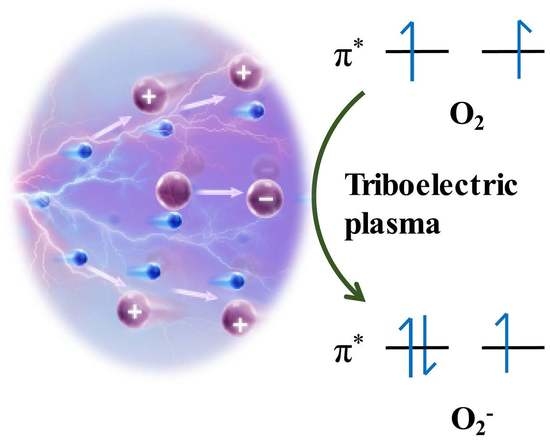
The Regulation of O2 Spin State and Direct Oxidation of CO at Room Temperature Using Triboelectric Plasma by Harvesting Mechanical Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Carbon Monoxide (CO) Oxidation Triggered by Mechanical Energy-Induced Triboelectric Plasma
2.2. Detection of 2O2− Radicals
2.3. Isotope Labeling Experiment
2.4. Isotope Kinetics Effect (KIE) Experiment
2.5. Density Functional Theory (DFT) Calculations
2.6. Triboelectric Plasma Simulation
3. Results and Discussion
3.1. CO Oxidation System Driven by Mechanical Energy-Induced Triboelectric Plasma
3.2. Influence of Different Parameters on the CO Oxidation Activity
3.3. Investigation of Dioxygen Activation by Triboelectric Plasma and Theoretical Calculations
3.4. Theoretical Simulation of Triboelectric Plasma
3.5. Mechanism of the Triboelectric Plasma-Triggered CO Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Behler, J.; Delley, B.; Lorenz, S.; Reuter, K.; Scheffler, M. Dissociation of O2 at Al(111): The role of spin selection rules. Phys. Rev. Lett. 2005, 94, 036104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.M.; Liu, W.Q.; Gao, X.J.; Lu, Z.H.; Wu, X.C.; Gao, X.F. Mechanisms of oxidase and superoxide dismutation-like activities of gold, silver, platinum, and palladium, and their alloys: A general way to the activation of molecular oxygen. J. Am. Chem. Soc. 2015, 137, 15882–15891. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Wang, Z.Y.U.; Wang, H.W.; Lu, J.L.; Yu, S.-H.; Jiang, H.-L. Singlet oxygen-engaged selective photo-oxidation over Pt nanocrystals/porphyrinic MOF: The roles of photothermal effect and Pt electronic state. J. Am. Chem. Soc. 2017, 139, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.S.; Luo, X.; Zhang, X.D.; Xie, J.F.; Jin, S.; Wang, H.; Zheng, X.S.; Wu, X.J.; Xie, Y. Enhanced superoxide generation on defective surfaces for selective photooxidation. J. Am. Chem. Soc. 2019, 141, 3797–3801. [Google Scholar] [CrossRef]
- Zhang, N.; Li, X.Y.; Ye, H.C.; Chen, S.M.; Ju, H.X.; Liu, D.B.; Lin, Y.; Ye, W.; Wang, C.M.; Xu, Q.; et al. Oxide defect engineering enables to couple solar energy into oxygen activation. J. Am. Chem. Soc. 2016, 138, 8928–8935. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.J.; Li, J.; Cheng, G. Selective aerobic oxidation of alkyl aromatics on Bi2MoO6 nanoplates decorated with Pt nanoparticles under visible light irradiation. Chem. Commun. 2018, 54, 12194–12197. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Gao, Y.Y.; Chong, R.F.; Wang, Z.L.; Guo, L.; Zhang, X.W.; Li, C. To boost photocatalytic activity in selective oxidation of alcohols on ultrathin Bi2MoO6 nanoplates with Pt nanoparticles as cocatalyst. J. Catal. 2017, 345, 96–103. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Zhang, B.Q.; Chong, R.F.; Li, R.G.; Yuan, B.; Lu, S.-M.; Li, C. Selective oxidation of sulfides on Pt/BiVO4 photocatalyst under visible light irradiation using water as the oxygen source and dioxygen as the electron acceptor. J. Catal. 2015, 332, 95–100. [Google Scholar] [CrossRef]
- Long, R.; Mao, K.K.; Gong, M.; Zhou, S.; Hu, J.H.; Zhi, M.; You, Y.; Bai, S.; Jiang, J.; Zhang, Q.; et al. Tunable oxygen activation for catalytic organic oxidation: Schottky junction versus plasmonic effects. Angew. Chem. Int. Ed. 2014, 53, 3205–3209. [Google Scholar] [CrossRef]
- Zhang, B.; Li, J.; Guo, L.; Chen, Z.P.; Li, C. Photothermally promoted cleavage of beta-1,4-glycosidic bonds of cellulosic biomass on Ir/HY catalyst under mild conditions. Appl. Catal. B 2018, 237, 660–664. [Google Scholar] [CrossRef]
- Hori, H.; Takashima, M.; Takase, M.; Ohtani, B. Kinetic analysis supporting multielectron reduction of oxygen in bismuth tungstate-photocatalyzed oxidation of organic compounds. Catal. Today 2018, 313, 218–223. [Google Scholar] [CrossRef]
- Setvín, M.; Aschauer, U.; Scheiber, P.; Li, Y.-F.; Hou, W.Y.; Schmid, M.; Selloni, A.; Diebold, U. Reaction of O2 with subsurface oxygen vacancies on TiO2 anatase (101). Science 2013, 341, 988–991. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Zhang, K.-X.; Zhang, B.; Wang, H.-H.; Yu, Q.Y.; Li, X.-H.; Antonietti, M.; Chen, J.S. Activating cobalt nanoparticles via the Mott-Schottky effect in nitrogen-rich carbon shells for base-free aerobic oxidation of alcohols to esters. J. Am. Chem. Soc. 2017, 139, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Jagadeesh, R.V.; Junge, H.; Pohl, M.M.; Radnik, J.; Bruckner, A.; Beller, M. Selective oxidation of alcohols to esters using heterogeneous Co3O4-N@C catalysts under mild conditions. J. Am. Chem. Soc. 2013, 135, 10776–10782. [Google Scholar] [CrossRef]
- Wu, W.T.; Zhang, Q.G.; Wang, X.K.; Han, C.C.; Shao, X.D.; Wang, Y.X.; Liu, J.L.; Li, Z.T.; Lu, X.Q.; Wu, M.B. Enhancing selective photooxidation through Co-Nx-doped carbon materials as singlet oxygen photosensitizers. ACS Catal. 2017, 7, 7267–7273. [Google Scholar] [CrossRef]
- Leung, S.-F.; Fu, H.-C.; Zhang, M.L.; Hassan, A.H.; Jiang, T.; Salama, K.N.; Wang, Z.L.; He, J.-H. Blue energy fuels: Converting ocean wave energy to carbon-based liquid fuels via CO2 reduction. Energy Environ. Sci. 2020, 13, 1300–1308. [Google Scholar] [CrossRef]
- You, H.L.; Wu, Z.; Zhang, L.H.; Ying, Y.R.; Liu, Y.; Fei, L.F.; Chen, X.X.; Jia, Y.M.; Wang, Y.J.; Wang, F.F.; et al. Harvesting the vibration energy of BiFeO3 nanosheets for hydrogen evolution. Angew. Chem. Int. Ed. 2019, 58, 11779–11784. [Google Scholar] [CrossRef]
- Gao, S.Y.; Zhu, Y.Z.; Chen, Y.; Tian, M.; Yang, Y.J.; Jiang, T.; Wang, Z.L. Self-power electroreduction of N2 into NH3 by 3D printed triboelectric nanogenerators. Mater. Today 2019, 28, 17–24. [Google Scholar] [CrossRef]
- Fan, F.R.; Tian, Z.Q.; Wang, Z.L. Flexible triboelectric generator! Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Sahu, M.Š.; Silvija, H.S.; Padhan, A.M.; Živković, P.; Jokić, S.; Kim, H.J. Development of triboelectric nanogenerator and mechanical energy harvesting using argon ion-implanted kapton, zinc oxide and kapton. Mater. Lett. 2021, 301, 130290. [Google Scholar] [CrossRef]
- Seo, J.H.; Sugato, S.; Manisha, K.; Hoe, J. Effect of cilia microstructure and ion injection upon single-electrode triboelectric nanogenerator for effective energy harvesting. Mater. Lett. 2021, 304, 130674. [Google Scholar] [CrossRef]
- Venugopal, K.; Panchatcharam, P.; Chandrasekhar, A.; Shanmugasundaram, V. Comprehensive review on triboelectric nanogenerator based wrist pulse measurement: Sensor fabrication and diagnosis of arterial pressure. ACS Sens. 2021, 6, 1681–1694. [Google Scholar] [CrossRef]
- Chandrasekhar, A.; Vivekananthan, V.; Khandelwal, G.; Kim, W.J.; Kim, S.J. Green energy from working surfaces: A contact electrification–enabled data theft protection and monitoring smart table. Mater. Today Energy 2020, 18, 100544. [Google Scholar] [CrossRef]
- Feng, Y.W.; Ling, L.L.; Nie, J.; Han, K.; Chen, X.Y.; Bian, Z.F.; Li, H.X.; Wang, Z.L. Self-powered electrostatic filter with enhanced photocatalytic degradation of formaldehyde based on built-in triboelectric nanogenerators. ACS Nano 2017, 11, 12411–12418. [Google Scholar] [CrossRef]
- Gao, S.Y.; Su, J.Z.; Wei, X.J.; Wang, M.; Tian, M.; Jiang, T.; Wang, Z.L. Self-powered electrochemical oxidation of 4-aminoazobenzene driven by a triboelectric nanogenerator. ACS Nano 2017, 11, 770–778. [Google Scholar] [CrossRef]
- Cheng, G.; Zheng, H.W.; Yang, F.; Zhao, L.; Zheng, M.L.; Yang, J.J.; Qin, H.F.; Du, Z.L.; Wang, Z.L. Managing and maximizing the output power of a triboelectric nanogenerator by controlled tip-electrode air-discharging and application for UV sensing. Nano Energy 2018, 44, 208–216. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, G.Q.; Zhang, Y.N.; Zhang, B.; Yang, F.; Zhao, L.; Zheng, M.L.; Cheng, G.; Du, Z.L. The self-powered CO2 gas sensor based on gas discharge induced by triboelectric nanogenerator. Nano Energy 2018, 53, 898–905. [Google Scholar] [CrossRef]
- Li, A.Y.; Zi, Y.L.; Guo, H.Y.; Wang, Z.L.; Fernandez, F.M. Triboelectric nanogenerators for sensitive nano-coulomb molecular mass spectrometry. Nat. Nanotechnol. 2017, 12, 481–487. [Google Scholar] [CrossRef]
- Han, K.; Luo, J.J.; Feng, Y.W.; Lai, Q.S.; Bai, Y.; Tang, W.; Wang, Z.L. Wind-driven radial-engine-shaped triboelectric nanogenerators for self-powered absorption and degradation of NOX. ACS Nano 2020, 14, 2751–2759. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, W.B.; Zi, Y.L.; Lu, Y.J.; Ji, L.J.; Liu, F.; Wu, C.S.; Wang, Z.L. Triboelectric microplasma powered by mechanical stimuli. Nat. Commun. 2018, 9, 3733. [Google Scholar] [CrossRef]
- Wong, M.-C.; Xu, W.; Hao, J.H. Microplasma-discharge-based nitrogen fixation driven by triboelectric nanogenerator toward self-powered mechano-nitrogenous fertilizer supplier. Adv. Funct. Mater. 2019, 29, 1904090. [Google Scholar] [CrossRef]
- Goto, H.; Hanada, Y.; Ohno, T.; Matsumura, M. Quantitative analysis of superoxide ion and hydrogen peroxide produced from molecular oxygen on photoirradiated TiO2 particles. J. Catal. 2004, 225, 223–229. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.F.; Shcherbanev, S.; Baron, B.; Starikovskaia, S. Nanosecond surface dielectric barrier discharge in atmospheric pressure air: I. measurements and 2D modeling of morphology, propagation and hydrodynamic perturbations. Plasma Sources Sci. Technol. 2017, 26, 125004. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Starikovskaia, S. Fast gas heating of nanosecond pulsed surface dielectric barrier discharge: Spatial distribution and fractional contribution from kinetics. Plasma Sources Sci. Technol. 2018, 27, 124007. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Wu, Y.; Wei, B.; Xu, H.J.; Liang, H.; Jia, M.; Song, H.M.; Li, Y.H. Nanosecond-pulsed dielectric barrier discharge-based plasma-assisted anti-icing: Modeling and mechanism analysis. J. Phys. D Appl. Phys. 2019, 53, 145205. [Google Scholar] [CrossRef]
- Kulikovsky, A.A. Positive streamer in a weak field in air: A moving avalanche-to-streamer transition. Phys. Rev. E 1997, 57, 7066–7074. [Google Scholar] [CrossRef]
- Flitti, A.; Pancheshnyi, S. Gas heating in fast pulsed discharges in N2–O2 mixtures. Phys. J. Appl. Phys. 2009, 45, 21001. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, A.; Neyts, E.C. Plasma technology: An emerging technology for energy storage. ACS Energy Lett. 2018, 3, 1013–1027. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Barboun, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Catalysis enabled by plasma activation of strong chemical bonds: A review. ACS Energy Lett. 2019, 4, 1115–1133. [Google Scholar] [CrossRef]
- Mehta, P.; Barboun, P.; Herrera, F.A.; Kim, J.; Rumbach, P.; Go, D.B.; Hicks, J.C.; Schneider, W.F. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis. Nat. Catal. 2018, 1, 269–275. [Google Scholar] [CrossRef]
- Chen, J.H.; Davidson, J.H. Model of the negative DC corona plasma comparison to the positive DC corona plasma. Plasma Chem. Plasma Process. 2003, 23, 83–102. [Google Scholar] [CrossRef]
- Denzler, D.N.; Frischkorn, C.; Hess, C.; Wolf, M.; Ertl, G. Electronic excitation and dynamic promotion of a surface reaction. Phys. Rev. Lett. 2003, 91, 226102. [Google Scholar] [CrossRef] [Green Version]
- Funk, S.; Bonn, M.; Denzler, D.N.; Hess, C.; Wolf, M.; Ertl, G. Desorption of CO from Ru(001) induced by near-infrared femtosecond laser pulses. J. Chem. Phys. 2000, 112, 9888–9897. [Google Scholar] [CrossRef]
- Bonn, M.; Funk, S.; Hess, C.; Denzler, D.N.; Stampfl, C.; Scheffler, M.; Wolf, M.; Ertl, C. Phonon-versus electron-mediated desorption and oxidation of CO on Ru(0001). Science 1999, 285, 1042–1045. [Google Scholar] [CrossRef] [Green Version]
- Das, A. A review of time economic innovative mnemonics in chemical education. Int. J. Phys. Chem. Educ. 2018, 10, 27–40. [Google Scholar] [CrossRef]
- Paulson, J.F. Some negative-ion reactions with CO2. J. Chem. Phys. 1970, 52, 963–964. [Google Scholar] [CrossRef]
- Christopher, P.; Xin, H.; Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 2011, 3, 467–472. [Google Scholar] [CrossRef]
- Li, S.M.; Zhang, B.; Gu, G.Q.; Xiang, X.C.; Zhang, W.H.; Shi, X.; Zhao, K.; Zhu, Y.F.; Guo, J.M.; Cui, P.; et al. Triboelectric plasma decomposition of CO2 at room temperature driven by mechanical energy. Nano Energy 2021, 88, 106287. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Li, S.; Zhang, B.; Wang, J.; Xiang, X.; Zhu, Y.; Zhao, K.; Shang, W.; Gu, G.; Guo, J.; et al. The Regulation of O2 Spin State and Direct Oxidation of CO at Room Temperature Using Triboelectric Plasma by Harvesting Mechanical Energy. Nanomaterials 2021, 11, 3408. https://doi.org/10.3390/nano11123408
Shi X, Li S, Zhang B, Wang J, Xiang X, Zhu Y, Zhao K, Shang W, Gu G, Guo J, et al. The Regulation of O2 Spin State and Direct Oxidation of CO at Room Temperature Using Triboelectric Plasma by Harvesting Mechanical Energy. Nanomaterials. 2021; 11(12):3408. https://doi.org/10.3390/nano11123408
Chicago/Turabian StyleShi, Xue, Sumin Li, Bao Zhang, Jiao Wang, Xiaochen Xiang, Yifei Zhu, Ke Zhao, Wanyu Shang, Guangqin Gu, Junmeng Guo, and et al. 2021. "The Regulation of O2 Spin State and Direct Oxidation of CO at Room Temperature Using Triboelectric Plasma by Harvesting Mechanical Energy" Nanomaterials 11, no. 12: 3408. https://doi.org/10.3390/nano11123408