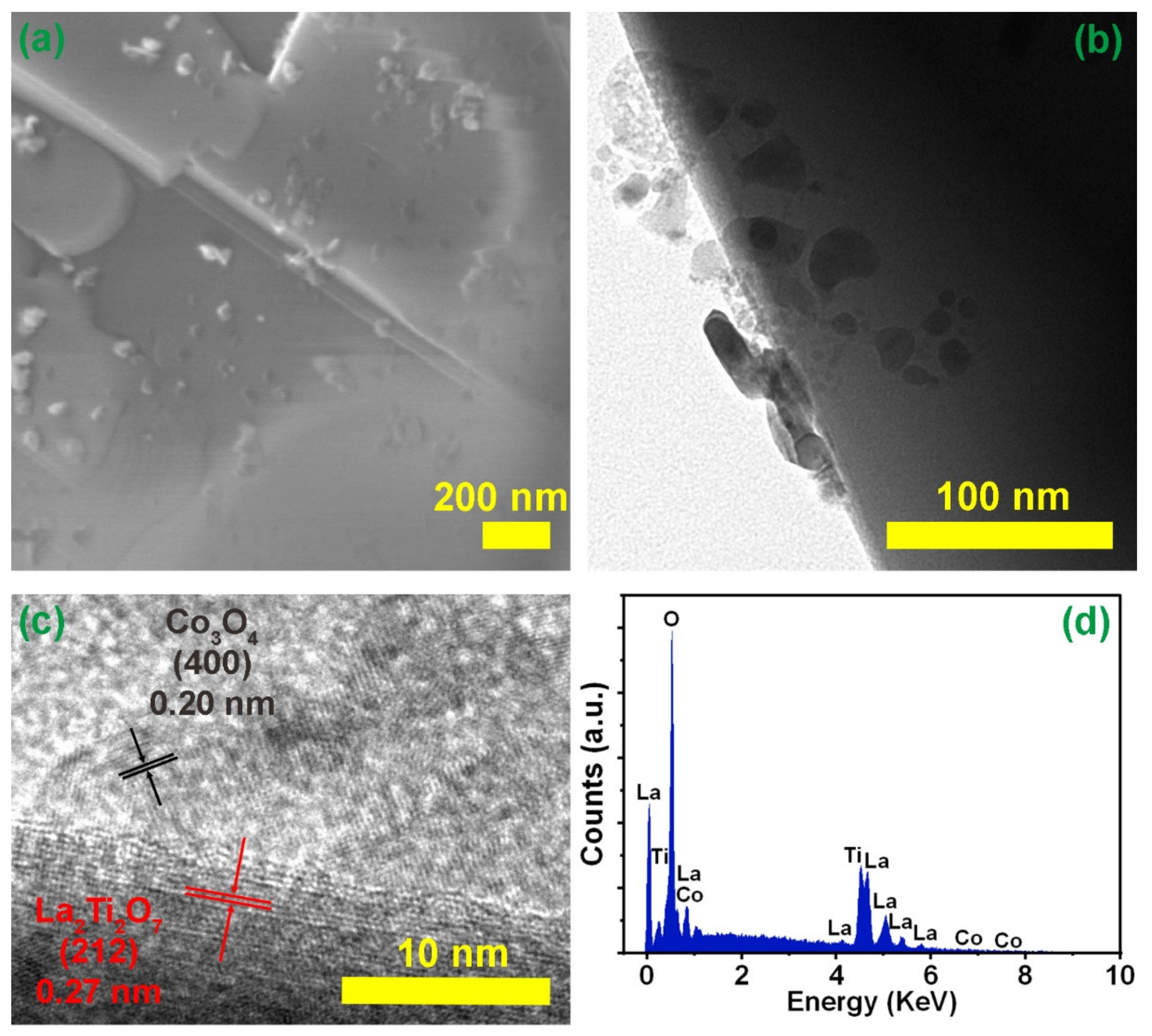
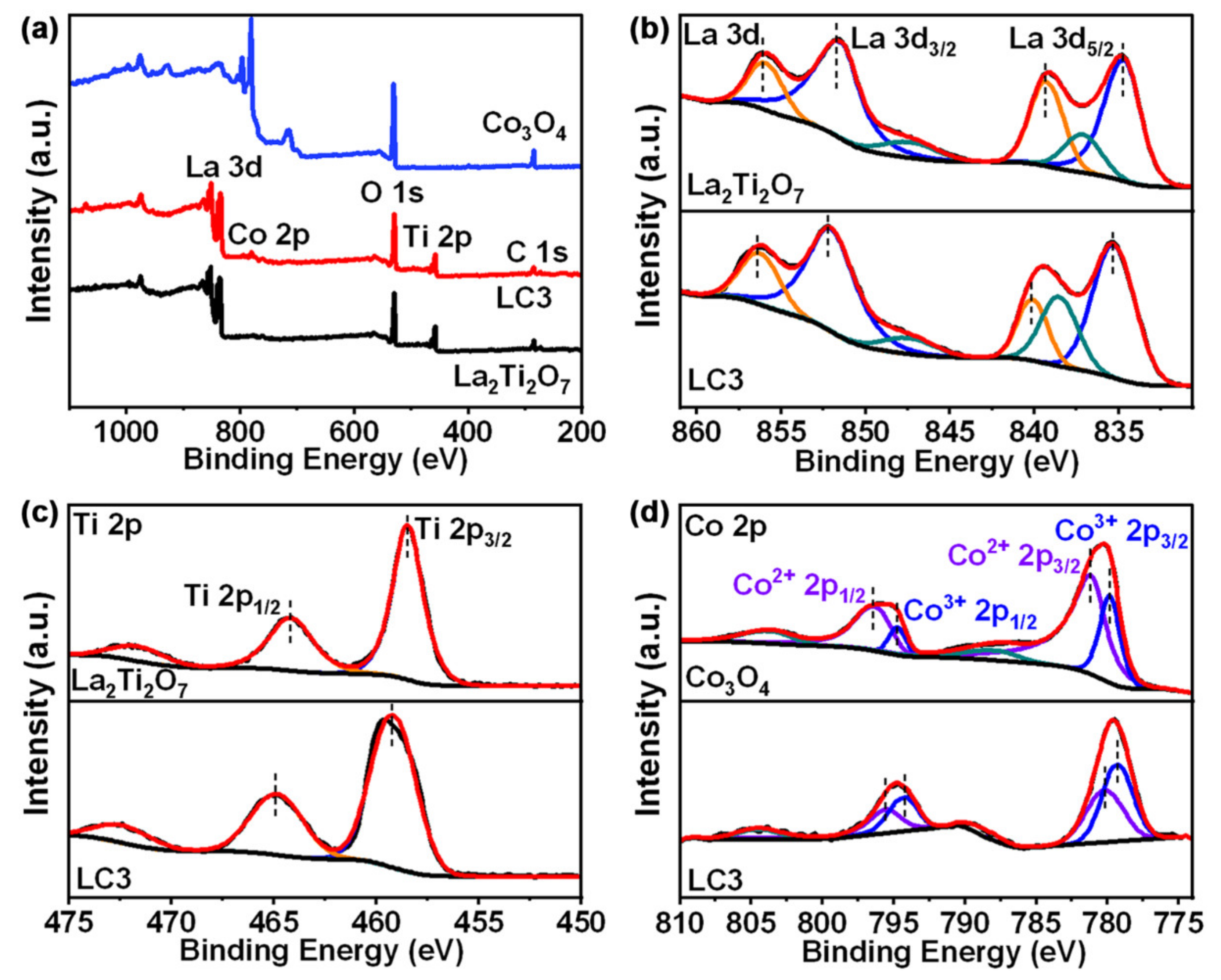
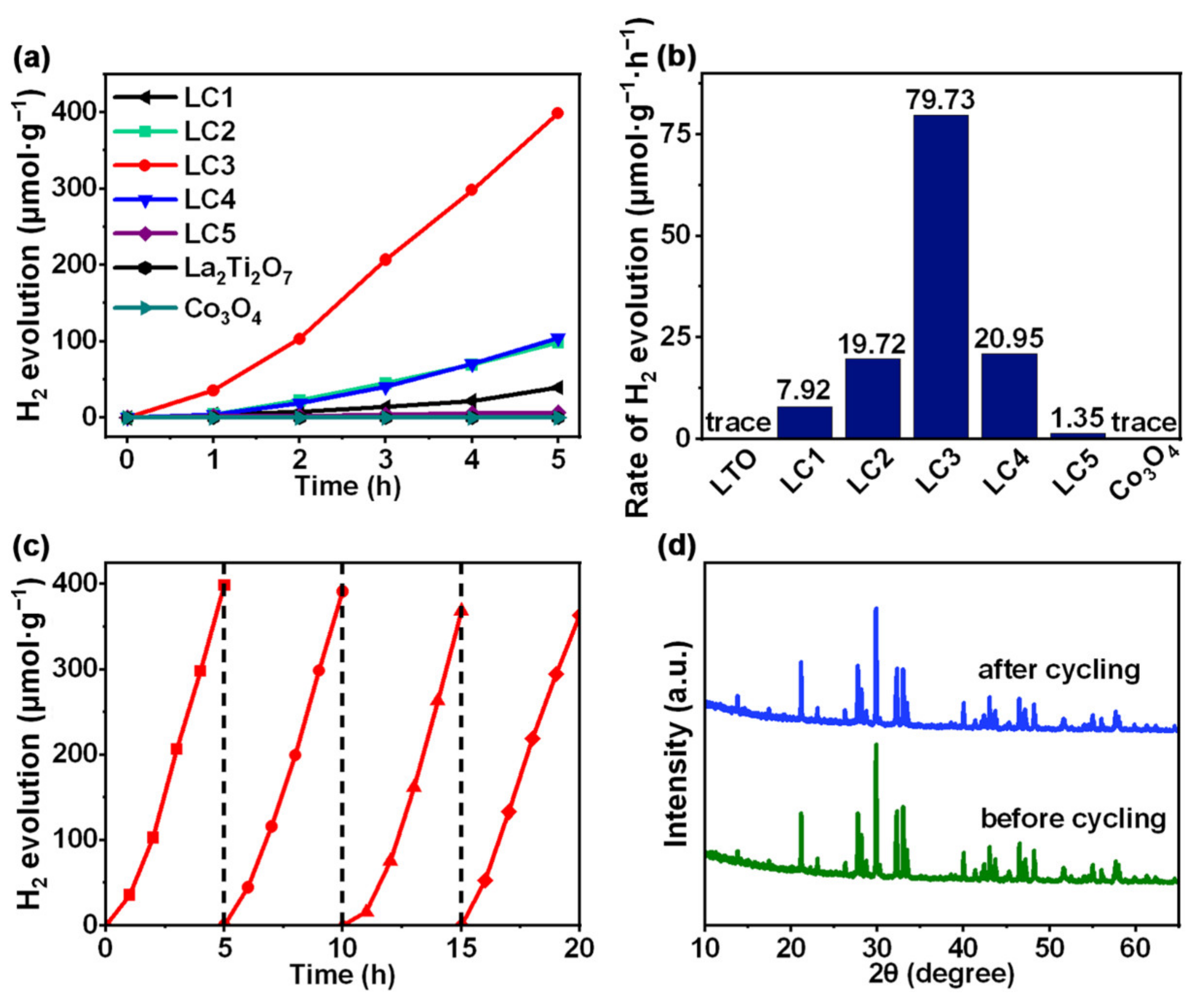
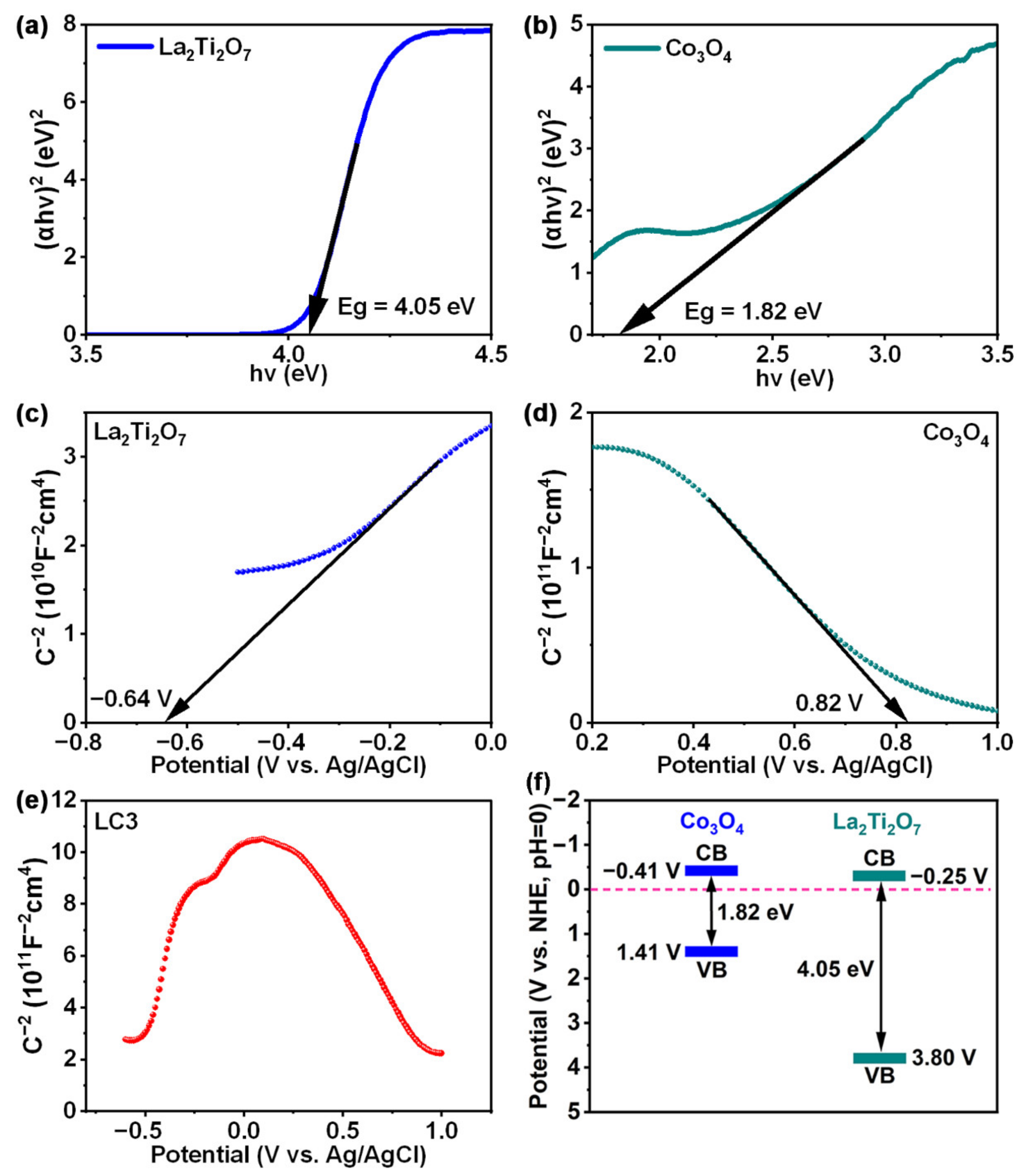
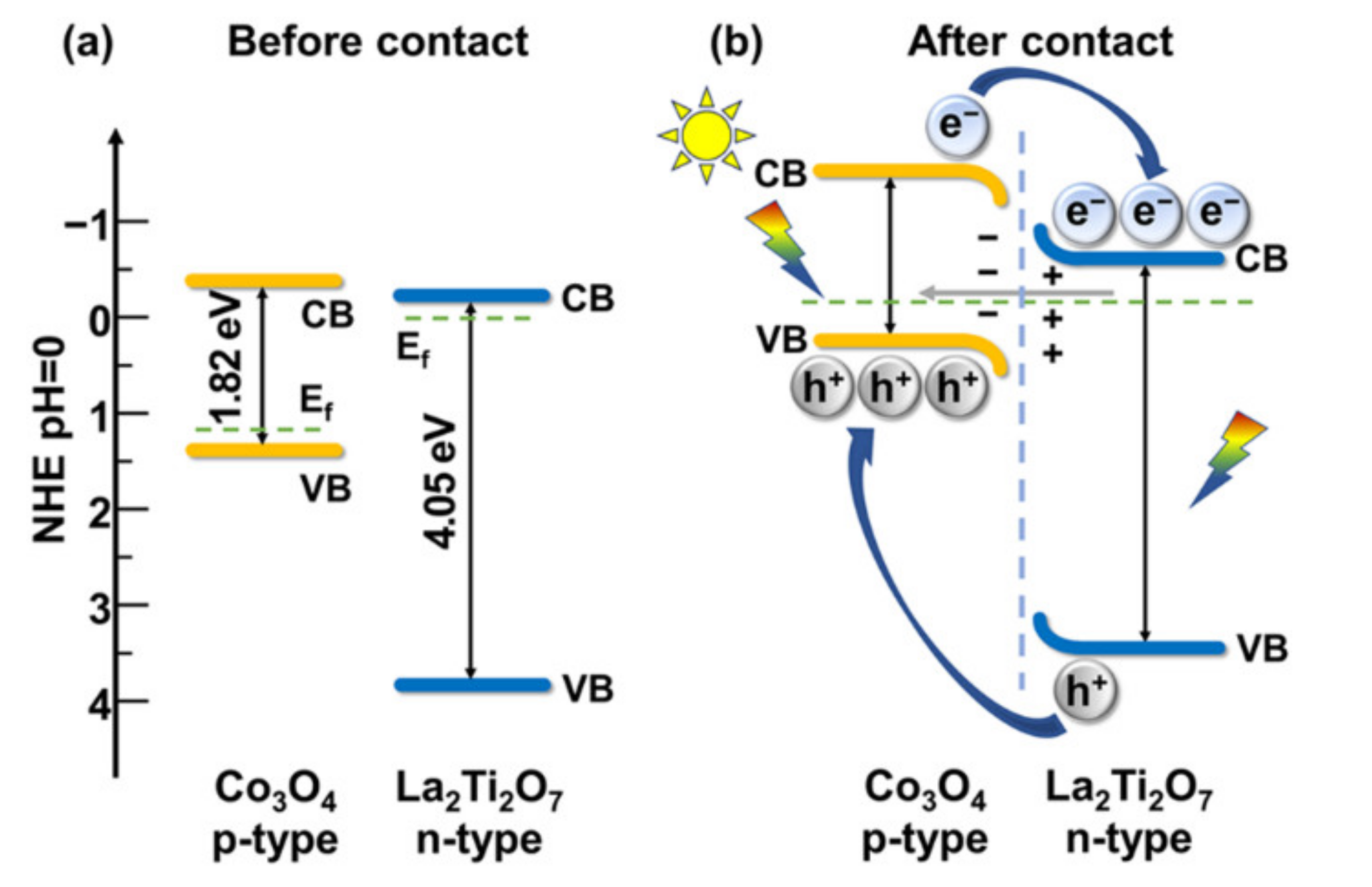
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hydrogen Basics. Available online: https://www.nrel.gov/research/eds-hydrogen.html (accessed on 1 April 2022).
- Sun, B.; Zhou, W.; Li, H.; Ren, L.; Qiao, P.; Li, W.; Fu, H. Synthesis of particulate hierarchical tandem heterojunctions toward optimized photocatalytic hydrogen production. Adv. Mater. 2018, 30, 1804282. [Google Scholar] [CrossRef] [PubMed]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Chi, B.; Pu, J.; Jian, L. Novel heterojunction photocatalysts based on lanthanum titanate nanosheets and indium oxide nanoparticles with enhanced photocatalytic hydrogen production activity. J. Mater. Chem. A 2014, 2, 19260–19267. [Google Scholar] [CrossRef]
- Yue, J.; Xu, J.; Niu, J.; Chen, M. Synergistic effects of multiple heterojunctions significantly enhance the photocatalytic H2 evolution rate CdS/La2Ti2O7/NiS2 ternary composites. Int. J. Hydrogen Energy 2019, 44, 19603–19613. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Xia, M.; An, H.; Bai, H.; Wei, J.; Yang, B.; Yang, G. Highly efficient charge transfer at 2D/2D layered P-La2Ti2O7/Bi2WO6 contact heterojunctions for upgraded visible-light-driven photocatalysis. Appl. Catal. B Environ. 2020, 261, 118244. [Google Scholar] [CrossRef]
- Wan, S.; Qi, F.; Jin, W.; Guo, X.; Liu, H.; Zhao, J.; Zhang, J.; Tang, C. Construction of ultrafine Ag3PO4 nanoparticle and La2Ti2O7 nanosheet 0D/2D heterojunctions with improved photocatalytic performance. J. Alloys Compd. 2018, 740, 901–909. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, X.; Bai, X.; Lv, L.; Ye, Z.; Huang, X. Nitrogen-doped perovskite-type La2Ti2O7 decorated on graphene composites exhibiting efficient photocatalytic activity toward bisphenol A in water. J. Colloid Interface Sci. 2015, 450, 45–53. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Wang, H.; Zhu, M.; Yan, H. Hydrothermal synthesis and photocatalytic properties of layered La2Ti2O7 nanosheets. Nanotechnology 2006, 17, 4863. [Google Scholar] [CrossRef]
- Yang, Q.-L.; Kang, S.-Z.; Chen, H.; Bu, W.; Mu, J. La2Ti2O7: An efficient and stable photocatalyst for the photoreduction of Cr (VI) ions in water. Desalination 2011, 266, 149–153. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, Y.; Li, S.; Zheng, H.; Wang, H.; Liu, J.; Li, K.; Yan, H. Synthesis and photocatalytic properties of the graphene–La2Ti2O7 nanocomposites. Chem. Eng. J. 2011, 178, 468–474. [Google Scholar] [CrossRef]
- Onozuka, K.; Kawakami, Y.; Imai, H.; Yokoi, T.; Tatsumi, T.; Kondo, J.N. Perovskite-type La2Ti2O7 mesoporous photocatalyst. J. Solid State Chem. 2012, 192, 87–92. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Z.; Tian, Y.; Hu, S.; Jia, L.; Chi, B.; Pu, J.; Li, J. Charge-compensated (Nb, Fe)-codoped La2Ti2O7 photocatalyst for photocatalytic H2 production and optical absorption. J. Alloys Compd. 2017, 709, 277–284. [Google Scholar] [CrossRef]
- Wang, R.; Xu, D.; Liu, J.; Li, K.; Wang, H. Preparation and photocatalytic properties of CdS/La2Ti2O7 nanocomposites under visible light. Chem. Eng. J. 2011, 168, 455–460. [Google Scholar] [CrossRef]
- Nashim, A.; Parida, K. n-La2Ti2O7/p-LaCrO3: A novel heterojunction based composite photocatalyst with enhanced photoactivity towards hydrogen production. J. Mater. Chem. A 2014, 2, 18405–18412. [Google Scholar] [CrossRef]
- Xia, M.; Yan, X.; Li, H.; Wells, N.; Yang, G. Well-designed efficient charge separation in 2D/2D N doped La2Ti2O7/ZnIn2S4 heterojunction through band structure/morphology regulation synergistic effect. Nano Energy 2020, 78, 105401. [Google Scholar] [CrossRef]
- Hao, X.; Xiang, D.; Jin, Z. Amorphous Co3O4 quantum dots hybridizing with 3D hexagonal CdS single crystals to construct a 0D/3D p–n heterojunction for a highly efficient photocatalytic H2 evolution. Dalton Trans. 2021, 50, 10501–10514. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Zuo, G.; Guo, Y.; Xiao, W.; Dai, Y.; Kong, J.; Xu, X.; Zhou, Y.; Xie, A.; et al. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B Environ. 2020, 278, 119298. [Google Scholar] [CrossRef]
- Shinde, V.R.; Mahadik, S.B.; Gujar, T.P.; Lokhande, C.D. Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl. Surf. Sci. 2006, 252, 7487–7492. [Google Scholar] [CrossRef]
- Huang, J.; Sheng, H.; Ross, R.D.; Han, J.; Wang, X.; Song, B.; Jin, S. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat. Commun. 2021, 12, 3036. [Google Scholar] [CrossRef] [PubMed]
- Tüysüz, H.; Hwang, Y.J.; Khan, S.B.; Asiri, A.M.; Yang, P. Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res. 2013, 6, 47–54. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Yang, L.; Zhang, M.; Zhu, H.; Wang, F.; Yin, J. Co3O4 imbedded g-C3N4 heterojunction photocatalysts for visible-light-driven hydrogen evolution. Renew. Energy 2020, 145, 691–698. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Wang, Y.; Jin, Z. Co3O4/CeO2 pn heterojunction construction and application for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 33809–33822. [Google Scholar] [CrossRef]
- Wang, L.; Tang, G.; Liu, S.; Dong, H.; Liu, Q.; Sun, J.; Tang, H. Interfacial active-site-rich 0D Co3O4/1D TiO2 pn heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 428, 131338. [Google Scholar] [CrossRef]
- Yu, C.; Li, M.; Yang, D.; Pan, K.; Yang, F.; Xu, Y.; Yuan, L.; Qu, Y.; Zhou, W. NiO nanoparticles dotted TiO2 nanosheets assembled nanotubes PN heterojunctions for efficient interface charge separation and photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 568, 150981. [Google Scholar] [CrossRef]
- Ao, Y.; Wang, K.; Wang, P.; Wang, C.; Hou, J. Synthesis of novel 2D-2D pn heterojunction BiOBr/La2Ti2O7 composite photocatalyst with enhanced photocatalytic performance under both UV and visible light irradiation. Appl. Catal. B Environ. 2016, 194, 157–168. [Google Scholar] [CrossRef]
- Dong, Y.; He, K.; Yin, L.; Zhang, A. A facile route to controlled synthesis of Co3O4 nanoparticles and their environmental catalytic properties. Nanotechnology 2007, 18, 435602. [Google Scholar] [CrossRef]
- Lou, X.; Han, J.; Chu, W.; Wang, X.; Cheng, Q. Synthesis and photocatalytic property of Co3O4 nanorods. Mater. Sci. Eng. B 2007, 137, 268–271. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2016, 128, 5363–5367. [Google Scholar] [CrossRef]
- Hu, S.; Jia, L.; Chi, B.; Pu, J.; Jian, L. Visible light driven (Fe, Cr)-codoped La2Ti2O7 photocatalyst for efficient photocatalytic hydrogen production. J. Power Source 2014, 266, 304–312. [Google Scholar] [CrossRef]
- Hsieh, S.-H.; Lee, G.-J.; Chen, C.-Y.; Chen, J.-H.; Ma, S.-H.; Horng, T.-L.; Chen, K.-H.; Wu, J.J. Hydrothermal synthesis of mesoporous Bi2O3/Co3O4 microsphere and photocatalytic degradation of orange II dyes by visible light. Top. Catal. 2013, 56, 623–629. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, M.; Shi, H.; Wang, C.; Fan, J.; Liu, E.; Hu, X. CuInS2 sensitized TiO2 for enhanced photodegradation and hydrogen production. Ceram. Int. 2019, 45, 6093–6101. [Google Scholar] [CrossRef]
- Meng, F.; Hong, Z.; Arndt, J.; Li, M.; Zhi, M.; Yang, F.; Wu, N. Visible light photocatalytic activity of nitrogen-doped La2Ti2O7 nanosheets originating from band gap narrowing. Nano Res. 2012, 5, 213–221. [Google Scholar] [CrossRef]
- Leng, M.; Huang, X.; Xiao, W.; Ding, J.; Liu, B.; Du, Y.; Xue, J. Enhanced oxygen evolution reaction by Co-OC bonds in rationally designed Co3O4/graphene nanocomposites. Nano Energy 2017, 33, 445–452. [Google Scholar] [CrossRef]
- Moon, H.S.; Yong, K. Noble-metal free photocatalytic hydrogen generation of CuPc/TiO2 nanoparticles under visible-light irradiation. Appl. Surf. Sci. 2020, 530, 147215. [Google Scholar] [CrossRef]
- Boppella, R.; Choi, C.H.; Moon, J.; Kim, D.H. Spatial charge separation on strongly coupled 2D-hybrid of rGO/La2Ti2O7/NiFe-LDH heterostructures for highly efficient noble metal free photocatalytic hydrogen generation. Appl. Catal. B Environ. 2018, 239, 178–186. [Google Scholar] [CrossRef]
- Huang, B.; Yang, W.; Wen, Y.; Shan, B.; Chen, R. Co3O4-modified TiO2 nanotube arrays via atomic layer deposition for improved visible-light photoelectrochemical performance. ACS Appl. Mater. Interfaces 2015, 7, 422–431. [Google Scholar] [CrossRef]
- Paramanik, L.; Reddy, K.H.; Parida, K.M. An energy band compactable B-rGO/PbTiO3 p–n junction: A highly dynamic and durable photocatalyst for enhanced photocatalytic H2 evolution. Nanoscale 2019, 11, 22328–22342. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Y.; Wei, Z.; Yang, S.; He, H.; Sun, C. Fabrication of a novel p–n heterojunction photocatalyst n-BiVO4@ p-MoS2 with core–shell structure and its excellent visible-light photocatalytic reduction and oxidation activities. Appl. Catal. B Environ. 2016, 185, 242–252. [Google Scholar] [CrossRef]
- Luo, S.; Ke, J.; Yuan, M.; Zhang, Q.; Xie, P.; Deng, L.; Wang, S. CuInS2 quantum dots embedded in Bi2WO6 nanoflowers for enhanced visible light photocatalytic removal of contaminants. Appl. Catal. B Environ. 2018, 221, 215–222. [Google Scholar] [CrossRef]
- Jin, Z.; Cao, Y. Cube Cu2O modified CoAL-LDH p-n heterojunction for photocatalytic hydrogen evolution. Int. J. Energy Res. 2021, 45, 19014–19027. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Zeng, G.-M. Novel p–n heterojunction BiOI/CeO2 photocatalyst for wider spectrum visible-light photocatalytic degradation of refractory pollutants. Dalton Trans. 2017, 46, 4982–4993. [Google Scholar] [CrossRef] [PubMed]
- Swain, G.; Sultana, S.; Parida, K. One-Pot-architectured Au-nanodot-promoted MoS2/ZnIn2S4: A novel p–n heterojunction photocatalyst for enhanced hydrogen production and phenol degradation. Inorg. Chem. 2019, 58, 9941–9955. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Zhao, W.; Han, X. Constructing Co3O4/La2Ti2O7 p-n Heterojunction for the Enhancement of Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 1695. https://doi.org/10.3390/nano12101695
Wen H, Zhao W, Han X. Constructing Co3O4/La2Ti2O7 p-n Heterojunction for the Enhancement of Photocatalytic Hydrogen Evolution. Nanomaterials. 2022; 12(10):1695. https://doi.org/10.3390/nano12101695
Chicago/Turabian StyleWen, Haodong, Wenning Zhao, and Xiuxun Han. 2022. "Constructing Co3O4/La2Ti2O7 p-n Heterojunction for the Enhancement of Photocatalytic Hydrogen Evolution" Nanomaterials 12, no. 10: 1695. https://doi.org/10.3390/nano12101695





