Molecular Linking Selectivity on Self-Assembled Metal-Semiconductor Nano-Hybrid Systems
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Materials Synthesis
2.3. Characterization
2.4. Catalytic Reduction of 4-Nitrophenol
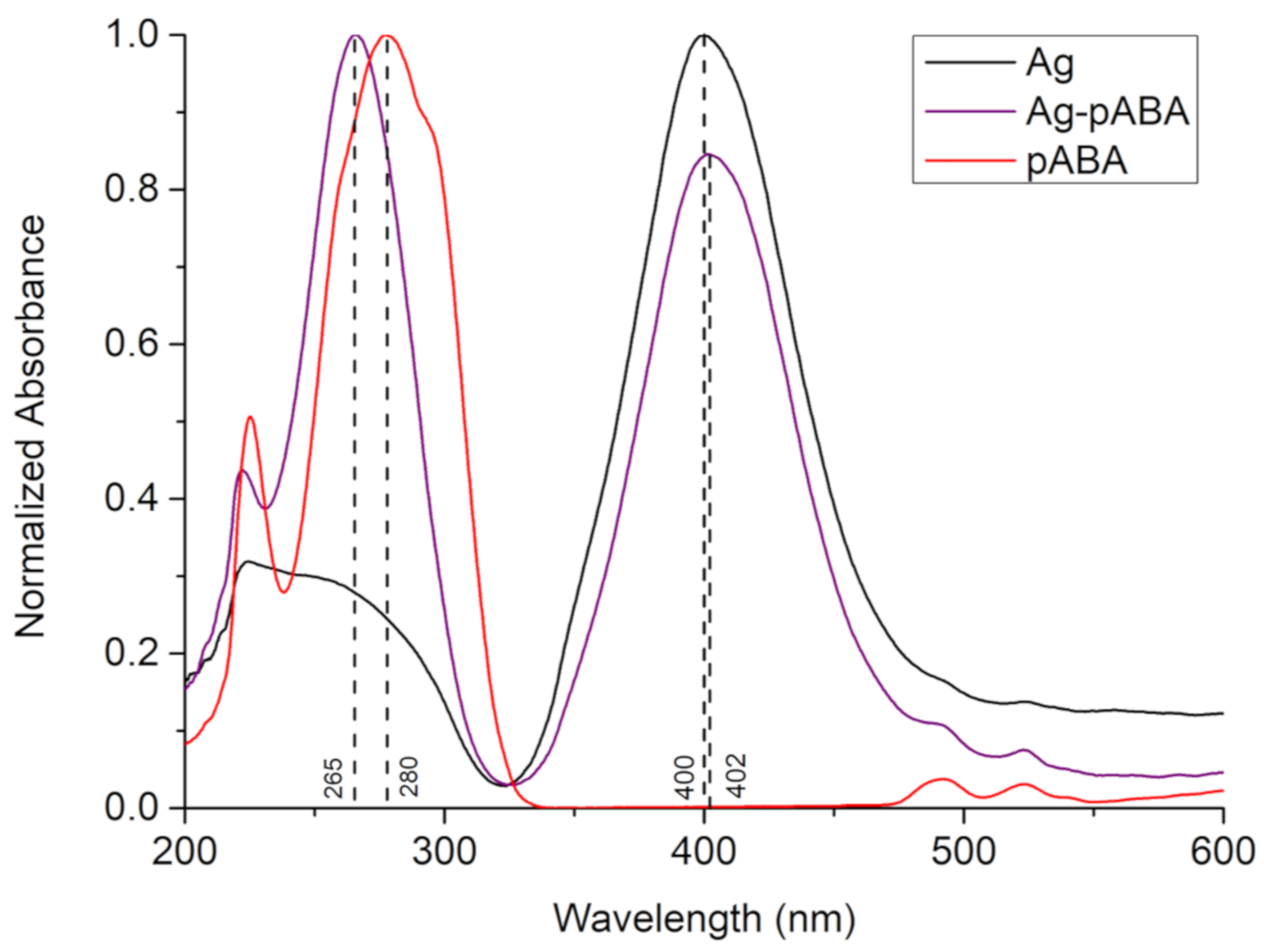
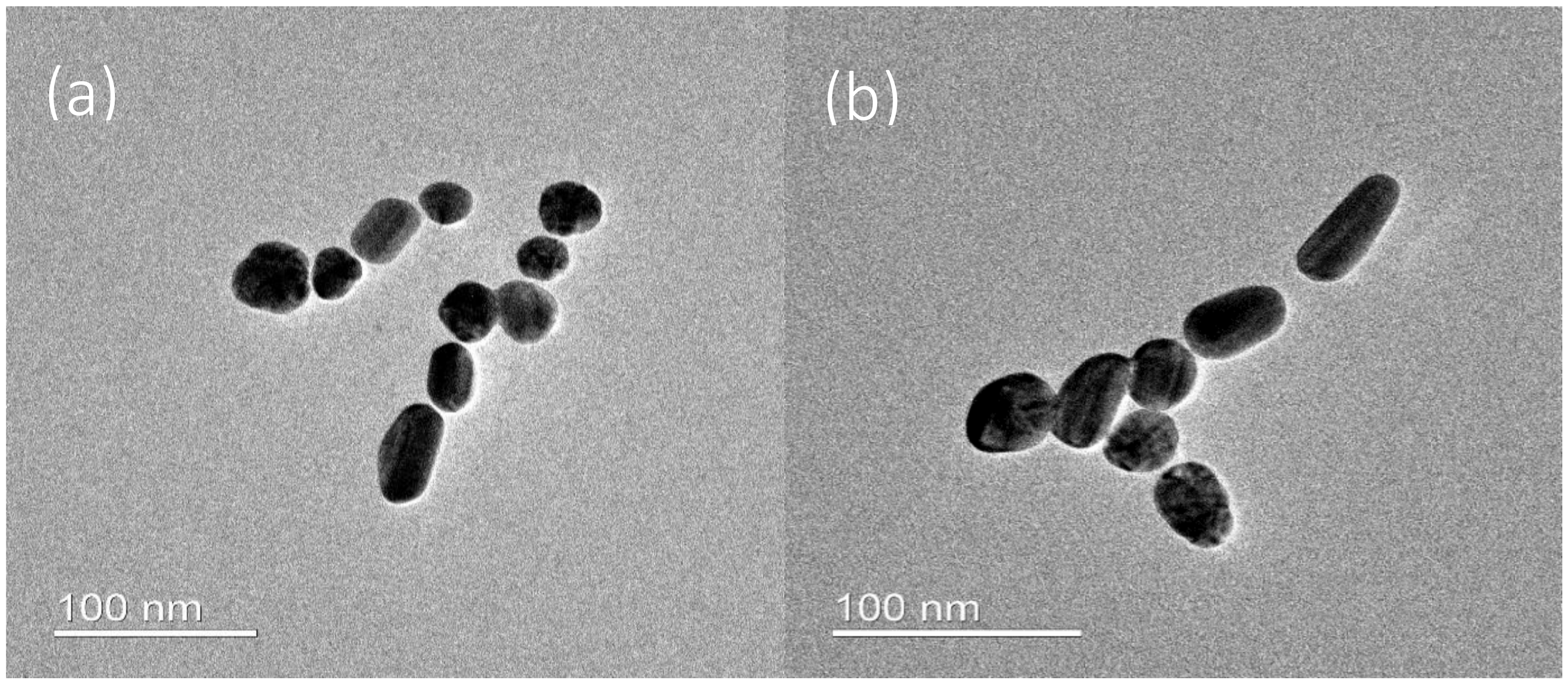
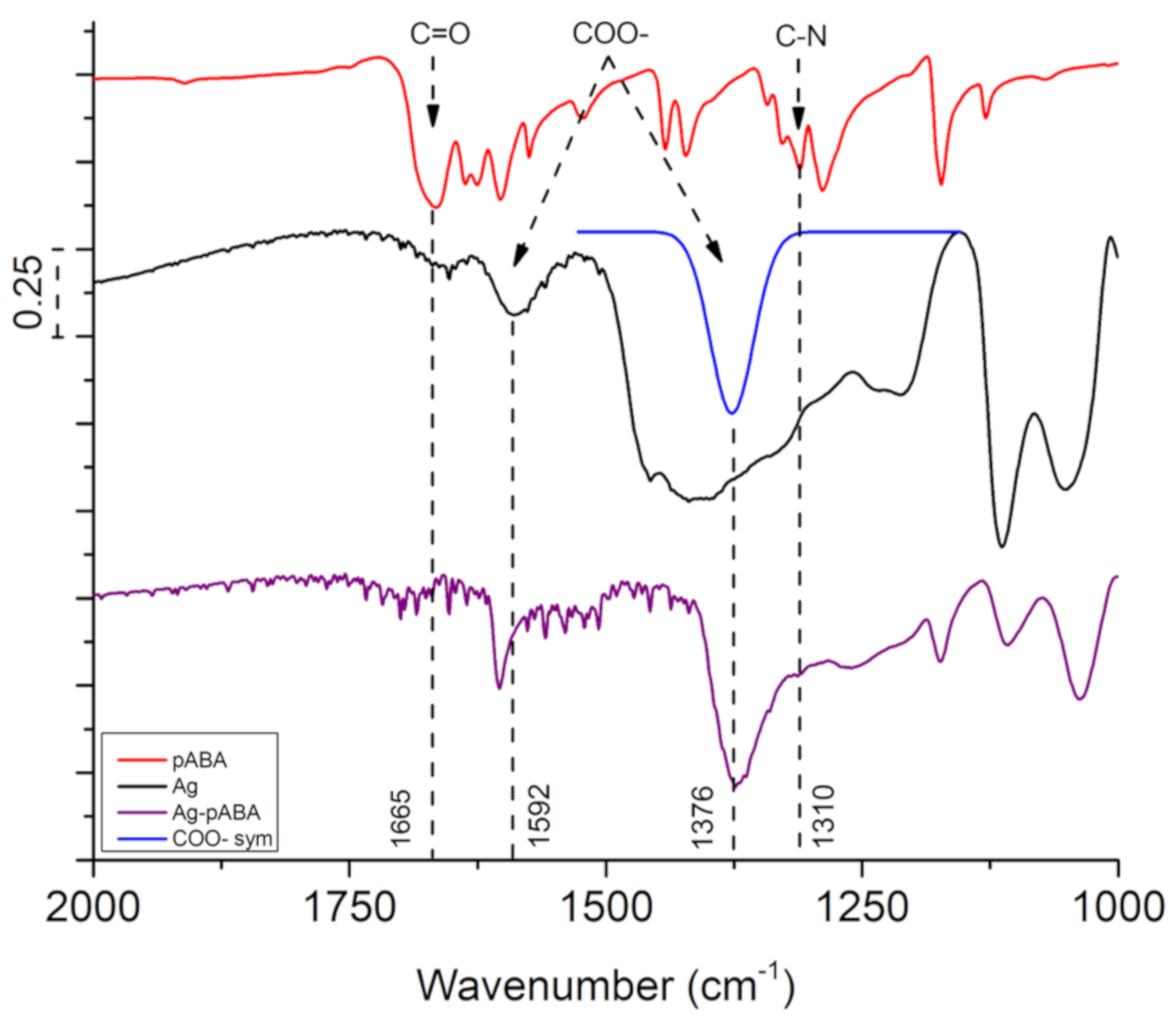
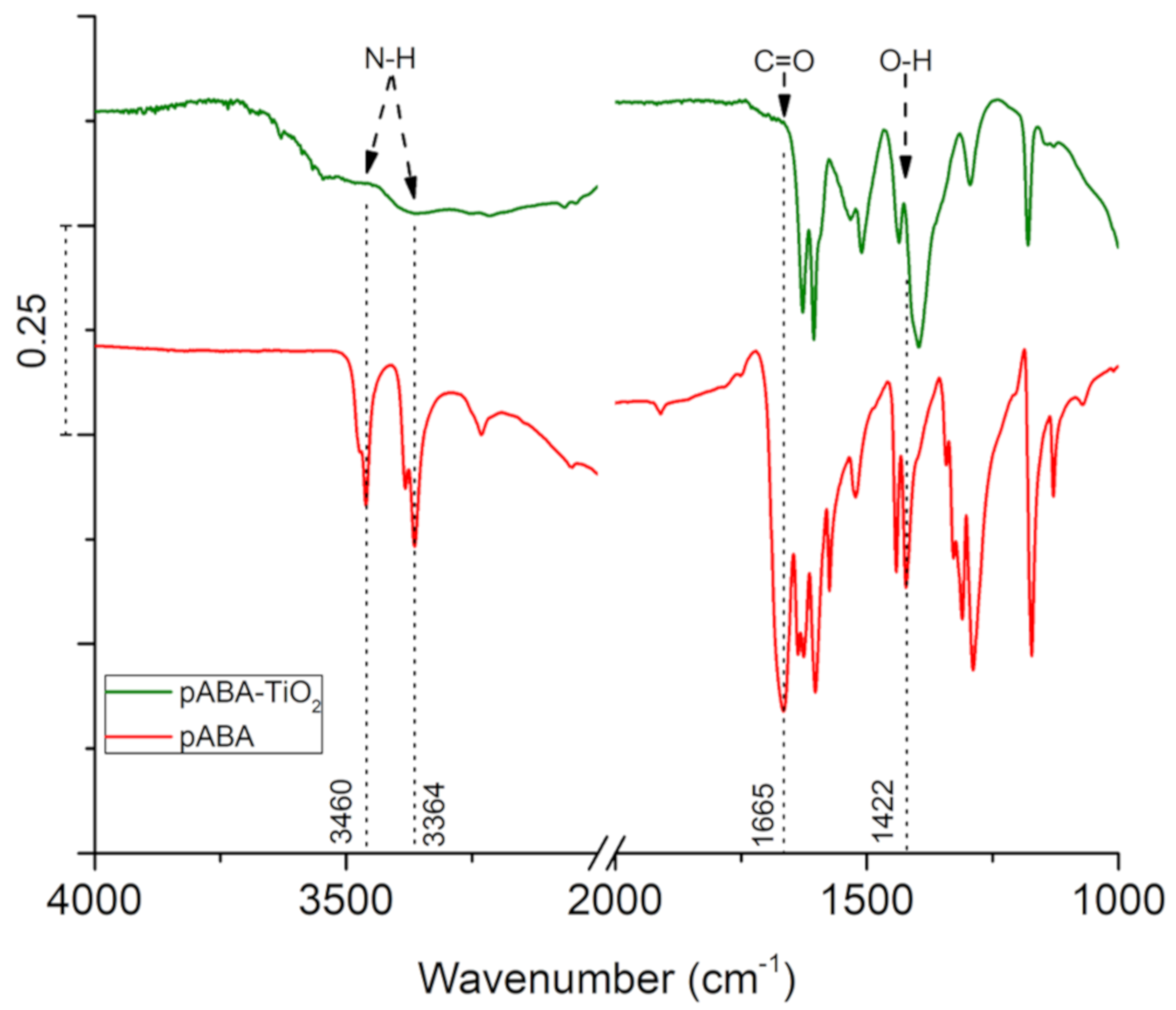
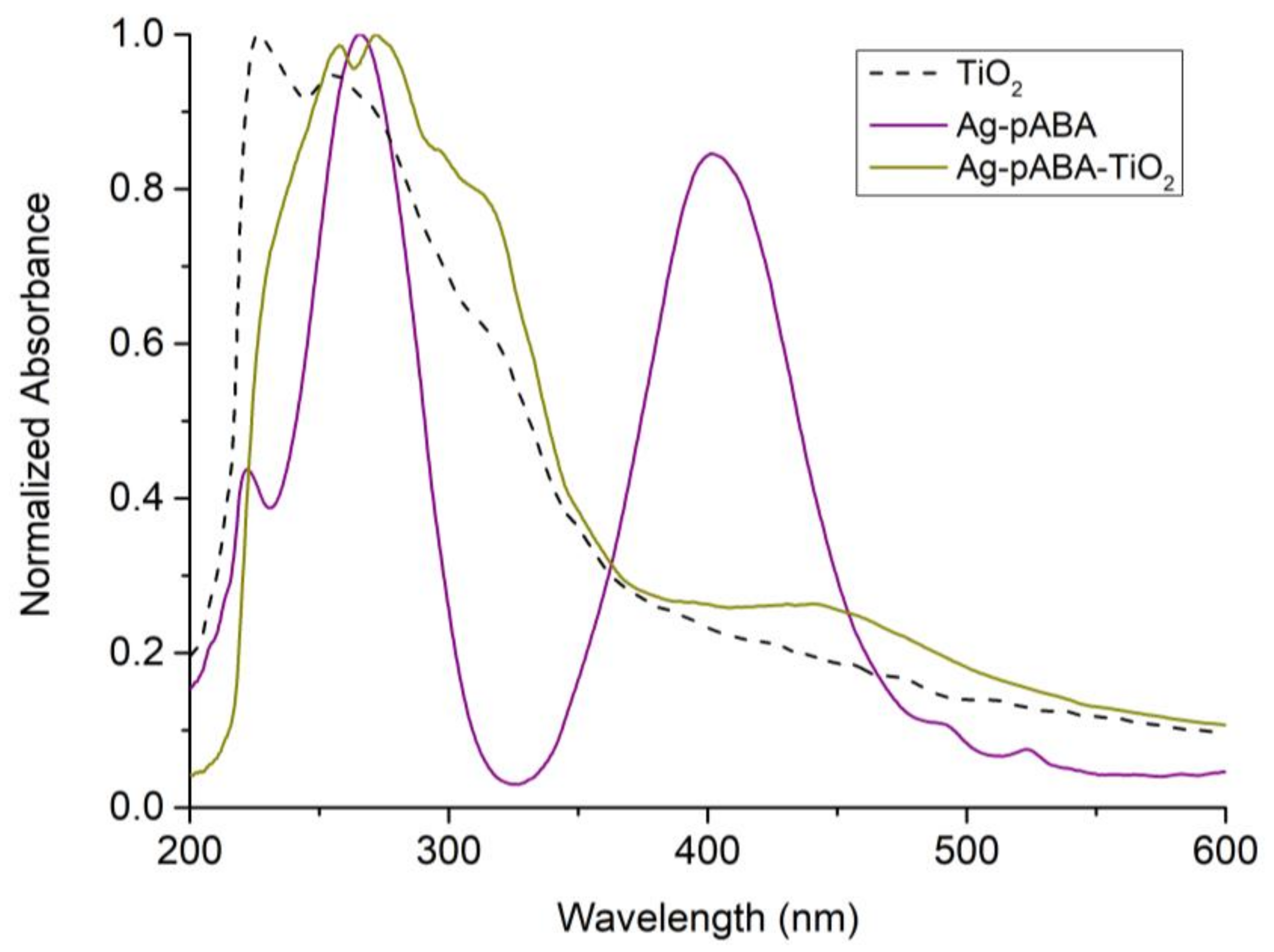
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brongersma, M.L.; Halas, N.J.; Nordlander, P. Plasmon-induced Hot Carrier Science and Technology. Nat. Nanotechnol. 2015, 10, 25–34. [Google Scholar] [CrossRef]
- Sá, J.; Tagliabue, G.; Friedli, P.; Szlachetko, J.; Rittmann-Frank, M.H.; Santomauro, F.G.; Milne, C.J.; Sigg, H. Direct observation of charge separation on Au localized surface plasmons. Energy Environ. Sci. 2013, 6, 3584–3588. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Mubeen, S.; Lee, J.; Singh, N.; Krämer, S.; Stucky, G.D.; Moskovits, M. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 2013, 8, 247–251. [Google Scholar] [CrossRef]
- Pavliuk, M.V.; Fernandes, A.B.; Abdellah, M.; Fernandes, D.L.A.; Machado, C.O.; Rocha, I.; Hattori, Y.; Paun, C.; Bastos, E.L.; Sá, J. Nano-hybrid plasmonic photocatalyst for hydrogen production at 20% efficiency. Sci. Rep. 2017, 7, 8670. [Google Scholar] [CrossRef] [Green Version]
- Pavliuk, M.V.; Gutiérrez Álvarez, S.; Hattori, Y.; Messing, M.E.; Czapla-Masztafiak, J.; Szlachetko, J.; Silva, J.L.; Araujo, C.M.; Fernandes, D.L.A.; Lu, L.; et al. Hydrated electron generation by excitation of copper localized surface plasmon resonance. J. Phys. Chem. Lett. 2019, 10, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.J.; DuChene, J.S.; Tagliabue, G.; Davoyan, A.; Cheng, W.-H.; Atwater, H.A. Nanoporous gold as a highly selective and active carbon dioxide reduction catalyst. ACS Appl. Energy Mater. 2019, 2, 164–170. [Google Scholar] [CrossRef] [Green Version]
- DuChene, J.S.; Tagliabue, G.; Welch, A.J.; Li, X.; Cheng, W.-H.; Atwater, H.A. Optical excitation of a nanoparticle Cu/p-NiO photocathode improves reaction selectivity for CO2 reduction in aqueous electrolytes. Nano Lett. 2020, 20, 2348–2358. [Google Scholar] [CrossRef] [Green Version]
- García de Arquer, F.P.; Mihi, A.; Kufer, D.; Konstantatos, G. Photoelectric energy conversion of plasmon-generated hot carriers in metal–insulator–semiconductor structures. ACS Nano 2013, 7, 3581–3588. [Google Scholar] [CrossRef]
- Nakamura, K.; Oshikiri, T.; Ueno, K.; Wang, Y.; Kamata, Y.; Kotake, Y.; Misawa, H. Properties of plasmon-induced photoelectric conversion on a TiO2/NiO p–n junction with Au nanoparticles. J. Phys. Chem. Lett. 2016, 7, 1004–1009. [Google Scholar] [CrossRef]
- Hattori, Y.; Abdellah, M.; Meng, J.; Zheng, K.; Sá, J. Simultaneous hot electron and hole injection upon excitation of gold surface plasmon. J. Phys. Chem. Lett. 2019, 10, 3140–3146. [Google Scholar] [CrossRef] [PubMed]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ultrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, G.; DuChene, J.S.; Abdellah, M.; Habib, A.; Hattori, Y.; Zheng, K.; Canton, S.E.; Gosztola, D.J.; Cheng, W.-H.; Sundararaman, R.; et al. Ultrafast studies of hot-hole dynamics in Au/p-GaN heterostructures. arXiv 2018, arXiv:1810.04238. [Google Scholar]
- Arquer, F.P.G.D.; Mihi, A.; Konstantatos, G. Molecular interfaces for plasmonic hot electron photovoltaics. Nanoscale 2015, 7, 2281–2288. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Chen, J.; McBride, J.R.; Lian, T. Molecular interfaces for plasmonic hot electron photovoltaics. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Grzeschik, R.; Schäfer, D.; Holtum, T.; Küpper, S.; Hoffmann, A.; Schlücker, S. On the overlooked critical role of the pH value on the kinetics of the 4-nitrophenol NaBH4-reduction catalyzed by noble-metal nanoparticles (Pt, Pd, and Au). J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, Y.; Yu, J. Spontaneous hydrolysis of borohydride required before its catalytic activation by metal nanoparticles. Catal. Commun. 2016, 84, 80–84. [Google Scholar] [CrossRef] [Green Version]
- Ayad, A.I.; Luart, D.; Dris, A.O.; Guénin, E. Kinetic analysis of 4-nitrophenol reduction by “water-soluble” palladium nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobley, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef]
- Steinigeweg, D.; Schlücker, S. Monodispersity and size control in the synthesis of 20–100 nm quasi-spherical silver nanoparticles by citrate and ascorbic acid reduction in glycerol–water mixtures. Chem. Commun. 2012, 48, 8682–8684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glycerin. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C56815&Type=IR-SPEC&Index=1 (accessed on 18 May 2020).
- Ranoszek-Soliwoda, K.; Tomaszewska, E.; Socha, E.; Krzyczmonik, P.; Ignaczak, A.; Orlowski, P.; Krzyzowska, M.; Celichowski, G.; Grobelny, J. The role of tannic acid and sodium citrate in the synthesis of silver nanoparticles. J. Nanopart. Res. 2017, 19, 273. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, P.; Nagahiro, T.; Fukada, N.; Kimura, Y.; Niwano, M.; Tamada, K. Characterization of citrates on gold and silver nanoparticles. J. Colloid Interface Sci. 2015, 438, 244–248. [Google Scholar] [CrossRef]
- Thomas, A.G.; Jackman, M.J.; Wagstaffe, M.; Radtke, H.; Syres, K.; Adell, J.; Lévy, A.; Martsinovich, N. Adsorption studies of p-aminobenzoic acid on the anatase TiO2 (101) surface. Langmuir 2014, 30, 12306–12314. [Google Scholar] [CrossRef] [PubMed]
- Dobson, K.D.; McQuillan, A. In situ infrared spectroscopic analysis of the adsorption of aromatic carboxylic acids to TiO2, ZrO2, Al2O3, and Ta2O5 from aqueous solutions. J. Spectrochim. Acta A 2000, 56, 557–565. [Google Scholar] [CrossRef]
- Tsoumachidou, S.; Velegraki, T.; Poulios, I. TiO2 photocatalytic degradation of UV filter para-aminobenzoic acid under artificial and solar illumination. J. Chem. Technol. Biotechnol. 2016, 91, 1773–1781. [Google Scholar] [CrossRef]
- Aditya, T.; Pal, A.; Pal, T. Nitroarene reduction: A trusted model reaction to test nanoparticle catalysts. Chem. Commun. 2015, 51, 9410–9431. [Google Scholar] [CrossRef]
- Cortés, E.; Xie, W.; Cambiasso, J.; Jermyn, A.S.; Sundararaman, R.; Narang, P.; Schlücker, S.; Maier, S.A. Plasmonic hot electron transport drives nano-localized chemistry. Nat. Commun. 2017, 8, 14880. [Google Scholar] [CrossRef]
- Gargiulo, J.; Berté, R.; Li, Y.; Maier, S.A.; Cortés, E. From optical to chemical hot spots in plasmonics. Acc. Chem. Res. 2019, 52, 2525–2535. [Google Scholar] [CrossRef]
- Abay, A.K.; Chen, X.; Kuo, D.-H. Highly efficient noble metal free copper nickel oxysulfide nanoparticles for catalytic reduction of 4-nitrophenol, methyl blue, and rhodamine-B organic pollutants. New J. Chem. 2017, 41, 5628–5638. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Zhao, D.; Chen, W.; Li, H.; Hou, C. Preparation of magnetic CuFe2O4@Ag@ZIF-8 nanocomposites with highly catalytic activity based on cellulose nanocrystals. Molecules 2019, 25, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.L.T.; Gascón Nicolás, A.; Edvinsson, T.; Meng, J.; Zheng, K.; Abdellah, M.; Sá, J. Molecular Linking Selectivity on Self-Assembled Metal-Semiconductor Nano-Hybrid Systems. Nanomaterials 2020, 10, 1378. https://doi.org/10.3390/nano10071378
Nguyen TLT, Gascón Nicolás A, Edvinsson T, Meng J, Zheng K, Abdellah M, Sá J. Molecular Linking Selectivity on Self-Assembled Metal-Semiconductor Nano-Hybrid Systems. Nanomaterials. 2020; 10(7):1378. https://doi.org/10.3390/nano10071378
Chicago/Turabian StyleNguyen, Thinh Luong The, Alba Gascón Nicolás, Tomas Edvinsson, Jie Meng, Kaibo Zheng, Mohamed Abdellah, and Jacinto Sá. 2020. "Molecular Linking Selectivity on Self-Assembled Metal-Semiconductor Nano-Hybrid Systems" Nanomaterials 10, no. 7: 1378. https://doi.org/10.3390/nano10071378





