Synthesis, Fabrication, and Characterization of Functionalized Polydiacetylene Containing Cellulose Nanofibrous Composites for Colorimetric Sensing of Organophosphate Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Aldehyde-Functionalized PCDA Ester (PCDA-HBA)
2.2.2. Fabrication of PCDA-HBA Containing Cellulose Acetate Nanocomposite Fibers
Preparation of Electrospinning Solution
Electrospinning of CA-PCDA-HBA Nanocomposite Fibers
Deacetylation of CA-PCDA-HBA into RC-PCDA-HBA Nanocomposite Fibers
Photopolymerization of RC-PCDA-HBA Nanocomposite Fibers
2.2.3. Characterization of Nanocomposite Fibers
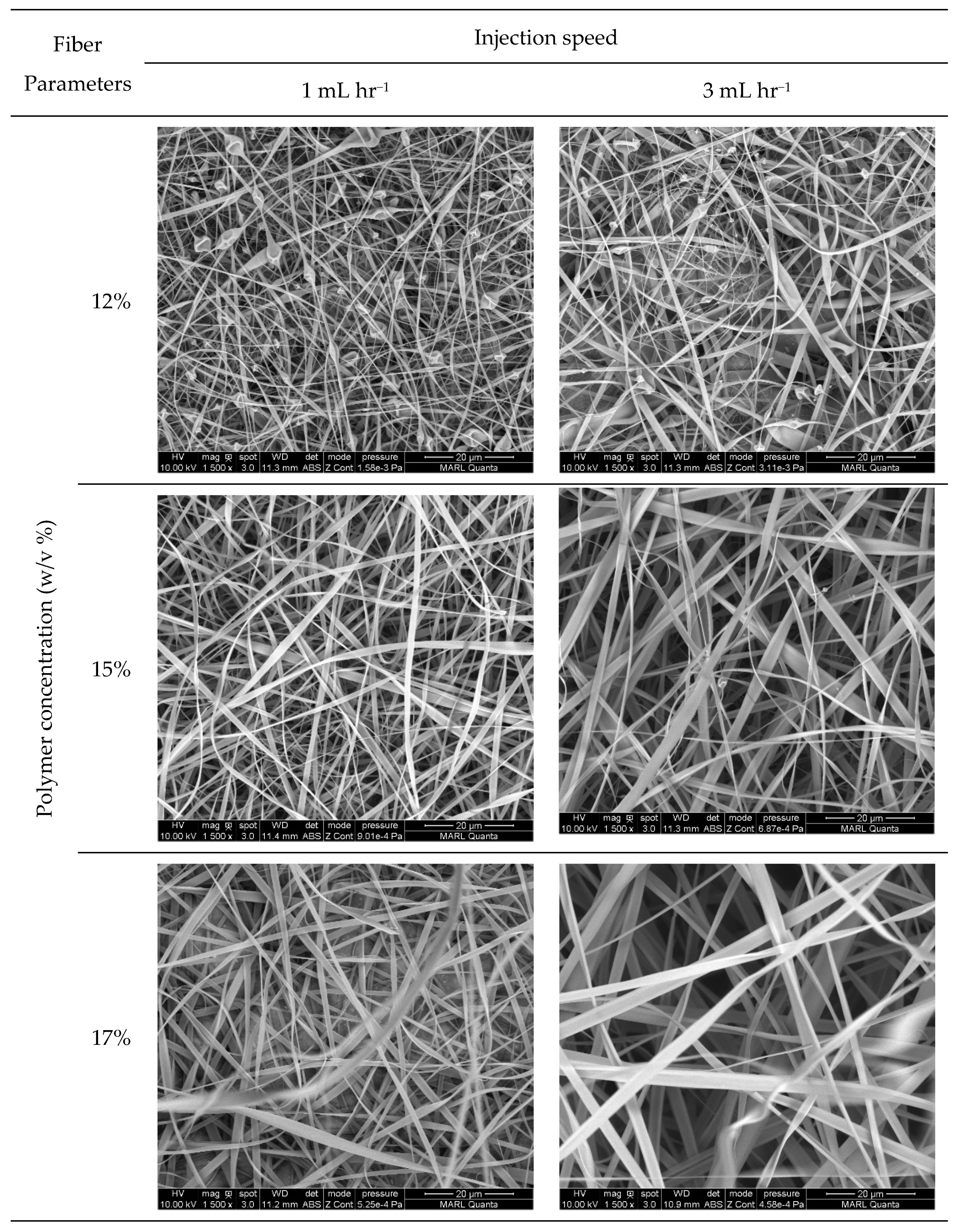
Morphology
Confirmation of the Regeneration of RC-PCDA-HBA Nanocomposites
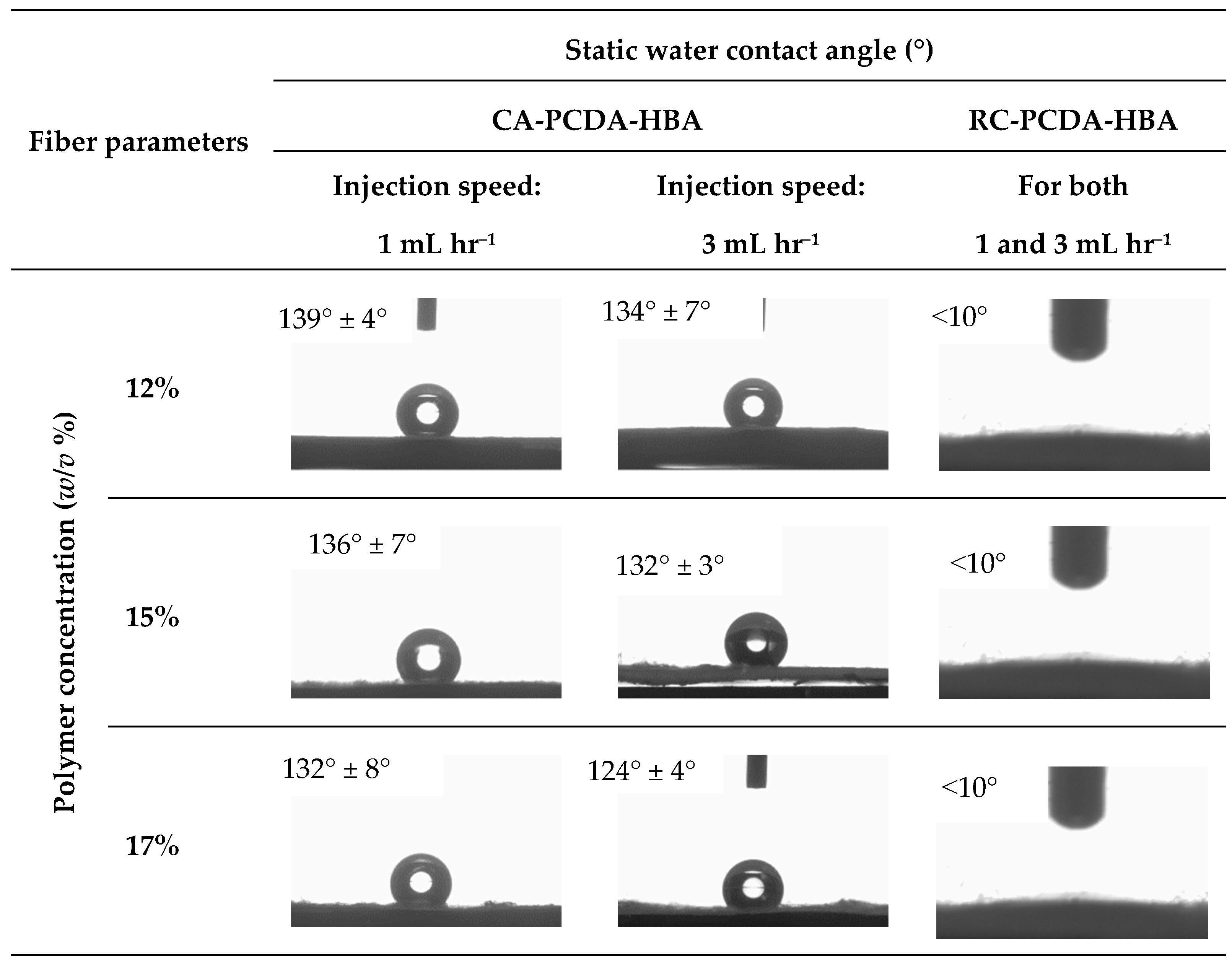
Contact Angle
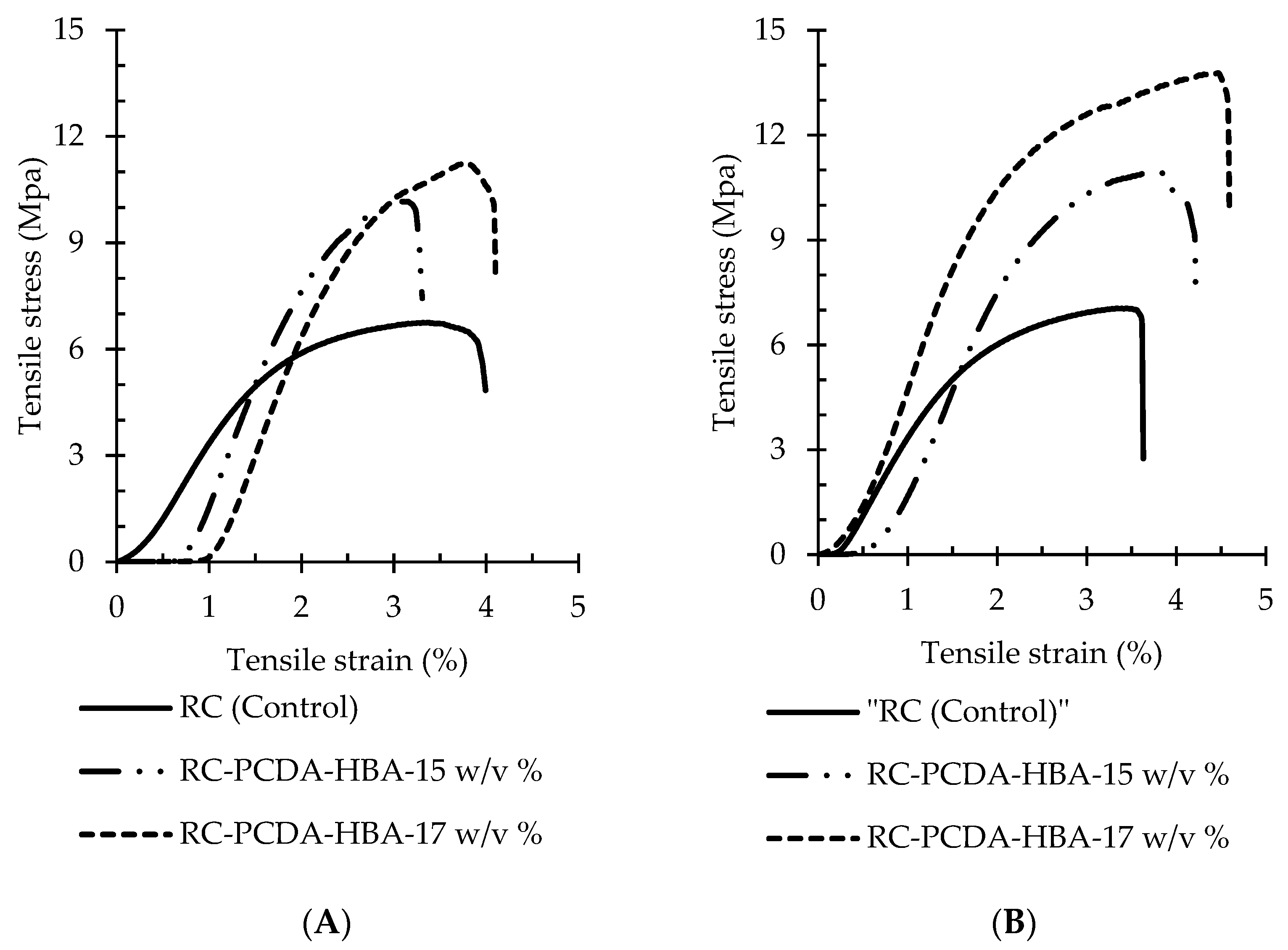
Tensile Properties
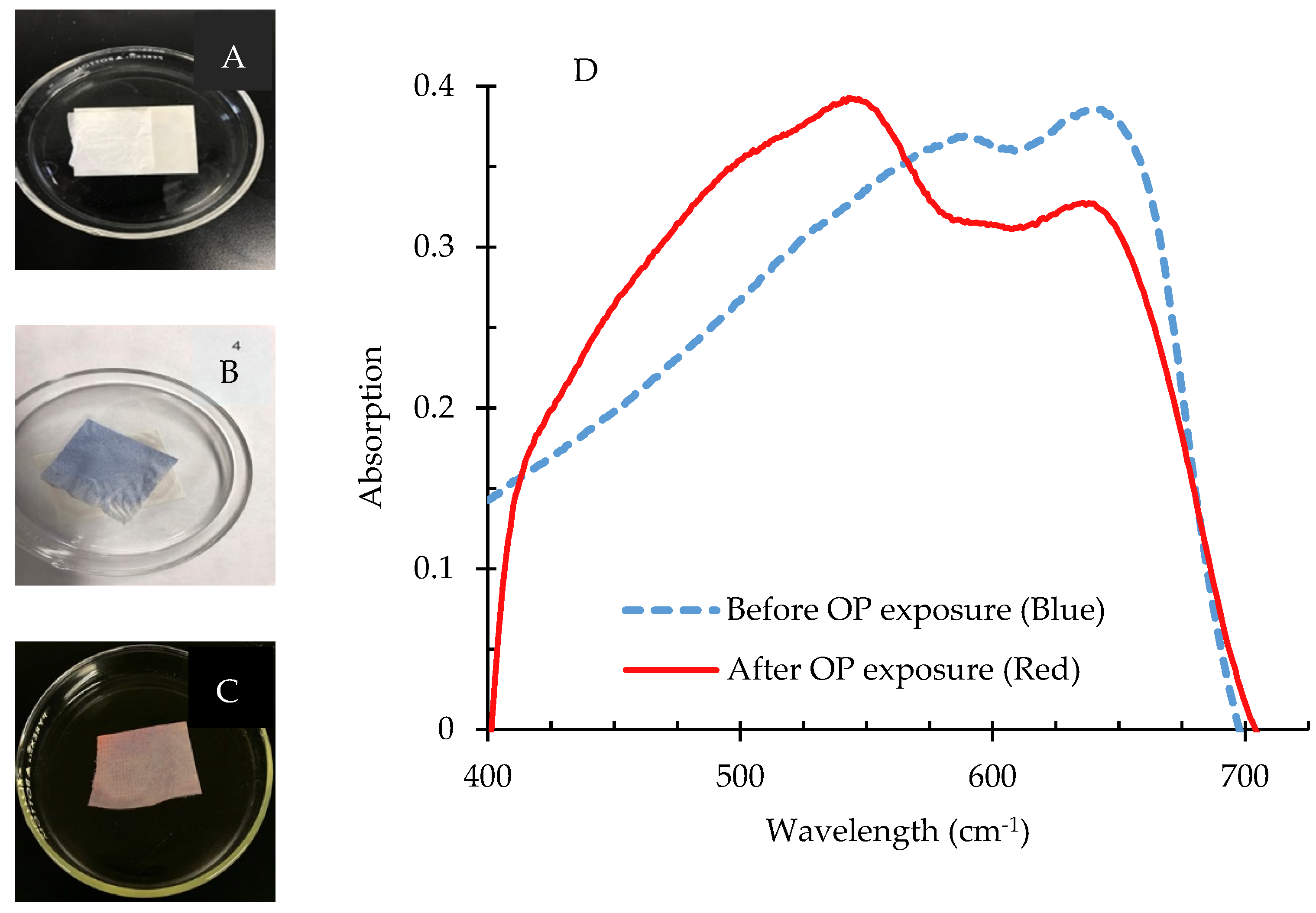
UV–Vis Spectroscopy
3. Results and Discussion
3.1. Synthesis of PCDA-HBA
3.2. Fabrication of CA-PCDA-HBA Composite NF
3.3. Characterizations
3.3.1. Morphology of the Nanocomposite Fibers
3.3.2. Regeneration of CA-PCDA-HBA Nanocomposite Fibers into RC-PCDA-HBA
3.3.3. Tensile Properties of the Nanocomposite Fibers
3.3.4. Application of PCDA-HBA for Sensing of OP Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, S.; Zhang, G.; Dennison, G.H.; FitzGerald, N.; Burn, P.L.; Gentle, I.R.; Shaw, P.E. Challenges in Fluorescence Detection of Chemical Warfare Agent Vapors Using Solid-State Films. Adv. Mater. 2020, 32, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Bromberg, L.; Lin, Z.; Brown, P.; Van Voorhis, T.; Hatton, T.A. Polydiacetylene Functionalized with Charged Termini for Device-Free Colorimetric Detection of Malathion. J. Colloid Interface Sci. 2018, 528, 27–35. [Google Scholar] [CrossRef]
- Bapat, G.; Labade, C.; Chaudhari, A.; Zinjarde, S. Silica Nanoparticle Based Techniques for Extraction, Detection, and Degradation of Pesticides. Adv. Colloid Interface Sci. 2016, 237, 1–14. [Google Scholar] [CrossRef]
- Vopham, T.; Brooks, M.M.; Yuan, J.-M.; Talbott, E.O.; Ruddell, D.; Hart, J.E.; Chang, C.-C.H.; Weissfeld, J.L. Pesticide Exposure and Hepatocellular Carcinoma Risk: A Case-Control Study Using a Geographic Information System (GIS) to Link SEER-Medicare and California Pesticide Data. Environ. Res. 2015, 143 Pt A, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Seo, S.; Kim, J. Colorimetric Detection of Warfare Gases by Polydiacetylenes toward Equipment-Free Detection. Adv. Funct. Mater. 2012, 22, 1632–1638. [Google Scholar] [CrossRef]
- Li, X.; Cui, H.; Zeng, Z. A Simple Colorimetric and Fluorescent Sensor to Detect Organophosphate Pesticides Based on Adenosine Triphosphate-Modified Gold Nanoparticles. Sensors 2018, 18, 4302. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon Dot-Based Bioplatform for Dual Colorimetric and Fluorometric Sensing of Organophosphate Pesticides. Sens. Actuators B. Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Su, X. Review of Optical Sensors for Pesticides. Fresenius’ Z. Anal. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Yapor, J.P.; Alharby, A.; Gentry-Weeks, C.; Reynolds, M.M.; Alam, A.M.; Li, Y.V. Polydiacetylene Nanofiber Composites as a Colorimetric Sensor Responding to Escherichia Coli and PH. ACS Omega 2017, 2, 7334–7342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragay, G.; Pino, F.; Merkoçi, A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef] [PubMed]
- Pavase, T.R.; Lin, H.; Shaikh, Q.-U.-A.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent Advances of Conjugated Polymer (CP) Nanocomposite-Based Chemical Sensors and Their Applications in Food Spoilage Detection: A Comprehensive Review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Alam, A.K.M.M. Development and Thermal Characterization of Polydiacetylene (PDA) Nanofiber Composites for Smart Wound Dressing Applications. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2016. [Google Scholar]
- Alam, A.M.; Yapor, J.P.; Reynolds, M.M.; Li, Y.V. Study of Polydiacetylene-Poly (Ethylene Oxide) Electrospun Fibers Used as Biosensors. Materials 2016, 9, 202. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Peng, X.; Yoon, J. Biosensors and Chemosensors Based on the Optical Responses of Polydiacetylenes. Chem. Soc. Rev. 2012, 41, 4610–4630. [Google Scholar] [CrossRef]
- Wang, D.E.; Wang, Y.; Tian, C.; Zhang, L.; Han, X.; Tu, Q.; Yuan, M.; Chen, S.; Wang, J. Polydiacetylene Liposome-Encapsulated Alginate Hydrogel Beads for Pb2+ Detection with Enhanced Sensitivity. J. Mater. Chem. A 2015, 3, 21690–21695. [Google Scholar] [CrossRef]
- Xia, H.; Li, J.; Zou, G.; Zhang, Q.; Jia, C. A Highly Sensitive and Reusable Cyanide Anion Sensor Based on Spiropyran Functionalized Polydiacetylene Vesicular Receptors. J. Mater. Chem. A 2013, 1, 10713–10719. [Google Scholar] [CrossRef]
- Chen, X.; Lee, J.; Jou, M.J.; Kim, J.M.; Yoon, J. Colorimetric and Fluorometric Detection of Cationic Surfactants Based on Conjugated Polydiacetylene Supramolecules. Chem. Commun. 2009, 3434–3436. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Lee, J.; Kim, M.H.; Yoon, J. Polydiacetylene-Based Electrospun Fibers for Detection of HCl Gas. Macromol. Rapid Commun. 2012, 33, 972–976. [Google Scholar] [CrossRef]
- Kim, M.; Shin, Y.J.; Hwang, S.W.; Shin, M.J.; Shin, J.S. Chromatic Detection of Glucose Using Polymerization of Diacetylene Vesicle. J. Appl. Polym. Sci. 2018, 135, 5–9. [Google Scholar] [CrossRef]
- Wang, D.E.; Zhang, Y.; Li, T.; Tu, Q.; Wang, J. Self-Immolative Trigger-Initiated Polydiacetylene Probe for β-Glucuronidase Activity. RSC Adv. 2014, 4, 16820–16823. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, T.; Chen, Y.; Chen, T.; Chi, B.; Wang, F.; Chen, X. A Polydiacetylenes-Based Colorimetric and Fluorescent Probe for L-Arginine and L-Lysine and Its Application for Logic Gate. Sensors Actuators B Chem. 2018, 255, 2211–2217. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, J.; Dong, W.; Wang, C.; Jin, W.; Xia, Y.; Wang, H.; Ding, H.; Jiang, L.; He, H. Development and Evaluation of a Polydiacetylene Based Biosensor for the Detection of H5 Influenza Virus. J. Virol. Methods 2015, 219, 38–45. [Google Scholar] [CrossRef]
- Wu, J.; Zawistowski, A.; Ehrmann, M.; Yi, T.; Schmuck, C. Peptide Functionalized Polydiacetylene Liposomes Act as a Fluorescent Turn-on Sensor for Bacterial Lipopolysaccharide. J. Am. Chem. Soc. 2011, 133, 9720–9723. [Google Scholar] [CrossRef]
- Verma, N.; Bhardwaj, A. Biosensor Technology for Pesticides—A Review. Appl. Biochem. Biotechnol. 2015, 175, 3093–3119. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What Are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Bastiaansen, C.W.M.; Peijs, T. High Strength and High Modulus Electrospun Nanofibers. Fibers 2014, 2, 158–187. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, J.M. Fabrication of Conjugated Polymer Supramolecules in Electrospun Micro/Nanofibers. Macromol. Chem. Phys. 2008, 209, 2194–2203. [Google Scholar] [CrossRef]
- Moazeni, N.; Sadrjahani, M.; Merati, A.A.; Latifi, M.; Rouhani, S. Effect of Stimuli-Responsive Polydiacetylene on the Crystallization and Mechanical Properties of PVDF Nanofibers. Polym. Bull. 2020, 77, 5373–5388. [Google Scholar] [CrossRef]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A.P. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose Acetate Electrospun Nanofibers for Drug Delivery Systems: Applications and Recent Advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crops Prod. 2017, 97, 646–671. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-Mediated Electroconductive Self-Healing Hydrogels with High Strength, Plasticity, Viscoelasticity, Stretchability, and Biocompatibility toward Multifunctional Applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Zhang, C.; Salick, M.R.; Cordie, T.M.; Ellingham, T.; Dan, Y.; Turng, L.S. Incorporation of Poly(Ethylene Glycol) Grafted Cellulose Nanocrystals in Poly(Lactic Acid) Electrospun Nanocomposite Fibers as Potential Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2015, 49, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Thyagarajan, R.; Hoen, M.E.; Yeung, H.F.; Ford, D.M.; Schiffman, J.D. Transport of Microorganisms into Cellulose Nanofiber Mats. RSC Adv. 2016, 6, 24438–24445. [Google Scholar] [CrossRef]
- Alam, A.K.M.M.; Ewaldz, E.; Xiang, C.; Qu, W.; Bai, X. Tunable Wettability of Biodegradable Multilayer Sandwich-Structured Electrospun Nanofibrous Membranes. Polymers 2020, 12, 2092. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Quan, Z.; Zhang, H.; Qin, X.; Wang, R.; Yu, J. Electrospun Cellulose Acetate Nanofiber Upscaling with a Metal Plate Needleless Spinneret. Mater. Res. Express 2020, 6, 1250e4. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.S. Development of Polydiacetylene Embedded Polyurethane Nanocomposites as a Mask for Sensing and Filtering Fine Dust. Fibers Polym. 2021, 22, 489–497. [Google Scholar] [CrossRef]
- Hassan, F.; Gentry-Weeks, C.; Reynolds, M.; Li, Y.V. Study on Microstructure and Mechanical Properties of Polydiacetylene Composite Biosensors. J. Appl. Polym. Sci. 2019, 136, 1–14. [Google Scholar] [CrossRef]
- Dale, T.J.; Rebek, J. Hydroxy Oximes as Organophosphorus Nerve Agent Sensors. Angew. Chem. Int. Ed. 2009, 48, 7850–7852. [Google Scholar] [CrossRef]



| Solution Concentration | Voltage | Distance | Injection Speed |
|---|---|---|---|
| (% w/v) | (kV) | (cm) | (mL h−1) |
| 12 | 15 | 10 | 1 |
| 12 | 15 | 10 | 3 |
| 15 | 15 | 10 | 1 |
| 15 | 15 | 10 | 3 |
| 17 | 15 | 10 | 1 |
| 17 | 15 | 10 | 3 |
| Fiber Parameters | Tensile Properties of the Nanocomposie Fibers | ||||
|---|---|---|---|---|---|
| Tensile Strength (MPa) | Young’s Modulus (MPa) | Breaking Elongation, % | |||
| RC | 15 w/v % | 1 mL h−1 | 6.68 ± 1.2 | 384 ± 86 | 3.0 ± 0.8 |
| 3 mL h−1 | 7.72 ± 1.5 | 405 ± 94 | 4.0 ± 0.9 | ||
| RC-PCDA-HBA | 15 w/v % | 1 mL h−1 | 10.08 ± 1.4 | 710 ± 45 | 3.7 ± 0.8 |
| 3 mL h−1 | 11.44 ± 1.7 | 712 ± 183 | 4.6 ± 0.9 | ||
| 17 w/v % | 1 mL h−1 | 11.71 ± 2.5 | 718 ± 82 | 4.1 ± 1.25 | |
| 3 mL h−1 | 14.26 ± 0.9 | 749 ± 114 | 5.26 ± 0.9 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, A.K.M.M.; Jenks, D.; Kraus, G.A.; Xiang, C. Synthesis, Fabrication, and Characterization of Functionalized Polydiacetylene Containing Cellulose Nanofibrous Composites for Colorimetric Sensing of Organophosphate Compounds. Nanomaterials 2021, 11, 1869. https://doi.org/10.3390/nano11081869
Alam AKMM, Jenks D, Kraus GA, Xiang C. Synthesis, Fabrication, and Characterization of Functionalized Polydiacetylene Containing Cellulose Nanofibrous Composites for Colorimetric Sensing of Organophosphate Compounds. Nanomaterials. 2021; 11(8):1869. https://doi.org/10.3390/nano11081869
Chicago/Turabian StyleAlam, A K M Mashud, Donovan Jenks, George A. Kraus, and Chunhui Xiang. 2021. "Synthesis, Fabrication, and Characterization of Functionalized Polydiacetylene Containing Cellulose Nanofibrous Composites for Colorimetric Sensing of Organophosphate Compounds" Nanomaterials 11, no. 8: 1869. https://doi.org/10.3390/nano11081869






