Efficient Hydrogen Generation and Total Nitrogen Removal for Urine Treatment in a Neutral Solution Based on a Self-Driving Nano Photoelectrocatalytic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Preparation of the Electrodes
2.3. Experimental Setup
2.4. Analytical Methods
3. Results and Discussion
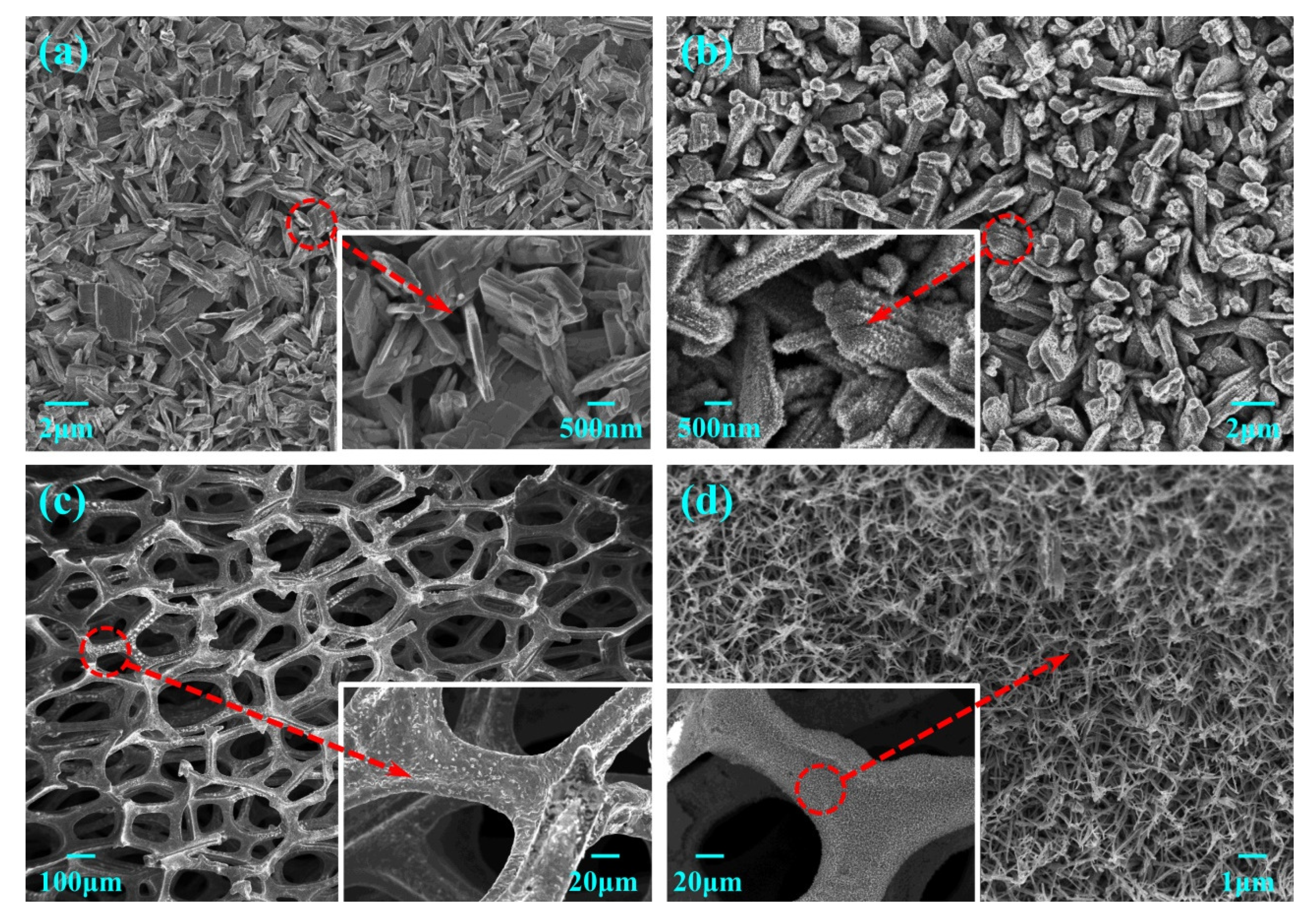
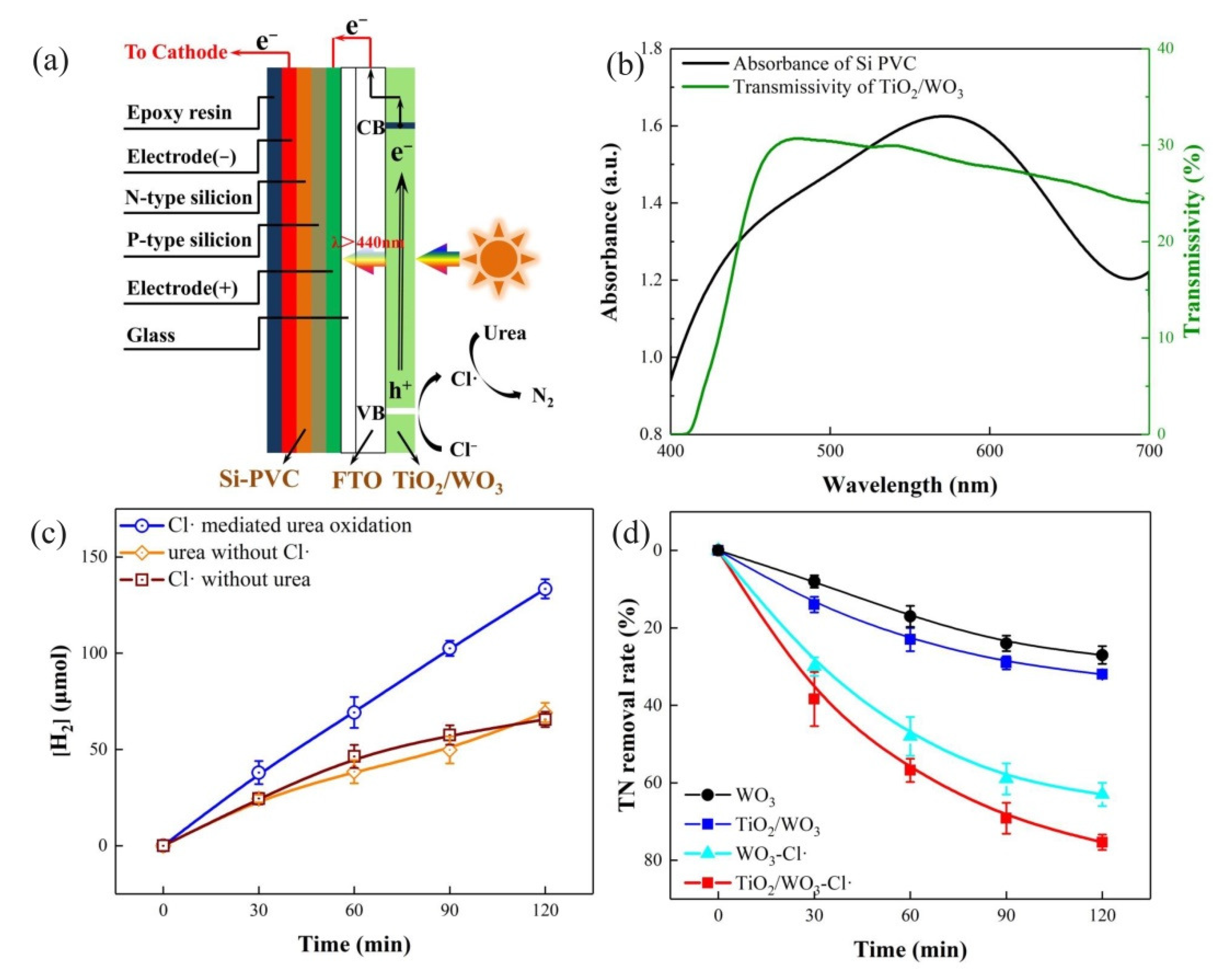
3.1. Characterization of the Electrodes
3.2. Hydrogen Generation and Total Nitrogen Removal
3.3. Influence Factors
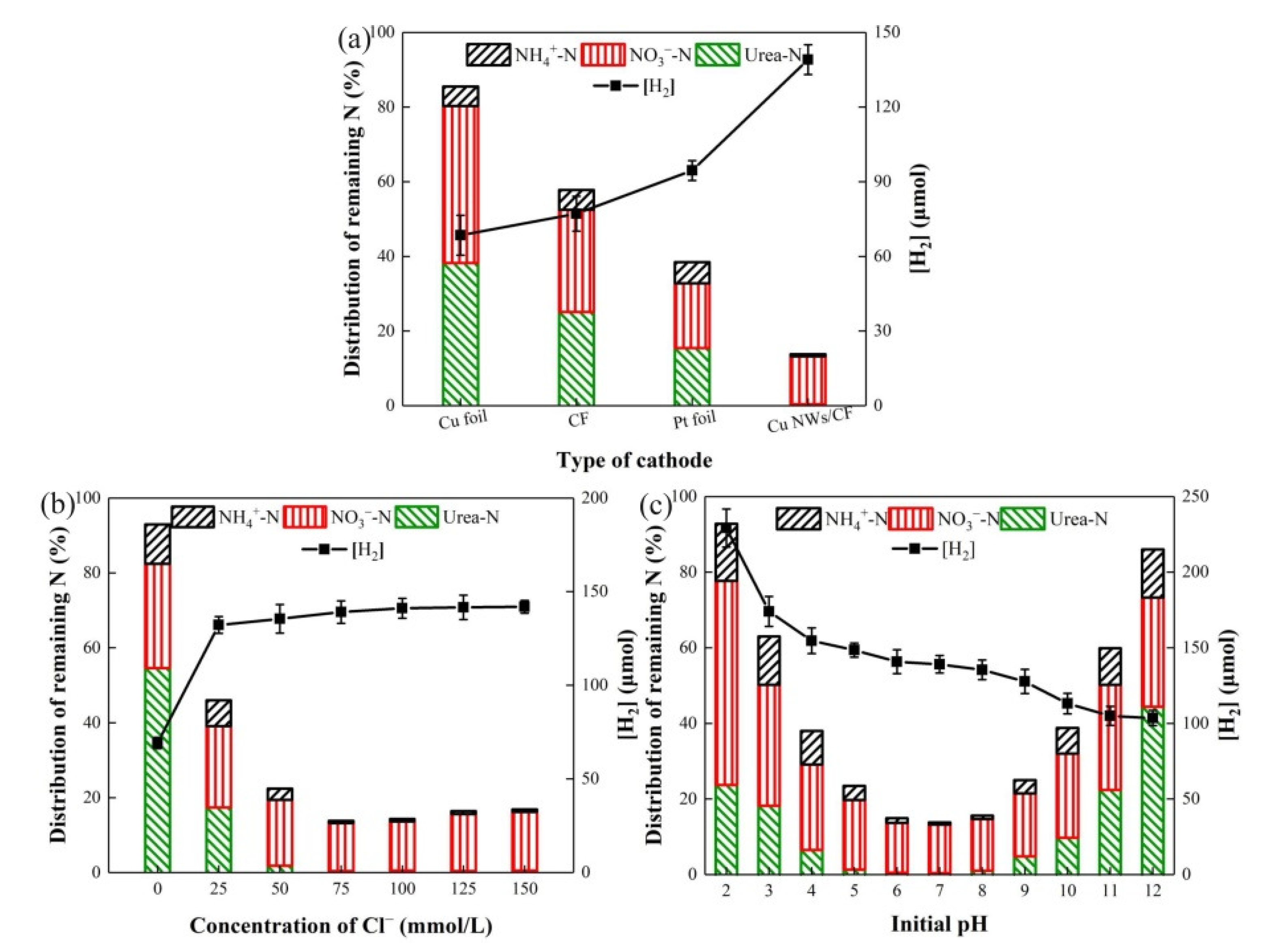
3.3.1. Effect of Cathode Type
3.3.2. Effect of Cl− Concentration
3.3.3. Effect of Initial pH
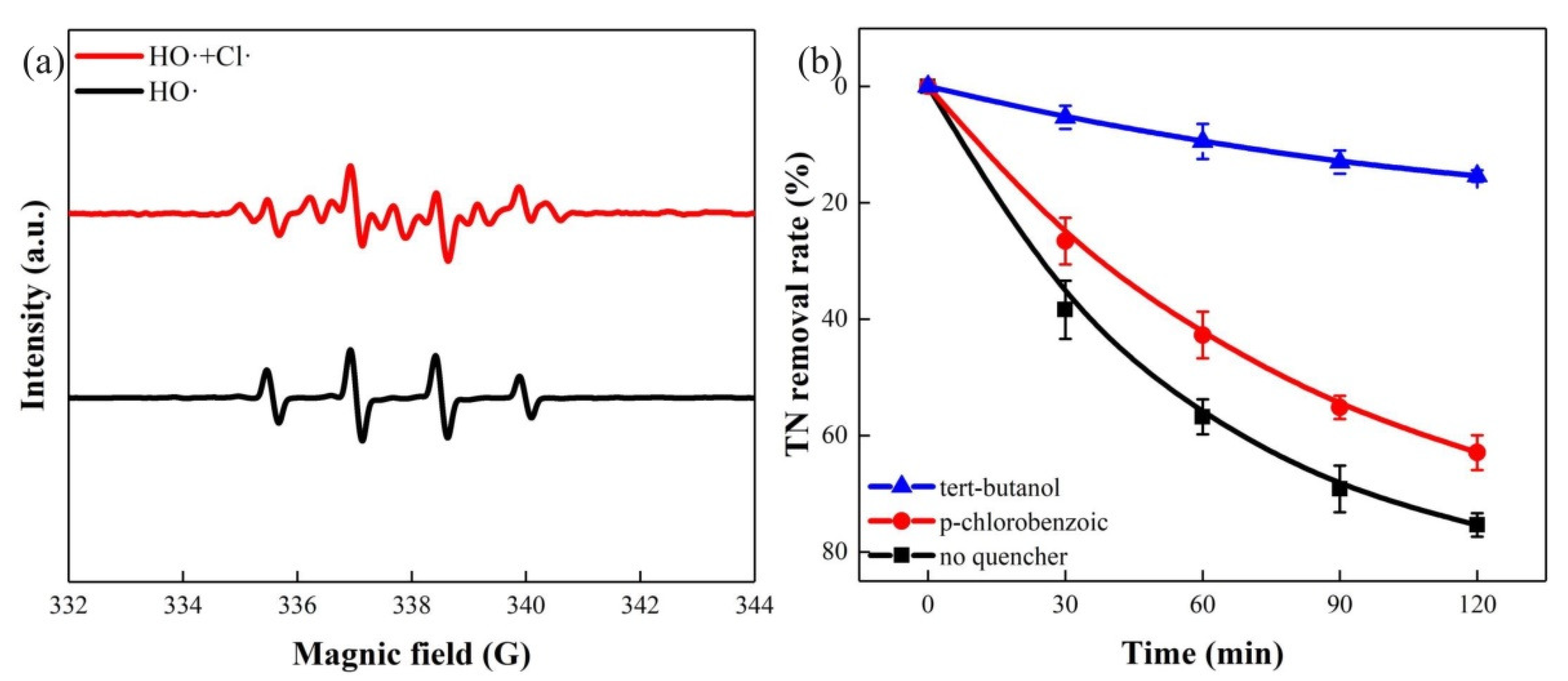
3.4. Mechanism of Hydrogen Generation and TN Removal
3.5. Application of Actual Urine and Stability of System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating worldwide use of urea—A global change contributing to coastal eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Lu, X.; Wang, H.; Qian, F.; Tong, Y.; Li, Y. Solar driven hydrogen releasing from urea and human urine. Energy Environ. Sci. 2012, 5, 8215–8219. [Google Scholar] [CrossRef]
- Rollinson, A.N.; Jones, J.; Dupont, V.; Twigg, M.V. Urea as a hydrogen carrier: A perspective on its potential for safe, sustainable and long-term energy supply. Energy Environ. Sci. 2011, 4, 1216–1224. [Google Scholar] [CrossRef] [Green Version]
- Larsen, T.A.; Alder, A.C.; Eggen, R.I.; Maurer, M.; Lienert, J. Source separation: Will we see a paradigm shift in wastewater handling? Environ. Sci. Technol. 2009, 43, 6121–6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spångberg, J.; Tidåker, P.; Jönsson, H. Environmental impact of recycling nutrients in human excreta to agriculture compared with enhanced wastewater treatment. Sci. Total Environ. 2014, 493, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Wilsenach, J.; Van Loosdrecht, M.C. Effects of separate urine collection on advanced nutrient removal processes. Environ. Sci. Technol. 2004, 38, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Wilsenach, J.; Schuurbiers, C.; Van Loosdrecht, M. Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res. 2007, 41, 458–466. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Boggs, B.K.; King, R.L.; Botte, G.G. Urea electrolysis: Direct hydrogen production from urine. Chem. Commun. 2009, 4859–4861. [Google Scholar] [CrossRef]
- Yan, X.; Hu, Q.-T.; Wang, G.; Zhang, W.-D.; Liu, J.; Li, T.; Gu, Z.-G. NiCo layered double hydroxide/hydroxide nanosheet heterostructures for highly efficient electro-oxidation of urea. Int. J. Hydrogen Energy 2020, 45, 19206–19213. [Google Scholar] [CrossRef]
- Gan, J.; Rajeeva, B.B.; Wu, Z.; Penley, D.; Zheng, Y. Plasmon-enhanced hierarchical photoelectrodes with mechanical flexibility for hydrogen generation from urea solution and human urine. J. Appl. Electrochem. 2020, 50, 63–69. [Google Scholar] [CrossRef]
- Liu, D.; Liu, T.; Zhang, L.; Qu, F.; Du, G.; Asiri, A.M.; Sun, X. High-performance urea electrolysis towards less energy-intensive electrochemical hydrogen production using a bifunctional catalyst electrode. J. Mater. Chem. A 2017, 5, 3208–3213. [Google Scholar] [CrossRef]
- Xie, J.; Gao, L.; Cao, S.; Liu, W.; Lei, F.; Hao, P.; Xia, X.; Tang, B. Copper-incorporated hierarchical wire-on-sheet α-Ni(OH)2 nanoarrays as robust trifunctional catalysts for synergistic hydrogen generation and urea oxidation. J. Mater. Chem. A 2019, 7, 13577–13584. [Google Scholar] [CrossRef]
- Song, M.; Zhang, Z.; Li, Q.; Jin, W.; Wu, Z.; Fu, G.; Liu, X. Ni-foam supported Co(OH)F and Co-P nanoarrays for energy-efficient hydrogen production via urea electrolysis. J. Mater. Chem. A 2019, 7, 3697–3703. [Google Scholar] [CrossRef]
- Urbańczyk, E.; Sowa, M.; Simka, W. Urea removal from aqueous solutions—A review. J. Appl. Electrochem. 2016, 46, 1011–1029. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Li, C.; Tang, R.; Lv, Z.; Ren, Y.; Wei, C.; Feng, C. Ion-exchange membrane bioelectrochemical reactor for removal of nitrate in the biological effluent from a coking wastewater treatment plant. Electrochem. Commun. 2014, 46, 99–102. [Google Scholar] [CrossRef]
- Lan, R.; Tao, S.; Irvine, J.T. A direct urea fuel cell–power from fertiliser and waste. Energy Environ. Sci. 2010, 3, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Ra, J.; Yoom, H.; Son, H.; Hwang, T.-M.; Lee, Y. Transformation of an amine moiety of atenolol during water treatment with chlorine/UV: Reaction kinetics, products, and mechanisms. Environ. Sci. Technol. 2019, 53, 7653–7662. [Google Scholar] [CrossRef]
- Deng, L.; Huang, C.-H.; Wang, Y.-L. Effects of combined UV and chlorine treatment on the formation of trichloronitromethane from amine precursors. Environ. Sci. Technol. 2014, 48, 2697–2705. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Blatchley III, E.R.; Wang, X.; Ren, P. UV/chlorine process for ammonia removal and disinfection by-product reduction: Comparison with chlorination. Water Res. 2015, 68, 804–811. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, L.; Liang, S.; Ye, J.; Yang, X.; Lei, Z.; Yan, Z.; Li, Y.; Wei, C.; Feng, C. Discovering the Importance of ClO• in a Coupled Electrochemical System for the Simultaneous Removal of Carbon and Nitrogen from Secondary Coking Wastewater Effluent. Environ. Sci. Technol. 2020, 54, 9015–9024. [Google Scholar] [CrossRef]
- Li, F.; Peng, X.; Liu, Y.; Mei, J.; Sun, L.; Shen, C.; Ma, C.; Huang, M.; Wang, Z.; Sand, W. A chloride-radical-mediated electrochemical filtration system for rapid and effective transformation of ammonia to nitrogen. Chemosphere 2019, 229, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Bai, J.; Li, X.; Shen, Z.; Xia, L.; Chen, S.; Xu, Q.; Zhou, B. Total organic carbon and total nitrogen removal and simultaneous electricity generation for nitrogen-containing wastewater based on the catalytic reactions of hydroxyl and chlorine radicals. Appl. Catal. B 2018, 238, 168–176. [Google Scholar] [CrossRef]
- Ji, Y.; Bai, J.; Li, J.; Luo, T.; Qiao, L.; Zeng, Q.; Zhou, B. Highly selective transformation of ammonia nitrogen to N2 based on a novel solar-driven photoelectrocatalytic-chlorine radical reactions system. Water Res. 2017, 125, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Qu, J.; Zhao, X.; Liu, H.; Wan, D. Electrochemical process combined with UV light irradiation for synergistic degradation of ammonia in chloride-containing solutions. Water Res. 2009, 43, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Ni, Y. Grass-like Ni/Cu nanosheet arrays grown on copper foam as efficient and non-precious catalyst for hydrogen evolution reaction. Appl. Catal. B 2020, 268, 118392. [Google Scholar] [CrossRef]
- Tian, Y.; Song, Y.; Liu, J.; Ji, J.; Wang, F. MoSx Coated Copper Nanowire on Copper Foam as A Highly Stable Photoelectrode for Enhanced Photoelectrocatalytic Hydrogen Evolution Reaction via. Plasmon-Induced Hot Carriers. Chem. Eng. J. 2020, 398, 125554. [Google Scholar] [CrossRef]
- Sivasakthi, P.; Premlatha, S.; Bapu, G.R.; Chandrasekaran, M. Pulse electrodeposited Ni-CeO2Gd doped nanocomposite on copper foam as an electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 4741–4750. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.; Yang, Y.; Zhang, G.; Gong, L.; Jing, L.; Fu, H.; Shi, K. Facile synthesis of Cu/CuxO nanoarchitectures with adjustable phase composition for effective NOx gas sensor at room temperature. Mater. Res. Bull. 2013, 48, 3657–3665. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Edit. 2020, 132, 5388–5392. [Google Scholar] [CrossRef]
- Zeng, Q.; Bai, J.; Li, J.; Li, L.; Xia, L.; Zhou, B.; Sun, Y. Highly-stable and efficient photocatalytic fuel cell based on an epitaxial TiO2/WO3/W nanothorn photoanode and enhanced radical reactions for simultaneous electricity production and wastewater treatment. Appl. Energy 2018, 220, 127–137. [Google Scholar] [CrossRef]
- Zeng, T.; Yu, M.; Zhang, H.; He, Z.; Chen, J.; Song, S. Fe/Fe3C@N-doped porous carbon hybrids derived from nano-scale MOFs: Robust and enhanced heterogeneous catalyst for peroxymonosulfate activation. Catal. Sci. Technol. 2017, 7, 396–404. [Google Scholar] [CrossRef]
- Ali, S.; Li, Z.; Chen, S.; Zada, A.; Khan, I.; Khan, I.; Ali, W.; Shaheen, S.; Qu, Y.; Jing, L. Synthesis of activated carbon-supported TiO2-based nano-photocatalysts with well recycling for efficiently degrading high-concentration pollutants. Catal. Today 2019, 335, 557–564. [Google Scholar] [CrossRef]
- Zeng, Q.; Bai, J.; Li, J.; Zhou, B.; Sun, Y. A low-cost photoelectrochemical tandem cell for highly-stable and efficient solar water splitting. Nano Energy 2017, 41, 225–232. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, J.; Bai, J.; Li, X.; Xia, L.; Zhou, B. Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance. Appl. Catal. B 2017, 202, 388–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Shen, Z.; Li, L.; Xia, L.; Chen, S.; Zhou, B. Exhaustive conversion of inorganic nitrogen to nitrogen gas based on a photoelectro-chlorine cycle reaction and a highly selective nitrogen gas generation cathode. Environ. Sci. Technol. 2018, 52, 1413–1420. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Hwang, Y.J.; Chae, S.Y.; Joo, O.S. Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity. J. Mater. Chem. A 2013, 1, 3479–3488. [Google Scholar] [CrossRef]
- Zhou, C.; Bai, J.; Zhang, Y.; Li, J.; Li, Z.; Jiang, P.; Fang, F.; Zhou, M.; Mei, X.; Zhou, B. Novel 3D Pd-Cu(OH)2/CF cathode for rapid reduction of nitrate-N and simultaneous total nitrogen removal from wastewater. J. Hazard. Mater. 2021, 401, 123232. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, H.; Qian, L.; Wang, Y.; Zhao, G. Design of a neutral photo-electro-Fenton system with 3D-ordered macroporous Fe2O3/carbon aerogel cathode: High activity and low energy consumption. Appl. Catal. B 2015, 174, 157–166. [Google Scholar] [CrossRef]
- Nagy, D.; Nagy, D.; Szilágyi, I.M.; Fan, X. Effect of the morphology and phases of WO3 nanocrystals on their photocatalytic efficiency. RSC Adv. 2016, 6, 33743. [Google Scholar] [CrossRef] [Green Version]
- Bayati, M.; Golestani-Fard, F.; Moshfegh, A. Visible photodecomposition of methylene blue over micro arc oxidized WO3–loaded TiO2 nano-porous layers. Appl. Catal. A 2010, 382, 322–331. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Zeng, G.; Liu, X.; Fang, C.; Li, C. A three-dimensional Cu nanobelt cathode for highly efficient electrocatalytic nitrate reduction. Nanoscale 2020, 12, 9385–9391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shen, Y.; Song, S.; He, Z. Enhanced electrocatalytic hydrodechlorination of 2, 4-dichlorophenoxyacetic acid by a Pd-Co3O4/Ni foam electrode. RSC Adv. 2019, 9, 12124–12133. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Xing, S.; Sun, B.; Cai, H.; Suo, H.; Zhao, C. Design and construction of three-dimensional flower-like CuO hierarchical nanostructures on copper foam for high performance supercapacitor. Electrochim. Acta 2016, 210, 639–645. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, Y.; Bai, J.; Li, J.; Mei, X.; Zhou, C.; Zhou, M.; Zhou, B. Efficient urine removal, simultaneous elimination of emerging contaminants, and control of toxic chlorate in a photoelectrocatalytic-chlorine system. Environ. Pollut. 2020, 267, 115605. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, L.; Lei, J.; Liu, Y.; Zhang, J. Photo-Fenton degradation of phenol by CdS/rGO/Fe2+ at natural pH with in situ-generated H2O2. Appl. Catal. B 2019, 241, 367–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, T.; Wang, J.; Xia, L.; Xu, Q.; Zhou, B. Extremely Efficient Decomposition of Ammonia N to N2 Using ClO• from Reactions of HO• and HOCl Generated in Situ on a Novel Bifacial Photoelectroanode. Environ. Sci. Technol. 2019, 53, 6945–6953. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Li, J.; Zhang, Y.; Shen, Z.; Qiao, L.; Xu, Q.; Zhou, B. Efficient TN removal and simultaneous TOC conversion for highly toxic organic amines based on a photoelectrochemical-chlorine radicals process. Catal. Today 2019, 335, 452–459. [Google Scholar] [CrossRef]
- McCurdy, D.; Lin, Z.; Inn, K.; Bell III, R.; Wagner, S.; Efurd, D.; Steiner, R.; Duffy, C.; Hamilton, T.; Brown, T. Second interlaboratory comparison study for the analysis of 239Pu in synthetic urine at the microBq (-100 aCi) level by mass spectrometry. J. Radioanal. Nucl. Chem. 2005, 263, 447–455. [Google Scholar] [CrossRef]
- Saetta, D.; Boyer, T.H. Mimicking and inhibiting urea hydrolysis in nonwater urinals. Environ. Sci. Technol. 2017, 51, 13850–13858. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.; Perreault, F.; Boyer, T.H. Urea recovery from fresh human urine by forward osmosis and membrane distillation (FO–MD). Environ. Sci. Water Res. Technol. 2019, 5, 1993–2003. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Li, J.; Xu, Y.; Zhou, C.; Zhang, Y.; Zha, L.; Zhang, B.; Bai, J.; Zhou, B. Efficient Hydrogen Generation and Total Nitrogen Removal for Urine Treatment in a Neutral Solution Based on a Self-Driving Nano Photoelectrocatalytic System. Nanomaterials 2021, 11, 2777. https://doi.org/10.3390/nano11112777
Wang P, Li J, Xu Y, Zhou C, Zhang Y, Zha L, Zhang B, Bai J, Zhou B. Efficient Hydrogen Generation and Total Nitrogen Removal for Urine Treatment in a Neutral Solution Based on a Self-Driving Nano Photoelectrocatalytic System. Nanomaterials. 2021; 11(11):2777. https://doi.org/10.3390/nano11112777
Chicago/Turabian StyleWang, Pengbo, Jinhua Li, Yang Xu, Changhui Zhou, Yan Zhang, Lina Zha, Bo Zhang, Jing Bai, and Baoxue Zhou. 2021. "Efficient Hydrogen Generation and Total Nitrogen Removal for Urine Treatment in a Neutral Solution Based on a Self-Driving Nano Photoelectrocatalytic System" Nanomaterials 11, no. 11: 2777. https://doi.org/10.3390/nano11112777





