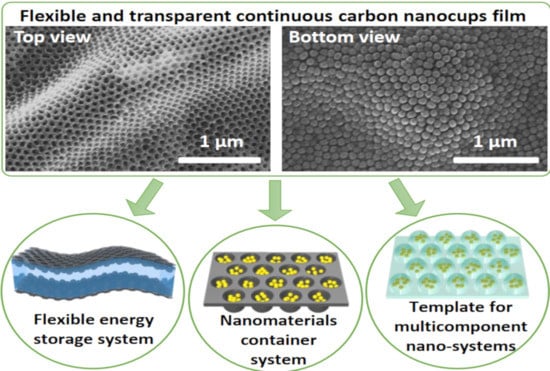
Graphitic Nanocup Architectures for Advanced Nanotechnology Applications
Abstract
:1. Introduction
2. Fabrication and Modification of Graphitic Nanoscale Architectures
2.1. Carbon Nanocups (CNC) Thin Film
2.2. Branched Carbon Nanocups (B-CNC) Thin Film
2.3. Carbon Nanotubes (CNTs)–CNC Hybrid Structure
3. Branched CNC for Electrodes of Flexible and Transparent Supercapacitors
4. CNTs–CNC Hybrid Structure Electrodes for High Power Supercapacitors
5. CNC Container System for Liquid Metal Nanodroplet Dynamics Study
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bendjemil, B.; Cleymand, F.; Pichler, T.; Knupfer, M.; Fink, J. Carbon Nanostructures in Cancer Diagnosis and Therapy. J. Nanom. Nanos Tech. JNNT-110 2019, 2019, 1–18. [Google Scholar]
- Bezzon, V.D.; Montanheiro, T.L.; de Menezes, B.R.; Ribas, R.G.; Righetti, V.A.; Rodrigues, K.F.; Thim, G.P. Carbon Nanostructure-based Sensors: A Brief Review on Recent Advances. Adv. Mater. Sci. Eng. 2019, 2019, 4293073. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.O.; Dimitrakopoulos, C.D.; Grill, A.; McArdle, T.J.; Pfeiffer, D.; Saenger, K.L.; Wisnieff, R.L. Carbon Nanostructure Device Fabrication Utilizing Protect Layers. U.S. Patent 9,768,288, 19 September 2017. [Google Scholar]
- Iglesias, D.; Bosi, S.; Melchionna, M.; Da Ros, T.; Marchesan, S. The glitter of carbon nanostructures in hybrid/composite hydrogels for medicinal use. Curr. Top. Med. Chem. 2016, 16, 1976–1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of carbon nanotubes in drug delivery: A comprehensive review. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135. [Google Scholar]
- Kim, H.; Gao, S.; Hong, S.; Lee, P.-C.; Kim, Y.L.; Ha, J.U.; Jeoung, S.K.; Jung, Y.J. Multifunctional primer film made from percolation enhanced CNT/Epoxy nanocomposite and ultrathin CNT network. Compos. Part B Eng. 2019, 175, 107107. [Google Scholar] [CrossRef]
- Hong, S.; Kim, H.; Gao, S.; Lavall, R.L.; Jung, H.Y.; Jung, Y.J. Reconfigurable solid-state electrolytes for high performance flexible supercapacitor. J. Power Sources 2019, 432, 16–23. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in carbon nanostructure–silica aerogel composites: A review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Yan, M.; Yu, J. Carbon nanostructures in biology and medicine. J. Mater. Chem. B 2017, 5, 6437–6450. [Google Scholar] [CrossRef]
- Mondal, K.; Balasubramaniam, B.; Gupta, A.; Lahcen, A.A.; Kwiatkowski, M. Carbon Nanostructures for Energy and Sensing Applications. J. Nanotechnol. 2019, 2019, 1454327. [Google Scholar] [CrossRef] [Green Version]
- Piovesana, S.; Iglesias, D.; Melle-Franco, M.; Kralj, S.; Cavaliere, C.; Melchionna, M.; Laganà, A.; Capriotti, A.L.; Marchesan, S. Carbon nanostructure morphology templates nanocomposites for phosphoproteomics. Nano Res. 2020, 13, 380–388. [Google Scholar] [CrossRef]
- Xiong, G.; He, P.; Lyu, Z.; Chen, T.; Huang, B.; Chen, L.; Fisher, T.S. Bioinspired leaves-on-branchlet hybrid carbon nanostructure for supercapacitors. Nat. Commun. 2018, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Hu, H.; Wu, H.B.; Lou, X.W. Complex hollow nanostructures: Synthesis and energy-related applications. Adv. Mater. 2017, 29, 1604563. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Jiang, Q.; Shi, Y.; Kim, H.; Busnaina, A.; Jung, H.Y.; Jung, Y.J. High-performance lithium battery driven by hybrid lithium storage mechanism in 3D architectured carbonized eggshell membrane anode. Carbon 2020, 166, 26–35. [Google Scholar] [CrossRef]
- Liu, J.; Rinzler, A.G.; Dai, H.; Hafner, J.H.; Bradley, R.K.; Boul, P.J.; Lu, A.; Iverson, T.; Shelimov, K.; Huffman, C.B. Fullerene pipes. Science 1998, 280, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lago, R.; Chen, Y.; Green, M.H.; Harris, P.F. Mechanical damage of carbon nanotubes by ultrasound. Carbon 1996, 34, 814–816. [Google Scholar] [CrossRef]
- Shelimov, K.B.; Esenaliev, R.O.; Rinzler, A.G.; Huffman, C.B.; Smalley, R.E. Purification of single-wall carbon nanotubes by ultrasonically assisted filtration. Chem. Phys. Lett. 1998, 282, 429–434. [Google Scholar] [CrossRef]
- Venema, L.C.; Wildöer, J.; Tuinstra, H.T.; Dekker, C.; Rinzler, A.; Smalley, R. Length control of individual carbon nanotubes by nanostructuring with a scanning tunneling microscope. Appl. Phys. Lett. 1997, 71, 2629–2631. [Google Scholar] [CrossRef] [Green Version]
- Gu, Q.; Gao, Z.; Zhao, H.; Lou, Z.; Liao, Y.; Xue, C. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. Rsc Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Subramanian, S.; Purdy, G. Graphite morphology control in cast iron. In Proceedings of the F. Weinberg International Symposium on Solidification Processing, Hamilton, ON, Canada, 27–29 August 1990; pp. 289–303. [Google Scholar]
- Xia, Y.; Yang, Z.; Mokaya, R. Simultaneous control of morphology and porosity in nanoporous carbon: Graphitic mesoporous carbon nanorods and nanotubules with tunable pore size. Chem. Mater. 2006, 18, 140–148. [Google Scholar] [CrossRef]
- Zhou, B.-X.; Ding, S.-S.; Zhang, B.-J.; Xu, L.; Chen, R.-S.; Luo, L.; Huang, W.-Q.; Xie, Z.; Pan, A.; Huang, G.-F. Dimensional transformation and morphological control of graphitic carbon nitride from water-based supramolecular assembly for photocatalytic hydrogen evolution: From 3D to 2D and 1D nanostructures. Appl. Catal. B Environ. 2019, 254, 321–328. [Google Scholar] [CrossRef]
- Liu, Y.; Che, Z.; Lu, X.; Zhou, X.; Han, M.; Bao, J.; Dai, Z. Nanostructured metal chalcogenides confined in hollow structures for promoting energy storage. Nanoscale Adv. 2020, 2, 583–604. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Meng, G.; Han, F.; Li, X.; Chen, B.; Xu, Q.; Zhu, X.; Chu, Z.; Kong, M.; Huang, Q. Nanocontainers made of various materials with tunable shape and size. Sci. Rep. 2013, 3, 2238. [Google Scholar] [PubMed] [Green Version]
- Kong, J.; Zhao, C.; Wei, Y.; Phua, S.L.; Dong, Y.; Lu, X. Nanocups-on-microtubes: A unique host towards high-performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 15191–15199. [Google Scholar]
- Lin, W.; Li, C.; Gao, F.; Zheng, J.; Wei, G.; Yang, W. Low-aspect ratio graphite hollow nanostructures. CrystEngComm 2013, 15, 8907–8911. [Google Scholar] [CrossRef]
- Chun, H.; Hahm, M.G.; Homma, Y.; Meritz, R.; Kuramochi, K.; Menon, L.; Ci, L.; Ajayan, P.M.; Jung, Y.J. Engineering low-aspect ratio carbon nanostructures: Nanocups, nanorings, and nanocontainers. ACS Nano 2009, 3, 1274–1278. [Google Scholar]
- Itaya, K.; Sugawara, S.; Arai, K.; Saito, S. Properties of porous anodic aluminum oxide films as membranes. J. Chem. Eng. Jpn. 1984, 17, 514–520. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.; Park, S.-J. Porous anodic aluminum oxide: Anodization and templated synthesis of functional nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Meng, G.; Cao, A.; Cheng, J.-Y.; Vijayaraghavan, A.; Jung, Y.J.; Shima, M.; Ajayan, P.M. Ordered Ni nanowire tip arrays sticking out of the anodic aluminum oxide template. J. Appl. Phys. 2005, 97, 064303. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in nano-engineered anodic aluminum oxide membrane development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [Green Version]
- Routkevitch, D.; Bigioni, T.; Moskovits, M.; Xu, J.M. Electrochemical fabrication of CdS nanowire arrays in porous anodic aluminum oxide templates. J. Phys. Chem. 1996, 100, 14037–14047. [Google Scholar] [CrossRef]
- Sui, Y.; Acosta, D.; Gonzalez-Leon, J.; Bermudez, A.; Feuchtwanger, J.; Cui, B.; Flores, J.; Saniger, J. Structure, thermal stability, and deformation of multibranched carbon nanotubes synthesized by CVD in the AAO template. J. Phys. Chem. B 2001, 105, 1523–1527. [Google Scholar] [CrossRef]
- Wang, X.; Han, G.-R. Fabrication and characterization of anodic aluminum oxide template. Microelectron. Eng. 2003, 66, 166–170. [Google Scholar] [CrossRef]
- Zhao, S.; Roberge, H.; Yelon, A.; Veres, T. New application of AAO template: A mold for nanoring and nanocone arrays. J. Am. Chem. Soc. 2006, 128, 12352–12353. [Google Scholar] [CrossRef] [PubMed]
- Hahm, M.G.; Leela Mohana Reddy, A.; Cole, D.P.; Rivera, M.; Vento, J.A.; Nam, J.; Jung, H.Y.; Kim, Y.L.; Narayanan, N.T.; Hashim, D.P. Carbon nanotube–nanocup hybrid structures for high power supercapacitor applications. Nano Lett. 2012, 12, 5616–5621. [Google Scholar] [CrossRef]
- Jung, H.Y.; Karimi, M.B.; Hahm, M.G.; Ajayan, P.M.; Jung, Y.J. Transparent, flexible supercapacitors from nano-engineered carbon films. Sci. Rep. 2012, 2, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, H.Y.; Chun, H.; Park, S.; Kang, S.-H.; Ahn, C.W.; Kwon, Y.-K.; Upmanyu, M.; Ajayan, P.M.; Jung, Y.J. Liquid metal nanodroplet dynamics inside nanocontainers. Sci. Rep. 2013, 3, 2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, C.V.; Ramos, D.; Petho, L.; Bürki, G.; Michler, J.; Philippe, L. Controlling the color and effective refractive index of metal-anodic aluminum oxide (AAO)–Al nanostructures: Morphology of AAO. J. Phys. Chem. C 2018, 122, 957–963. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Lei, Y. A mini review: Functional nanostructuring with perfectly-ordered anodic aluminum oxide template for energy conversion and storage. Front. Chem. Sci. Eng. 2018, 12, 481–493. [Google Scholar] [CrossRef]
- Bruera, F.A.; Kramer, G.R.; Vera, M.L.; Ares, A.E. Evaluation of the influence of synthesis conditions on the morphology of nanostructured anodic aluminum oxide coatings on Al 1050. Surf. Interfaces 2020, 18, 100448. [Google Scholar] [CrossRef]
- Jo, Y.-W.; Lee, S.-G.; Yeo, J.-H.; Kim, K.-M. Fabrication and Characterization of an Anodic Aluminum Oxide Template with Variations in the Anodization Solution for pH Sensors. J. Nanosci. Nanotechnol. 2016, 16, 11779–11782. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, T.; Gao, Y.; Long, H.; Xi, S.; Tang, Z. Fabrication of a 3D micro/nano dual-scale carbon array and its demonstration as the microelectrodes for supercapacitors. J. Micromech. Microeng. 2014, 24, 045001. [Google Scholar] [CrossRef]
- Ohtani, M.; Fukuzumi, S. Photoelectrochemical Cell Based on Cup-Shaped Nanocarbon–Fullerene Composite Nanocluster Film: Enhancement of Photocurrent Generation by Cup-Shaped Nanocarbons as an Electron Transporter. Fuller. Nanotub. Carbon Nanostruct. 2010, 18, 251–260. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Star, A. Synthesis, characterization, and manipulation of nitrogen-doped carbon nanotube cups. ACS Nano 2008, 2, 1914–1920. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Xie, S.; Liu, W.; Niu, C. Template synthesis of graphitic hollow carbon nanoballs as supports for SnO x nanoparticles towards enhanced lithium storage performance. Nanoscale 2018, 10, 6159–6167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Saito, R.; Oyama, Y.; Jiang, J.; Cancado, L.; Pimenta, M.; Jorio, A.; Samsonidze, G.G.; Dresselhaus, G.; Dresselhaus, M. D-band Raman intensity of graphitic materials as a function of laser energy and crystallite size. Chem. Phys. Lett. 2006, 427, 117–121. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751–758. [Google Scholar] [CrossRef]
- Izadi-Najafabadi, A.; Yasuda, S.; Kobashi, K.; Yamada, T.; Futaba, D.N.; Hatori, H.; Yumura, M.; Iijima, S.; Hata, K. Extracting the full potential of single-walled carbon nanotubes as durable supercapacitor electrodes operable at 4 V with high power and energy density. Adv. Mater. 2010, 22, E235–E241. [Google Scholar] [CrossRef]
- Zeng, Y.; Ci, L.; Carey, B.J.; Vajtai, R.; Ajayan, P.M. Design and reinforcement: Vertically aligned carbon nanotube-based sandwich composites. ACS Nano 2010, 4, 6798–6804. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free single-walled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [Green Version]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Bacsa, R.; Laurent, C.; Peigney, A.; Bacsa, W.; Vaugien, T.; Rousset, A. High specific surface area carbon nanotubes from catalytic chemical vapor deposition process. Chem. Phys. Lett. 2000, 323, 566–571. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.J.; Balakrishnan, K.; Huang, J.; Meunier, V.; Sumpter, B.G.; Srivastava, A.; Conway, M.; Mohana Reddy, A.L.; Yu, J.; Vajtai, R. Ultrathin planar graphene supercapacitors. Nano Lett. 2011, 11, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496. [Google Scholar] [PubMed]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- In, H.J.; Kumar, S.; Shao-Horn, Y.; Barbastathis, G. Origami fabrication of nanostructured, three-dimensional devices: Electrochemical capacitors with carbon electrodes. Appl. Phys. Lett. 2006, 88, 083104. [Google Scholar] [CrossRef] [Green Version]
- Pech, D.; Brunet, M.; Taberna, P.-L.; Simon, P.; Fabre, N.; Mesnilgrente, F.; Conédéra, V.; Durou, H. Elaboration of a microstructured inkjet-printed carbon electrochemical capacitor. J. Power Sources 2010, 195, 1266–1269. [Google Scholar] [CrossRef] [Green Version]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-engineerable and highly densely packed single-walled carbon nanotubes and their application as super-capacitor electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359–4363. [Google Scholar] [CrossRef]
- Dash, R.; Chmiola, J.; Yushin, G.; Gogotsi, Y.; Laudisio, G.; Singer, J.; Fischer, J.; Kucheyev, S. Titanium carbide derived nanoporous carbon for energy-related applications. Carbon 2006, 44, 2489–2497. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Lian, K.; Gogotsi, Y. Pseudocapacitive behavior of carbon nanoparticles modified by phosphomolybdic acid. J. Electrochem. Soc. 2009, 156, A921–A926. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Ramaprabhu, S. Nanocrystalline metal oxides dispersed multiwalled carbon nanotubes as supercapacitor electrodes. J. Phys. Chem. C 2007, 111, 7727–7734. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.-L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Gao, S.; Hahm, M.G.; Ahn, C.W.; Jung, H.Y.; Jung, Y.J. Graphitic Nanocup Architectures for Advanced Nanotechnology Applications. Nanomaterials 2020, 10, 1862. https://doi.org/10.3390/nano10091862
Kim H, Gao S, Hahm MG, Ahn CW, Jung HY, Jung YJ. Graphitic Nanocup Architectures for Advanced Nanotechnology Applications. Nanomaterials. 2020; 10(9):1862. https://doi.org/10.3390/nano10091862
Chicago/Turabian StyleKim, Hyehee, Sen Gao, Myung Gwan Hahm, Chi Won Ahn, Hyun Young Jung, and Yung Joon Jung. 2020. "Graphitic Nanocup Architectures for Advanced Nanotechnology Applications" Nanomaterials 10, no. 9: 1862. https://doi.org/10.3390/nano10091862