Linking Bi-Metal Distribution Patterns in Porous Carbon Nitride Fullerene to Its Catalytic Activity toward Gas Adsorption
Abstract
:1. Introduction
2. Computational Details
3. Results
3.1. Geometry of Pristine C24N24
3.2. Metal Distribution Patterns
3.2.1. Geometry and Electronic Properties of Bi-Metal Complexes
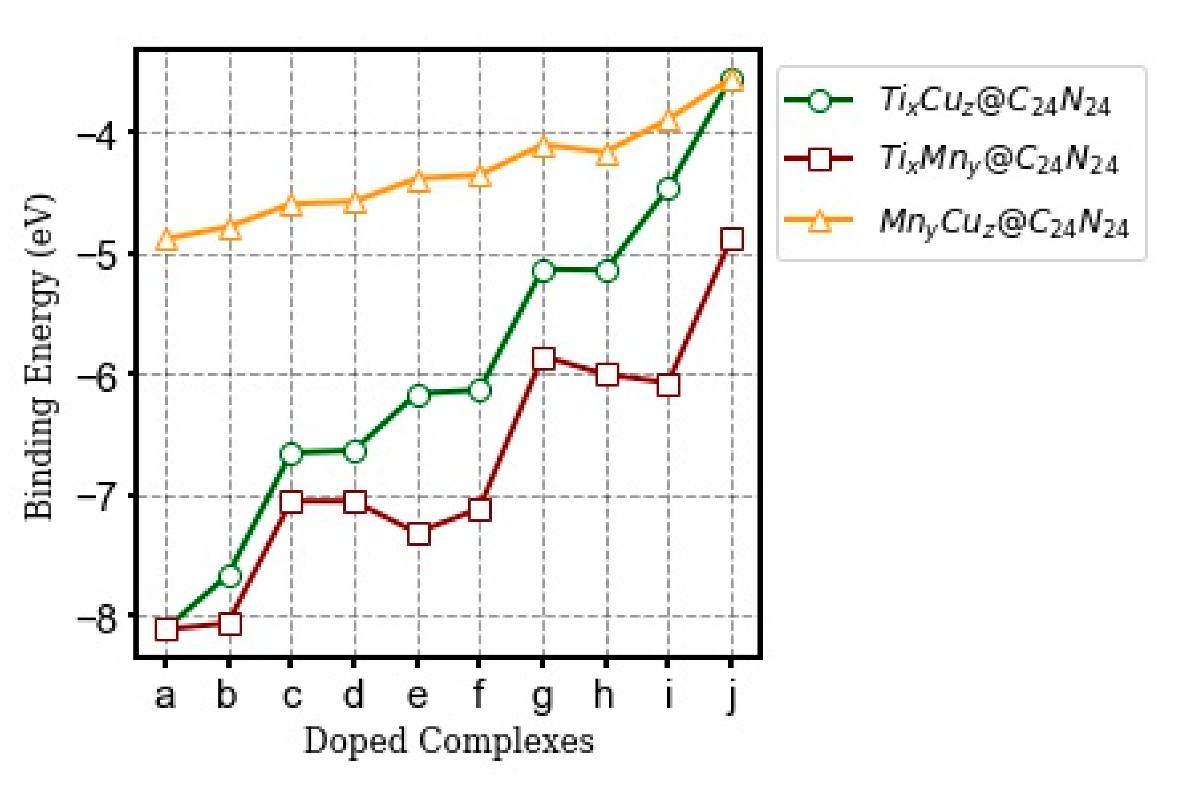
3.2.2. Binding Energy (Eb)
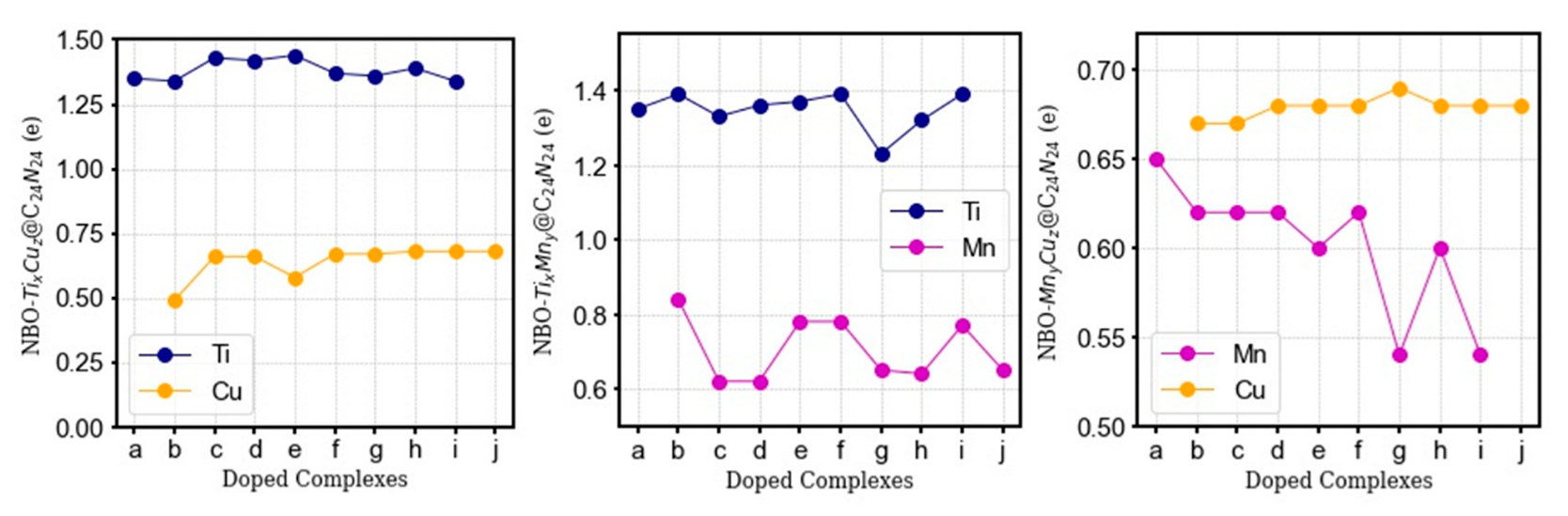
3.2.3. NBO Charge Analysis
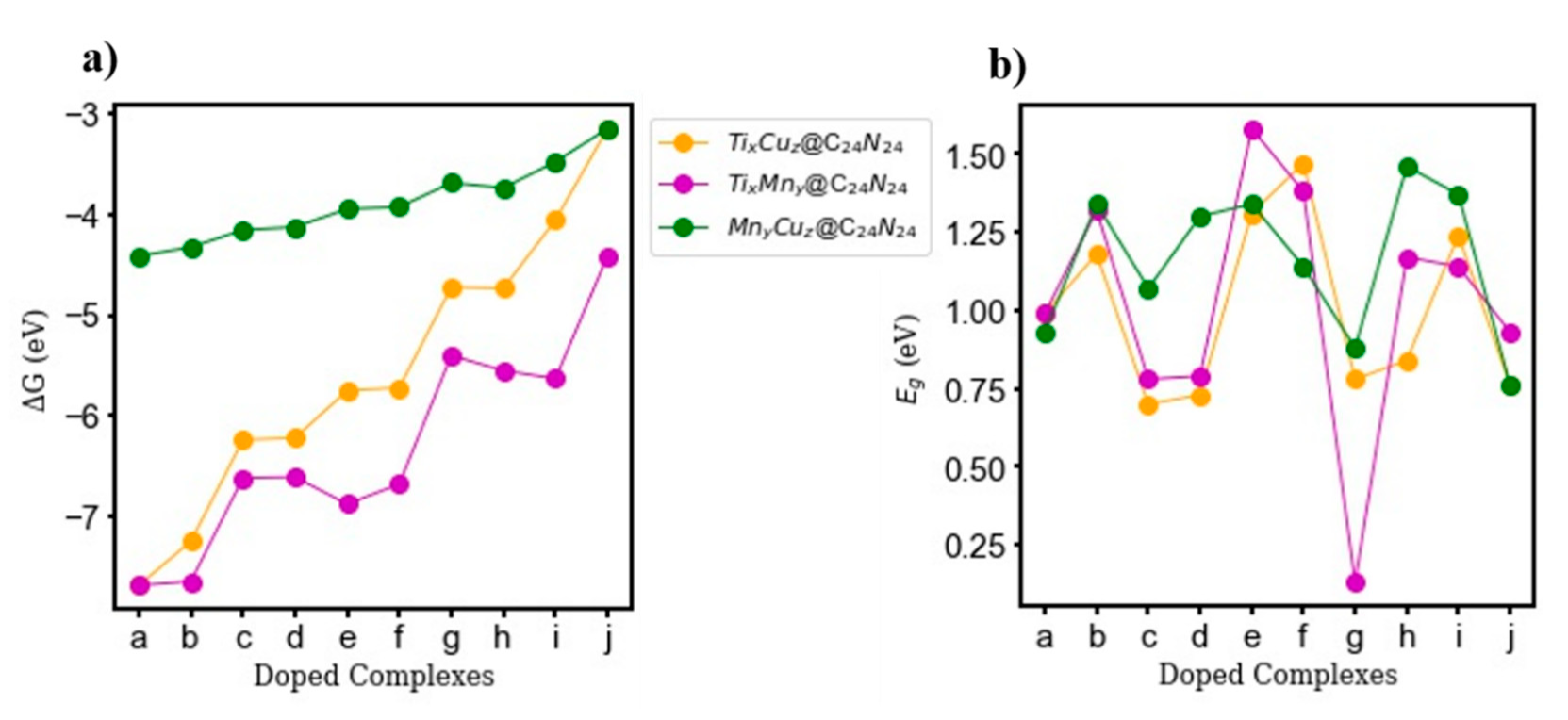
3.2.4. Thermodynamic Properties and Energy Gap (Eg)
4. Catalytic Behavior of Bi-Metal Complexes toward Adsorption of Gas Species
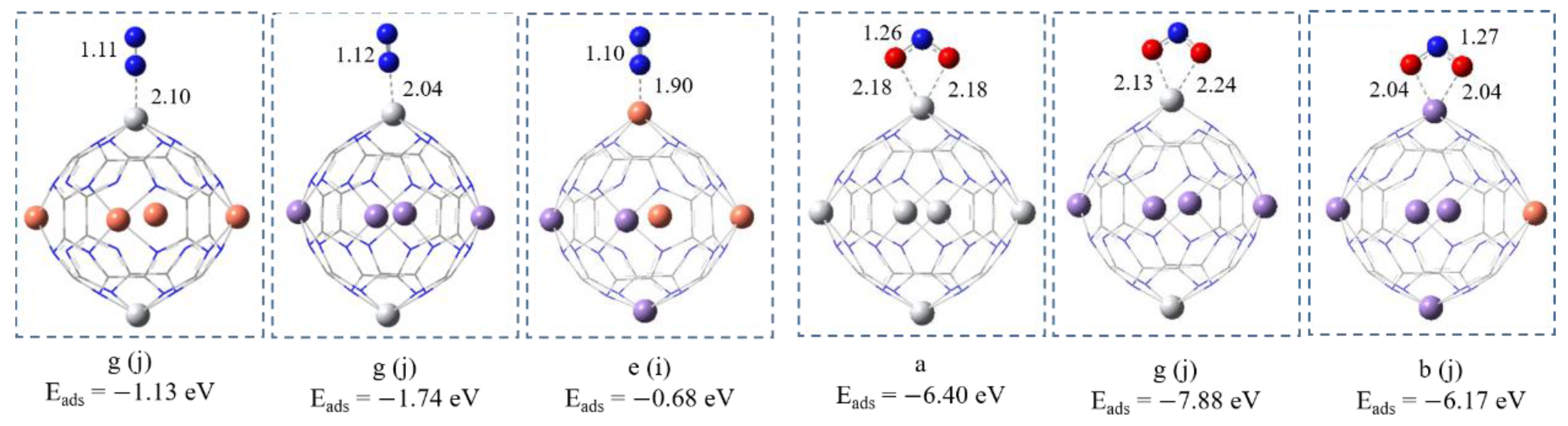
4.1. Geometric Properties
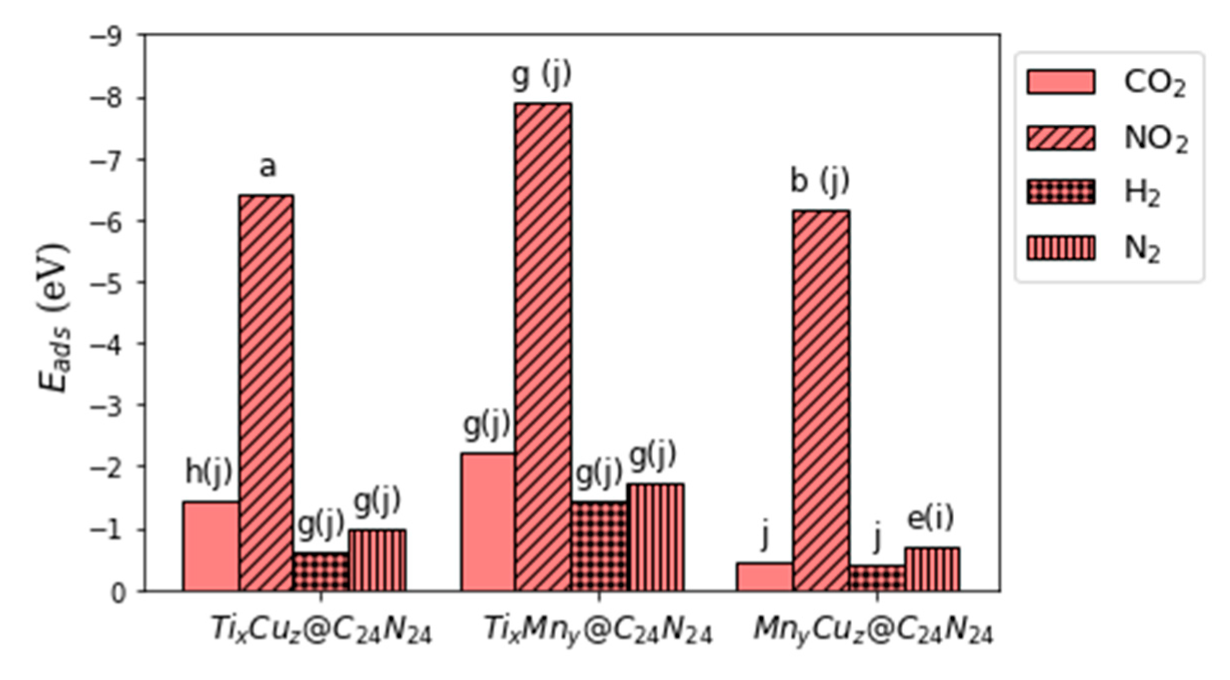
4.2. Adsorption of CO2 and H2
4.3. Adsorption of N2 and NO2
4.4. Electronic and Thermodynamic Properties
4.5. Lifetime of the Adsorbed Gas Species on Bi-Metal Complexes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trogadas, P.; Fuller, T.F.; Strasser, P. Carbon as catalyst and support for electrochemical energy conversion. Carbon 2014, 75, 5–42. [Google Scholar] [CrossRef]
- Srinivasu, K.; Modak, B.; Ghosh, S.K. Porous graphitic carbon nitride: A possible metal-free photocatalyst for water splitting. J. Phys. Chem. C 2014, 118, 26479–26484. [Google Scholar] [CrossRef]
- Albero, J.; Vidal, A.; Migani, A.; Concepción, P.; Blancafort, L.; García, H. Phosphorus-doped graphene as a metal-free material for thermochemical water reforming at unusually mild conditions. ACS Sustain. Chem. Eng. 2018, 7, 838–846. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; He, Q.; Yuan, X.; Huang, D.; et al. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B Environ. 2020, 265, 118579. [Google Scholar] [CrossRef]
- Han, F.; Wang, R.; Feng, Y.; Wang, S.; Liu, L.; Li, X.; Han, Y.; Chen, H. On demand synthesis of hollow fullerene nanostructures. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zettergren, H.; Alcami, M.; Martín, F. First- and second-electron affinities ofC60andC70isomers. Phys. Rev. A 2007, 76, 043205. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, L.; Wang, X.; Hu, Z. Carbon-based nanocages: A new platform for advanced energy storage and conversion. Adv. Mater. 2020, 32, e1904177. [Google Scholar] [CrossRef]
- El Mahdy, A.M. Density functional investigation of CO and NO adsorption on TM-decorated C60 fullerene. Appl. Surf. Sci. 2016, 383, 353–366. [Google Scholar] [CrossRef]
- Coro, J.; Suárez, M.; Silva, L.S.; Eguiluz, K.; Banda, G. Fullerene applications in fuel cells: A review. Int. J. Hydrogen Energy 2016, 41, 17944–17959. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, M.; Song, W.; Wu, Z. Doped fullerene as a metal-free electrocatalyst for oxygen reduction reaction: A first-principles study. Carbon 2017, 114, 393–401. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, Z.; Zhang, Q.; Li, B.; Shen, Z. Fullerene (C60)/CdS nanocomposite with enhanced photocatalytic activity and stability. Appl. Surf. Sci. 2017, 403, 151–158. [Google Scholar] [CrossRef]
- Sood, P.; Kim, K.C.; Jang, S.S. Electrochemical properties of boron-doped fullerene derivatives for lithium-ion battery ap-plications. ChemPhysChem 2018, 19, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Chang, J.; Ke, Q. Probing the activity of pure and N-doped fullerenes towards oxygen reduction reaction by density functional theory. Carbon 2018, 126, 53–57. [Google Scholar] [CrossRef]
- Hultman, L.; Stafström, S.; Czigány, Z.; Neidhardt, J.; Hellgren, N.; Brunell, I.F.; Suenaga, K.; Colliex, C. Cross-linked nano-onions of carbon nitride in the solid phase: Existence of a novel C 48 N 12 Aza-fullerene. Phys. Rev. Lett. 2001, 87, 225503. [Google Scholar] [CrossRef] [PubMed]
- Otero, G.; Biddau, G.; Sanchez, C.S.; Caillard, R.; López, M.F.; Rogero, C.; Palomares, F.J.; Cabello, N.; Basanta, M.A.; Ortega, J.; et al. Fullerenes from aromatic precursors by surface-catalysed cyclodehydrogenation. Nat. Cell Biol. 2008, 454, 865–868. [Google Scholar] [CrossRef]
- Usachov, D.; Vilkov, O.; Grüneis, A.; Haberer, D.; Fedorov, A.; Adamchuk, V.; Preobrajenski, A.B.; Dudin, P.; Barinov, A.; Oehzelt, M.; et al. Nitrogen-doped graphene: Efficient growth, structure, and electronic properties. Nano Lett. 2011, 11, 5401–5407. [Google Scholar] [CrossRef]
- Zhai, Z.; Shen, H.; Chen, J.; Li, X.; Jiang, Y. Direct growth of nitrogen-doped graphene films on glass by plasma-assisted hot filament CVD for enhanced electricity generation. J. Mater. Chem. A 2019, 7, 12038–12049. [Google Scholar] [CrossRef]
- Hayashi, A.; Xie, Y.; Poblet, J.M.; Campanera, J.M.; Lebrilla, C.B.; Balch, A.L. Mass spectrometric and computational studies of heterofullerenes ([C58Pt]-, [C59Pt]+) Obtained by laser ablation of electrochemically deposited films. J. Phys. Chem. A 2004, 108, 2192–2198. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, J.-X.; Cai, Q.-H.; Wang, X.-G.; Wang, X.-Z. Doping of calcium in C60Fullerene for enhancing CO2Capture and N2O transformation: A theoretical study. J. Phys. Chem. A 2011, 115, 9969–9976. [Google Scholar] [CrossRef]
- He, T.; Santiago, A.R.P.; Du, A. Atomically embedded asymmetrical dual-metal dimers on N-doped graphene for ultra-efficient nitrogen reduction reaction. J. Catal. 2020, 388, 77–83. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, T.; Liang, X.; Deng, X.; Guo, W.; Wang, Z.; Lu, X.; Wu, C.-M.L. Single transition metal atoms on nitrogen-doped carbon for CO2 electrocatalytic reduction: CO production or further CO reduction? Appl. Surf. Sci. 2020, 533, 147466. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Y.; Liu, S.; Cheng, Z.; Tan, Y.; Shen, Z. Single cobalt atom anchored on N-doped graphyne for boosting the overall water splitting. Appl. Surf. Sci. 2020, 502, 144155. [Google Scholar] [CrossRef]
- Asset, T.; Atanassov, P. Iron-nitrogen-carbon catalysts for proton exchange membrane fuel cells. Joule 2020, 4, 33–44. [Google Scholar] [CrossRef]
- Nematollahi, P.; Neyts, E. Direct methane conversion to methanol on M and MN4 embedded graphene (M=Ni and Si): A comparative DFT study. Appl. Surf. Sci. 2019, 496, 143618. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, L.; Li, K.; Yin, C.; Tang, H.; Wang, Y.; Wu, Z. RuN4 doped graphene oxide, a highly efficient bifunctional catalyst for oxygen reduction and CO2 reduction from computational study. ACS Sustain. Chem. Eng. 2019, 7, 8136–8144. [Google Scholar] [CrossRef]
- Holst-Olesen, K.; Silvioli, L.; Rossmeisl, J.; Arenz, M. Enhanced oxygen reduction reaction on Fe/N/C catalyst in acetate buffer electrolyte. ACS Catal. 2019, 9, 3082–3089. [Google Scholar] [CrossRef]
- Jia, Y.; Xiong, X.; Wang, D.; Duan, X.; Sun, K.; Li, Y.; Zheng, L.; Lin, W.; Dong, M.; Zhang, G.; et al. Atomically dispersed Fe-N4 modified with precisely located S for highly efficient oxygen reduction. Nano-Micro Lett. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Chung, H.T.; Cullen, D.A.; Higgins, D.; Sneed, B.T.; Holby, E.F.; More, K.L.; Zelenay, P. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 2017, 357, 479–484. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Zitolo, A.; Ranjbar-Sahraie, N.; Mineva, T.; Emiliano, F.; Jia, Q.; Stamatin, S.; Harrington, G.F.; Lyth, S.M.; Krtil, P.; Mukerjee, S.; et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kattel, S.; Wang, G. A density functional theory study of oxygen reduction reaction on Me–N4 (Me = Fe, Co, or Ni) clusters between graphitic pores. J. Mater. Chem. A 2013, 1. [Google Scholar] [CrossRef]
- Kattel, S.; Atanassov, P.; Kiefer, B. Density functional theory study of Ni–Nx/C electrocatalyst for oxygen reduction in al-kaline and acidic media. J. Phys. Chem. C. 2012, 116, 17378–17383. [Google Scholar] [CrossRef]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef]
- Bi, W.; Li, X.; You, R.; Chen, M.; Yuan, R.; Huang, W.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. Surface immobilization of transition metal ions on nitrogen-doped graphene realizing high-efficient and selective CO2 reduction. Adv. Mater. 2018, 30, e1706617. [Google Scholar] [CrossRef]
- Zhang, C.; Sha, J.; Fei, H.; Liu, M.; Yazdi, S.; Zhang, J.; Zhong, Q.; Zou, X.; Zhao, N.; Yu, H.; et al. Single-atomic ruthenium catalytic sites on nitrogen-doped graphene for oxygen reduction reaction in acidic medium. ACS Nano 2017, 11, 6930–6941. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Liu, Z.; Liu, S.; Zhao, X.; Huang, K. High-capacity hydrogen storage medium: Ti doped fullerene. Appl. Phys. Lett. 2011, 98, 023107. [Google Scholar] [CrossRef]
- Tang, C.; Chen, S.; Zhu, W.; Kang, J.; He, X.; Zhang, Z. Transition metal Ti coated porous fullerene C24B24: Potential material for hydrogen storage. Int. J. Hydrogen Energy 2015, 40, 16271–16277. [Google Scholar] [CrossRef]
- Ma, L.-J.; Hao, W.; Han, T.; Rong, Y.; Jia, J.; Wu, H.-S. Sc/Ti decorated novel C24N24 cage: Promising hydrogen storage materials. Int. J. Hydrogen Energy 2021, 46, 7390–7401. [Google Scholar] [CrossRef]
- Modak, B.; Srinivasu, K.; Ghosh, S.K. Exploring metal decorated porphyrin-like porous fullerene as catalyst for oxygen re-duction reaction: A DFT study. Int. J. Hydrogen Energy 2017, 42, 2278–2287. [Google Scholar] [CrossRef]
- Shakerzadeh, E.; Hamadi, H.; Esrafili, M.D. Computational mechanistic insights into CO oxidation reaction over Fe decorated C24N24 fullerene. Inorg. Chem. Commun. 2019, 106, 190–196. [Google Scholar] [CrossRef]
- Ogliaro, F.; Bearpark, M.; Heyd, J.; Brothers, E.; Kudin, K.; Staroverov, V.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K. Gaussian 16; Revision A. 03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- De Lara-Castells, M.P.; Stoll, H.; Civalleri, B.; Causà, M.; Voloshina, E.; Mitrushchenkov, A.O.; Pi, M. Communication: A combined periodic density functional and incremental wave-function-based approach for the dispersion-accounting time-resolved dynamics of 4He nanodroplets on surfaces: 4He/graphene. J. Chem. Phys. 2014, 141, 151102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasu, K.; Ghosh, S.K. Transition metal decorated porphyrin-like porous fullerene: Promising materials for molecular hydrogen adsorption. J. Phys. Chem. C 2012, 116, 25184–25189. [Google Scholar] [CrossRef]
- Ochterski, J.W. Thermochemistry in Gaussian; Gaussian Inc.: Wallingford, CT, USA, 2000. [Google Scholar]
- Wiberg, K. Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 1968, 24, 1083–1096. [Google Scholar] [CrossRef]
- Song, Y.-D.; Wang, L.; Wu, L.-M. How the alkali metal atoms affect electronic structure and the nonlinear optical properties of C24N24 nanocage. Optik 2017, 135, 139–152. [Google Scholar] [CrossRef]
- Holcomb, D.; Kittel, C. Introduction to solid state physics. Am. J. Phys. 1967, 35. [Google Scholar] [CrossRef] [Green Version]
- Philipsen, P.; Baerends, E. Cohesive energy of 3D transition metals: Density functional theory atomic and bulk calculations. Phys. Rev. B 1996, 54, 5326. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; He, J.; Yang, Y.; Xia, Z.; Feng, Y.; Ma, J. Fe, V-co-doped C2N for electrocatalytic N2-to-NH3 conversion. J. Energy Chem. 2021, 53, 303–308. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, H.; Chen, J.; Wu, M.; Leung, D.Y.; Grimes, C.A.; Lu, Z.; Xu, Z.; Feng, S.-P. Synergistic effects of Pd–Ag bimetals and g-C3N4 photocatalysts for selective and efficient conversion of gaseous CO2. J. Power Sources 2020, 466, 228306. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A review on bimetallic nickel-based catalysts for CO2 reforming of methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Yang, X.-F.; Wang, A.-Q.; Li, L.; Liu, X.-Y.; Zhang, T.; Mou, C.-Y.; Li, J. Bimetallic Au–Pd alloy catalysts for N2O decomposition: Effects of surface structures on catalytic activity. J. Phys. Chem. C. 2012, 116, 6222–6232. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, M.; Li, Z.; Du, A.; Searles, D.J. Carbon dioxide capture and gas separation on B80Fullerene. J. Phys. Chem. C 2014, 118, 2170–2177. [Google Scholar] [CrossRef]
- Li, J.; Hou, M.; Chen, Y.; Cen, W.; Chu, Y.; Yin, S. Enhanced CO2 capture on graphene via N, S dual-doping. Appl. Surf. Sci. 2017, 399, 420–425. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Deshpande, M.D.; Hussain, T.; Ahuja, R. Investigating CO2 storage properties of C2N monolayer functionalized with small metal clusters. J. CO2 Util. 2020, 35, 1–13. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, H.; Cheng, X.; Tang, Y. Transition metal atom Fe, Co, Ni decorated B38 fullerene: Potential material for hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 15256–15261. [Google Scholar] [CrossRef]
- Chakraborty, B.; Ray, P.; Garg, N.; Banerjee, S. High capacity reversible hydrogen storage in titanium doped 2D carbon allotrope Ψ-graphene: Density Functional Theory investigations. Int. J. Hydrogen Energy 2021, 46, 4154–4167. [Google Scholar] [CrossRef]
- Ding, B.; Ong, W.-J.; Jiang, J.; Chen, X.; Li, N. Uncovering the electrochemical mechanisms for hydrogen evolution reaction of heteroatom doped M2C MXene (M = Ti, Mo). Appl. Surf. Sci. 2020, 500, 143987. [Google Scholar] [CrossRef]
- Wang, K.; Gu, G.; Hu, S.; Zhang, J.; Sun, X.; Wang, F.; Li, P.; Zhao, Y.; Fan, Z.; Zou, X. Molten salt assistant synthesis of three-dimensional cobalt doped graphitic carbon nitride for photocatalytic N2 fixation: Experiment and DFT simulation analysis. Chem. Eng. J. 2019, 368, 896–904. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Y.; Wang, S.; Wang, C.; Ma, J. Fe-doped phosphorene for the nitrogen reduction reaction. J. Mater. Chem. A 2018, 6, 13790–13796. [Google Scholar] [CrossRef]
- Qu, M.; Qin, G.; Fan, J.; Du, A.; Sun, Q. Theoretical insights into the performance of single and double transition metal atoms doped on N-graphenes for N2 electroreduction. Appl. Surf. Sci. 2021, 537, 148012. [Google Scholar] [CrossRef]
- DeBoer, J.H. The Dynamical Character of Adsorption. Soil Sci. 1953, 76, 166. [Google Scholar] [CrossRef]

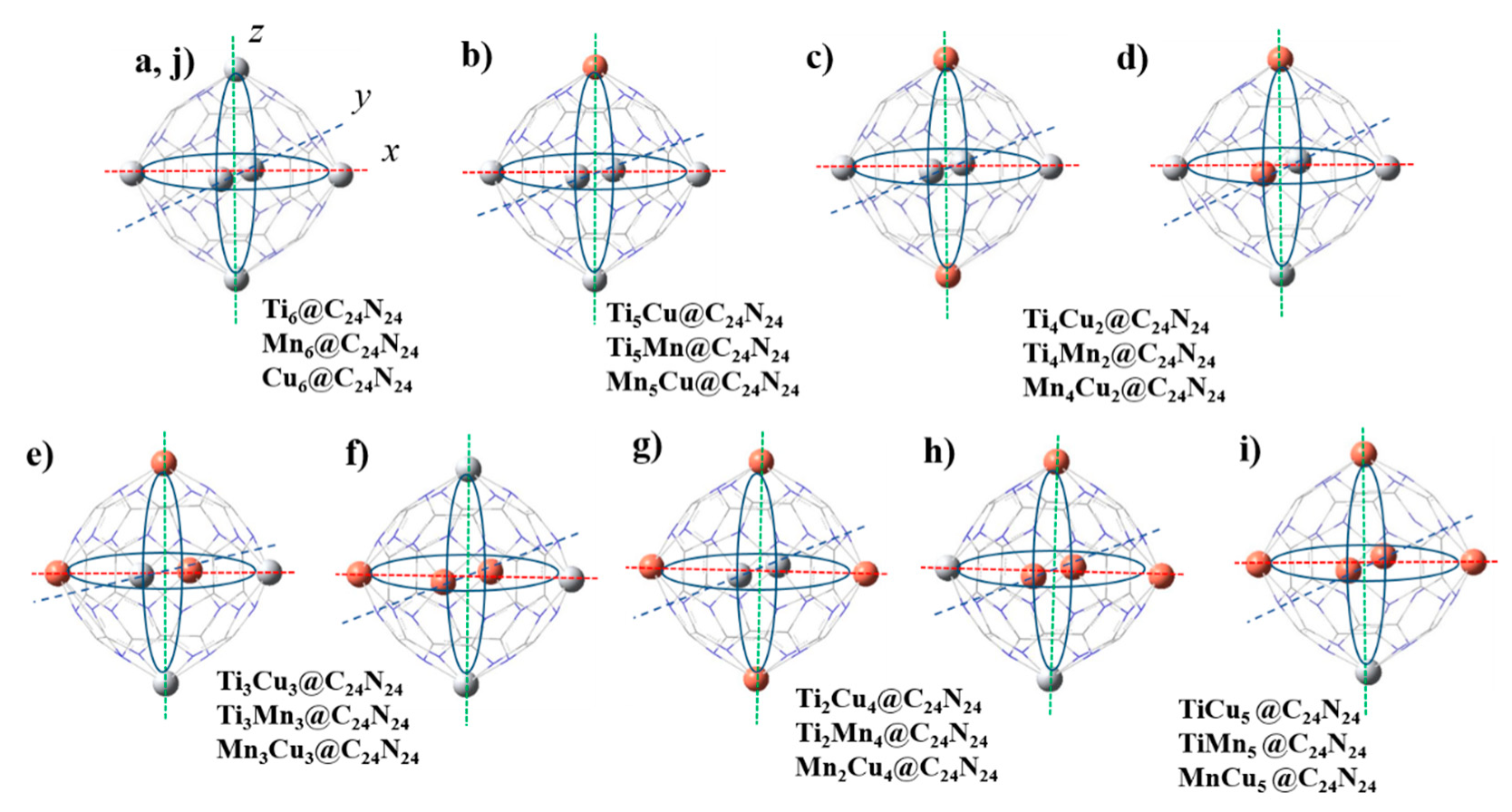


| Complex/Metal-Ratio | 6:0 | 5:1 | 4:2 | 3:3 | 2:4 | 1:5 | 0:6 |
|---|---|---|---|---|---|---|---|
| a | b | c, d | e, f | g, h | i | j | |
| TixCuz@C24N24 | Ti6 | Ti5Cu | Ti4Cu2 | Ti3Cu3 | Ti2Cu4 | TiCu5 | Cu6 |
| TixMny@C24N24 | Ti6 | Ti5Mn | Ti4Mn2 | Ti3Mn3 | Ti2Mn4 | TiMn5 | Mn6 |
| MnyCuz@C24N24 | Mn6 | Mn5Cu | Mn4Cu2 | Mn3Cu3 | Mn2Cu4 | MnCu5 | Cu6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nematollahi, P.; Neyts, E.C. Linking Bi-Metal Distribution Patterns in Porous Carbon Nitride Fullerene to Its Catalytic Activity toward Gas Adsorption. Nanomaterials 2021, 11, 1794. https://doi.org/10.3390/nano11071794
Nematollahi P, Neyts EC. Linking Bi-Metal Distribution Patterns in Porous Carbon Nitride Fullerene to Its Catalytic Activity toward Gas Adsorption. Nanomaterials. 2021; 11(7):1794. https://doi.org/10.3390/nano11071794
Chicago/Turabian StyleNematollahi, Parisa, and Erik C. Neyts. 2021. "Linking Bi-Metal Distribution Patterns in Porous Carbon Nitride Fullerene to Its Catalytic Activity toward Gas Adsorption" Nanomaterials 11, no. 7: 1794. https://doi.org/10.3390/nano11071794







