Mimicking Natural Photosynthesis: Designing Ultrafast Photosensitized Electron Transfer into Multiheme Cytochrome Protein Nanowires
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Protein Expression, Purifiction and Photosensitizer Labeling
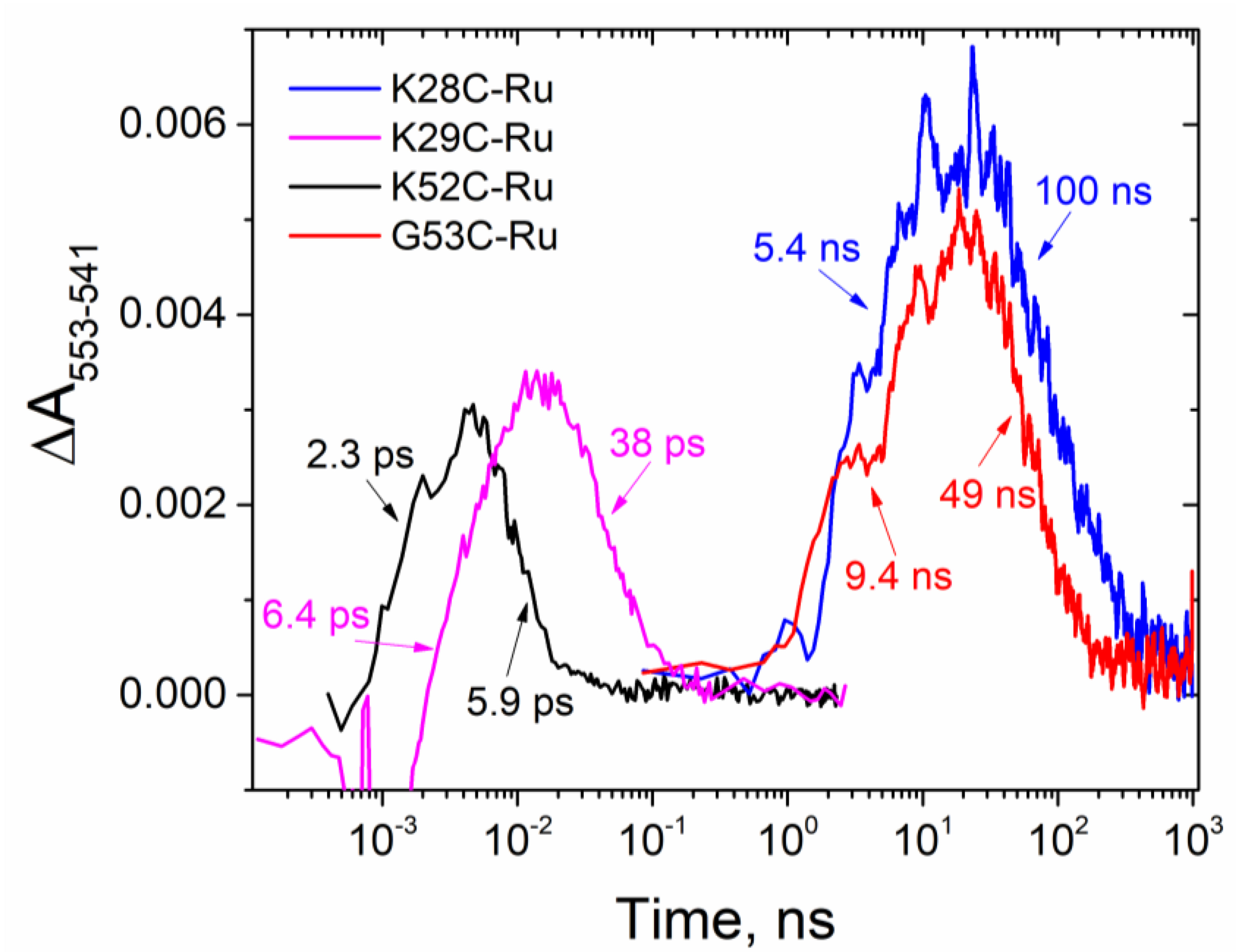
3.2. Kinetics of Electron Transfer
3.3. Molecular Dynamics Modeling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thapper, A.; Styring, S.; Saracco, G.; Rutherford, A.W.; Robert, B.; Magnuson, A.; Lubitz, W.; Llobet, A.; Kurz, P.; Holzwarth, A.; et al. Artificial Photosynthesis for Solar Fuels—An Evolving Research Field within AMPEA, a Joint Programme of the European Energy Research Alliance. Green 2013, 3, 43. [Google Scholar] [CrossRef]
- Faunce, T.; Styring, S.; Wasielewski, M.R.; Brudvig, G.W.; Rutherford, A.W.; Messinger, J.; Lee, A.F.; Hill, C.L.; deGroot, H.; Fontecave, M.; et al. Artificial photosynthesis as a frontier technology for energy sustainability. Energy Environ. Sci. 2013, 6, 1074–1076. [Google Scholar] [CrossRef] [Green Version]
- Alivisatos, P.; Buchanan, M. Basic Research Needs for Carbon Capture: Beyond 2020; USDOE Office of Science (SC): Washington, DC, USA, 2010.
- Fukuzumi, S. Production of Liquid Solar Fuels and Their Use in Fuel Cells. Joule 2017, 1, 689–738. [Google Scholar] [CrossRef] [Green Version]
- Tumas, B.; Dempsey, J.L.; Mallouk, T.E. Report of the Basic Energy Sciences Roundtable on Liquid Solar Fuels; USDOE Office of Science (SC): Washington, DC, USA, 2019.
- Askerka, M.; Brudvig, G.W.; Batista, V.S. The O-2-Evolving Complex of Photosystem II: Recent Insights from Quantum Mechanics/Molecular Mechanics (QM/MM), Extended X-ray Absorption Fine Structure (EXAFS), and Femtosecond X-ray Crystallography Data. Acc. Chem. Res. 2017, 50, 41–48. [Google Scholar] [CrossRef]
- Barber, J. Photosynthetic energy conversion: Natural and artificial. Chem. Soc. Rev. 2009, 38, 185–196. [Google Scholar] [CrossRef]
- Junge, W. Oxygenic photosynthesis: History, status and perspective. Q. Rev. Biophys. 2019, 52, e1. [Google Scholar] [CrossRef]
- Kreisbeck, C.; Aspuru-Guzik, A. Efficiency of energy funneling in the photosystem II supercomplex of higher plants. Chem. Sci. 2016. [Google Scholar] [CrossRef] [Green Version]
- Lubitz, W.; Chrysina, M.; Cox, N. Water oxidation in photosystem II. Photosynth. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Blankenship, R.E.; Tiede, D.M.; Barber, J.; Brudvig, G.W.; Fleming, G.; Ghirardi, M.; Gunner, M.R.; Junge, W.; Kramer, D.M.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332, 805–809. [Google Scholar] [CrossRef] [Green Version]
- Utschig, L.M.; Soltau, S.R.; Mulfort, K.L.; Niklas, J.; Poluektov, O.G. Z-scheme solar water splitting via self-assembly of photosystem I-catalyst hybrids in thylakoid membranes. Chem. Sci. 2018, 9, 8504–8512. [Google Scholar] [CrossRef] [Green Version]
- Utschig, L.M.; Soltau, S.R.; Tiede, D.M. Light-driven hydrogen production from Photosystem I-catalyst hybrids. Curr. Opin. Chem. Biol. 2015, 25, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Brahmachari, U.; Pokkuluri, P.R.; Tiede, D.M.; Niklas, J.; Poluektov, O.G.; Mulfort, K.L.; Utschig, L.M. Interprotein electron transfer biohybrid system for photocatalytic H2 production. Photosynth. Res. 2020, 143, 183–192. [Google Scholar] [CrossRef]
- Mukherjee, A.; Kokhan, O.; Huang, J.; Niklas, J.; Chen, L.X.; Tiede, D.M.; Mulfort, K.L. Detection of a charge-separated catalyst precursor state in a linked photosensitizer-catalyst assembly. Phys. Chem. Chem. Phys. 2013, 15, 21070–21076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulfort, K.L.; Tiede, D.M. Supramolecular cobaloxime assemblies for H2 photocatalysis: An initial solution state structure-function analysis. J. Phys. Chem. B 2010, 114, 14572–14581. [Google Scholar] [CrossRef] [PubMed]
- Soltau, S.R.; Niklas, J.; Dahlberg, P.D.; Poluektov, O.G.; Tiede, D.M.; Mulfort, K.L.; Utschig, L.M. Aqueous light driven hydrogen production by a Ru-ferredoxin-Co biohybrid. Chem. Commun. 2015, 51, 10628–10631. [Google Scholar] [CrossRef]
- Utschig, L.M.; Silver, S.C.; Mulfort, K.L.; Tiede, D.M. Nature-driven photochemistry for catalytic solar hydrogen production: A Photosystem I-transition metal catalyst hybrid. J. Am. Chem. Soc. 2011, 133, 16334–16337. [Google Scholar] [CrossRef]
- Belliston-Bittner, W.; Dunn, A.R.; Nguyen, Y.H.; Stuehr, D.J.; Winkler, J.R.; Gray, H.B. Picosecond photoreduction of inducible nitric oxide synthase by rhenium(I)-diimine wires. J. Am. Chem. Soc. 2005, 127, 15907–15915. [Google Scholar] [CrossRef] [Green Version]
- Berglund, J.; Pascher, T.; Winkler, J.R.; Gray, H.B. Photoinduced Oxidation of Horseradish Peroxidase. J. Am. Chem. Soc. 1997, 119, 2464–2469. [Google Scholar] [CrossRef]
- Chang, I.J.; Gray, H.B.; Winkler, J.R. High-driving-force electron transfer in metalloproteins: Intramolecular oxidation of ferrocytochrome c by Ru(2,2′-bpy)2(im)(his-33)3+. J. Am. Chem. Soc. 1991, 113, 7056–7057. [Google Scholar] [CrossRef]
- Dmochowski, I.J.; Winkler, J.R.; Gray, H.B. Enantiomeric discrimination of Ru-substrates by cytochrome P450cam. J. Inorg. Biochem. 2000, 81, 221–228. [Google Scholar] [CrossRef]
- Dunn, A.R.; Dmochowski, I.J.; Winkler, J.R.; Gray, H.B. Nanosecond photoreduction of cytochrome p450cam by channel-specific Ru-diimine electron tunneling wires. J. Am. Chem. Soc. 2003, 125, 12450–12456. [Google Scholar] [CrossRef] [Green Version]
- Durham, B.; Pan, L.P.; Long, J.E.; Millett, F. Photoinduced electron-transfer kinetics of singly labeled ruthenium bis(bipyridine) dicarboxybipyridine cytochrome c derivatives. Biochemistry 1989, 28, 8659–8665. [Google Scholar] [CrossRef]
- Engstrom, G.; Rajagukguk, R.; Saunders, A.J.; Patel, C.N.; Rajagukguk, S.; Merbitz-Zahradnik, T.; Xiao, K.; Pielak, G.J.; Trumpower, B.; Yu, C.A.; et al. Design of a ruthenium-labeled cytochrome c derivative to study electron transfer with the cytochrome bc1 complex. Biochemistry 2003, 42, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Geren, L.; Hahm, S.; Durham, B.; Millett, F. Photoinduced electron transfer between cytochrome c peroxidase and yeast cytochrome c labeled at Cys 102 with (4-bromomethyl-4′-methylbipyridine)[bis(bipyridine)]ruthenium2+. Biochemistry 1991, 30, 9450–9457. [Google Scholar] [CrossRef]
- Hartings, M.R.; Kurnikov, I.V.; Dunn, A.R.; Winkler, J.R.; Gray, H.B.; Ratner, M.A. Electron tunneling through sensitizer wires bound to proteins. Coord. Chem. Rev. 2010, 254, 248–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mines, G.A.; Bjerrum, M.J.; Hill, M.G.; Casimiro, D.R.; Chang, I.J.; Winkler, J.R.; Gray, H.B. Rates of Heme Oxidation and Reduction in Ru(His33)cytochromecat Very High Driving Forces. J. Am. Chem. Soc. 1996, 118, 1961–1965. [Google Scholar] [CrossRef]
- Pan, L.P.; Durham, B.; Wolinska, J.; Millett, F. Preparation and characterization of singly labeled ruthenium polypyridine cytochrome c derivatives. Biochemistry 1988, 27, 7180–7184. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.J.; Herrera, N.; Hill, M.G.; Winkler, J.R.; Gray, H.B. Electron flow through nitrotyrosinate in Pseudomonas aeruginosa azurin. J. Am. Chem. Soc. 2013, 135, 11151–11158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wuttke, D.S.; Bjerrum, M.J.; Winkler, J.R.; Gray, H.B. Electron-tunneling pathways in cytochrome C. Science 1992, 256, 1007–1009. [Google Scholar] [CrossRef]
- Wuttke, D.S.; Gray, H.B.; Fisher, S.L.; Imperiali, B. Semisynthesis of bipyridyl-alanine cytochrome c mutants: Novel proteins with enhanced electron-transfer properties. J. Am. Chem. Soc. 1993, 115, 8455–8456. [Google Scholar] [CrossRef]
- Winkler, J.R.; Gray, H.B. Electron transfer in ruthenium-modified proteins. Chem. Rev. 1992, 92, 369–379. [Google Scholar] [CrossRef]
- Winkler, J.R.; Gray, H.B. Electron flow through metalloproteins. Chem. Rev. 2014, 114, 3369–3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winkler, J.R.; Gray, H.B. Long-range electron tunneling. J. Am. Chem. Soc. 2014, 136, 2930–2939. [Google Scholar] [CrossRef] [PubMed]
- Moser, C.C.; Keske, J.M.; Warncke, K.; Farid, R.S.; Dutton, P.L. Nature of biological electron transfer. Nature 1992, 355, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Page, C.C.; Moser, C.C.; Chen, X.; Dutton, P.L. Natural engineering principles of electron tunnelling in biological oxidation-reduction. Nature 1999, 402, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Beratan, D.N.; Liu, C.; Migliore, A.; Polizzi, N.F.; Skourtis, S.S.; Zhang, P.; Zhang, Y. Charge transfer in dynamical biosystems, or the treachery of (static) images. Acc. Chem. Res. 2015, 48, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Beratan, D.N.; Betts, J.N.; Onuchic, J.N. Protein electron transfer rates set by the bridging secondary and tertiary structure. Science 1991, 252, 1285–1288. [Google Scholar] [CrossRef]
- Beratan, D.N.; Onuchic, J.N.; Winkler, J.R.; Gray, H.B. Electron-tunneling pathways in proteins. Science 1992, 258, 1740–1741. [Google Scholar] [CrossRef]
- Lin, J.; Balabin, I.A.; Beratan, D.N. The nature of aqueous tunneling pathways between electron-transfer proteins. Science 2005, 310, 1311–1313. [Google Scholar] [CrossRef] [Green Version]
- Gruschus, J.M.; Kuki, A. New Hamiltonian model for long-range electronic superexchange in complex molecular structures. J. Phys. Chem. 1993, 97, 5581–5593. [Google Scholar] [CrossRef]
- Gruschus, J.M.; Kuki, A. Ellipsoidal Delocalization of Tunneling Electrons in Long-Range Electron Transfer Proteins. J. Phys. Chem. B 1999, 103, 11407–11414. [Google Scholar] [CrossRef]
- Cave, R.J.; Newton, M.D. Calculation of electronic coupling matrix elements for ground and excited state electron transfer reactions: Comparison of the generalized Mulliken–Hush and block diagonalization methods. J. Chem. Phys. 1997, 106, 9213–9226. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Stuchebrukhov, A.A. Electron tunneling in respiratory complex I. Proc. Natl. Acad. Sci. USA 2010, 107, 19157–19162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliore, A.; Sit, P.H.; Klein, M.L. Evaluation of Electronic Coupling in Transition-Metal Systems Using DFT: Application to the Hexa-Aquo Ferric-Ferrous Redox Couple. J. Chem. Theory Comput. 2009, 5, 307–323. [Google Scholar] [CrossRef]
- Oberhofer, H.; Blumberger, J. Electronic coupling matrix elements from charge constrained density functional theory calculations using a plane wave basis set. J. Chem. Phys. 2010, 133, 244105. [Google Scholar] [CrossRef]
- Pacher, T.; Cederbaum, L.S.; Köppel, H. Approximately diabatic states from block diagonalization of the electronic Hamiltonian. J. Chem. Phys. 1988, 89, 7367–7381. [Google Scholar] [CrossRef]
- Voityuk, A.A.; Rösch, N. Fragment charge difference method for estimating donor–acceptor electronic coupling: Application to DNA π-stacks. J. Chem. Phys. 2002, 117, 5607–5616. [Google Scholar] [CrossRef]
- Wu, Q.; Cheng, C.L.; Van Voorhis, T. Configuration interaction based on constrained density functional theory: A multireference method. J. Chem. Phys. 2007, 127, 164119. [Google Scholar] [CrossRef]
- Kokhan, O.; Ponomarenko, N.S.; Pokkuluri, P.R.; Schiffer, M.; Mulfort, K.L.; Tiede, D.M. Bidirectional Photoinduced Electron Transfer in Ruthenium(II)-Tris-bipyridyl-Modified PpcA, a Multi-heme c-Type Cytochrome from Geobacter sulfurreducens. J. Phys. Chem. B 2015, 119, 7612–7624. [Google Scholar] [CrossRef]
- Ponomarenko, N.S.; Kokhan, O.; Pokkuluri, P.R.; Mulfort, K.L.; Tiede, D.M. Examination of abiotic cofactor assembly in photosynthetic biomimetics: Site-specific stereoselectivity in the conjugation of a ruthenium(II) tris(bipyridine) photosensitizer to a multi-heme protein. Photosynth. Res. 2020, 143, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Berglund, H.; Davydov, R.; Norrby, T.; Hammarström, L.; Korall, P.; Börje, A.; Philouze, C.; Berg, K.; Tran, A.; et al. Binuclear Ruthenium−Manganese Complexes as Simple Artificial Models for Photosystem II in Green Plants. J. Am. Chem. Soc. 1997, 119, 6996–7004. [Google Scholar] [CrossRef]
- Pokkuluri, P.R.; Londer, Y.Y.; Duke, N.E.; Erickson, J.; Pessanha, M.; Salgueiro, C.A.; Schiffer, M. Structure of a novel c7-type three-heme cytochrome domain from a multidomain cytochrome c polymer. Protein Sci. 2004, 13, 1684–1692. [Google Scholar] [CrossRef] [Green Version]
- Arslan, E.; Schulz, H.; Zufferey, R.; Kunzler, P.; Thony-Meyer, L. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 1998, 251, 744–747. [Google Scholar] [CrossRef]
- Kokhan, O.; Ponomarenko, N.; Pokkuluri, P.R.; Schiffer, M.; Tiede, D.M. Multimerization of solution-state proteins by tetrakis(4-sulfonatophenyl)porphyrin. Biochemistry 2014, 53, 5070–5079. [Google Scholar] [CrossRef]
- Dantas, J.M.; Morgado, L.; Catarino, T.; Kokhan, O.; Pokkuluri, P.R.; Salgueiro, C.A. Evidence for interaction between the triheme cytochrome PpcA from Geobacter sulfurreducens and anthrahydroquinone-2,6-disulfonate, an analog of the redox active components of humic substances. Biochim. Biophys. Acta 2014, 1837, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kale, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef] [Green Version]
- van Wonderen, J.H.; Hall, C.R.; Jiang, X.; Adamczyk, K.; Carof, A.; Heisler, I.; Piper, S.E.H.; Clarke, T.A.; Watmough, N.J.; Sazanovich, I.V.; et al. Ultrafast Light-Driven Electron Transfer in a Ru(II)tris(bipyridine)-Labeled Multiheme Cytochrome. J. Am. Chem. Soc. 2019, 141, 15190–15200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado, L.; Bruix, M.; Londer, Y.Y.; Pokkuluri, P.R.; Schiffer, M.; Salgueiro, C.A. Redox-linked conformational changes of a multiheme cytochrome from Geobacter sulfurreducens. Biochem. Biophys. Res. Commun. 2007, 360, 194–198. [Google Scholar] [CrossRef]
- Morgado, L.; Bruix, M.; Orshonsky, V.; Londer, Y.Y.; Duke, N.E.; Yang, X.; Pokkuluri, P.R.; Schiffer, M.; Salgueiro, C.A. Structural insights into the modulation of the redox properties of two Geobacter sulfurreducens homologous triheme cytochromes. Biochim. Biophys. Acta 2008, 1777, 1157–1165. [Google Scholar] [CrossRef] [Green Version]
- Morgado, L.; Bruix, M.; Pessanha, M.; Londer, Y.Y.; Salgueiro, C.A. Thermodynamic characterization of a triheme cytochrome family from Geobacter sulfurreducens reveals mechanistic and functional diversity. Biophys. J. 2010, 99, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgado, L.; Bruix, M.; Pokkuluri, P.R.; Salgueiro, C.A.; Turner, D.L. Redox- and pH-linked conformational changes in triheme cytochrome PpcA from Geobacter sulfurreducens. Biochem. J. 2017, 474, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Morgado, L.; Paixao, V.B.; Schiffer, M.; Pokkuluri, P.R.; Bruix, M.; Salgueiro, C.A. Revealing the structural origin of the redox-Bohr effect: The first solution structure of a cytochrome from Geobacter sulfurreducens. Biochem. J. 2012, 441, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbo, J.K.; Xu, Y.; Zhang, P.; Straub, J.E.; Leitner, D.M. Vibrational energy flow across heme–cytochrome c and cytochrome c–water interfaces. Theor. Chem. Acc. 2014, 133, s00214–s014. [Google Scholar] [CrossRef]
- Anfinrud, P.A.; Han, C.; Hochstrasser, R.M. Direct observations of ligand dynamics in hemoglobin by subpicosecond infrared spectroscopy. Proc. Natl. Acad. Sci. USA 1989, 86, 8387–8391. [Google Scholar] [CrossRef] [Green Version]
- Barns, K.J.; Lampa-Pastirk, S.; Dillman, K.L.; Beck, W.F. Intramolecular vibrational excitation of unfolding reactions in ZnII-substituted and metal-free cytochromes c: Activation enthalpies from integrated fluorescence stokes shift and line shape excitation profiles. J. Phys. Chem. B 2008, 112, 15108–15115. [Google Scholar] [CrossRef]
- Bram, O.; Consani, C.; Cannizzo, A.; Chergui, M. Femtosecond UV studies of the electronic relaxation processes in Cytochrome c. J. Phys. Chem. B 2011, 115, 13723–13730. [Google Scholar] [CrossRef]
- Bu, L.; Straub, J.E. Vibrational Energy Relaxation of “Tailored” Hemes in Myoglobin Following Ligand Photolysis Supports Energy Funneling Mechanism of Heme “Cooling”. J. Phys. Chem. B 2003, 107, 10634–10639. [Google Scholar] [CrossRef]
- Chin, J.K.; Jimenez, R.; Romesberg, F.E. Direct observation of protein vibrations by selective incorporation of spectroscopically observable carbon-deuterium bonds in cytochrome c. J. Am. Chem. Soc. 2001, 123, 2426–2427. [Google Scholar] [CrossRef]
- Fujii, N.; Mizuno, M.; Ishikawa, H.; Mizutani, Y. Observing Vibrational Energy Flow in a Protein with the Spatial Resolution of a Single Amino Acid Residue. J. Phys. Chem. Lett. 2014, 5, 3269–3273. [Google Scholar] [CrossRef]
- Fujii, N.; Mizuno, M.; Mizutani, Y. Direct observation of vibrational energy flow in cytochrome c. J. Phys. Chem. B 2011, 115, 13057–13064. [Google Scholar] [CrossRef]
- Fujisaki, H.; Straub, J.E. Vibrational energy relaxation in proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 6726–6731. [Google Scholar] [CrossRef] [Green Version]
- Genberg, L.; Richard, L.; McLendon, G.; Miller, R.J. Direct observation of global protein motion in hemoglobin and myoglobin on picosecond time scales. Science 1991, 251, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, M.; Mizuno, M.; Mizutani, Y. Importance of Atomic Contacts in Vibrational Energy Flow in Proteins. J. Phys. Chem. Lett. 2016, 7, 1950–1954. [Google Scholar] [CrossRef] [PubMed]
- Lampa-Pastirk, S.; Beck, W.F. Intramolecular vibrational preparation of the unfolding transition state of Zn(II)-substituted cytochrome c. J. Phys. Chem. B 2006, 110, 22971–22974. [Google Scholar] [CrossRef] [PubMed]
- Leitner, D.M. Energy flow in proteins. Annu. Rev. Phys. Chem. 2008, 59, 233–259. [Google Scholar] [CrossRef]
- Lian, T.; Locke, B.; Kholodenko, Y.; Hochstrasser, R.M. Energy Flow from Solute to Solvent Probed by Femtosecond IR Spectroscopy: Malachite Green and Heme Protein Solutions. J. Phys. Chem. 1994, 98, 11648–11656. [Google Scholar] [CrossRef]
- Marcus, G.A.; Schwettman, H.A. Picosecond optical thermometry of protein in H2O. J. Phys. Chem. B 2007, 111, 3048–3054. [Google Scholar] [CrossRef] [PubMed]
- Negrerie, M.; Cianetti, S.; Vos, M.H.; Martin, J.L.; Kruglik, S.G. Ultrafast heme dynamics in ferrous versus ferric cytochrome c studied by time-resolved resonance Raman and transient absorption spectroscopy. J. Phys. Chem. B 2006, 110, 12766–12781. [Google Scholar] [CrossRef]
- Oladepo, S.A.; Xiong, K.; Hong, Z.; Asher, S.A.; Handen, J.; Lednev, I.K. UV resonance Raman investigations of peptide and protein structure and dynamics. Chem. Rev. 2012, 112, 2604–2628. [Google Scholar] [CrossRef] [Green Version]
- Rubtsov, I.V.; Burin, A.L. Ballistic and diffusive vibrational energy transport in molecules. J. Chem. Phys. 2019, 150, 020901. [Google Scholar] [CrossRef]
- Rubtsova, N.I.; Rubtsov, I.V. Vibrational energy transport in molecules studied by relaxation-assisted two-dimensional infrared spectroscopy. Annu. Rev. Phys. Chem. 2015, 66, 717–738. [Google Scholar] [CrossRef]
- Vos, M.H.; Martin, J.L. Femtosecond processes in proteins. Biochim. Biophys. Acta 1999, 1411, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, S.; Mizuno, M.; Tran, D.P.; Dokainish, H.; Kitao, A.; Mizutani, Y. Vibrational Energy Transfer from Heme through Atomic Contacts in Proteins. J. Phys. Chem. B 2018, 122, 5877–5884. [Google Scholar] [CrossRef]
- Yu, X.; Leitner, D.M. Vibrational Energy Transfer and Heat Conduction in a Protein. J. Phys. Chem. B 2003, 107, 1698–1707. [Google Scholar] [CrossRef]
- Zhang, Y.; Fujisaki, H.; Straub, J.E. Molecular dynamics study on the solvent dependent heme cooling following ligand photolysis in carbonmonoxy myoglobin. J. Phys. Chem. B 2007, 111, 3243–3250. [Google Scholar] [CrossRef] [PubMed]
- Pessanha, M.; Londer, Y.Y.; Long, W.C.; Erickson, J.; Pokkuluri, P.R.; Schiffer, M.; Salgueiro, C.A. Redox characterization of Geobacter sulfurreducens cytochrome c7: Physiological relevance of the conserved residue F15 probed by site-specific mutagenesis. Biochemistry 2004, 43, 9909–9917. [Google Scholar] [CrossRef]
- Pessanha, M.; Morgado, L.; Louro, R.O.; Londer, Y.Y.; Pokkuluri, P.R.; Schiffer, M.; Salgueiro, C.A. Thermodynamic characterization of triheme cytochrome PpcA from Geobacter sulfurreducens: Evidence for a role played in e-/H+ energy transduction. Biochemistry 2006, 45, 13910–13917. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzolf, D.R.; McKenzie, A.M.; O’Malley, M.C.; Ponomarenko, N.S.; Swaim, C.M.; Brittain, T.J.; Simmons, N.L.; Pokkuluri, P.R.; Mulfort, K.L.; Tiede, D.M.; et al. Mimicking Natural Photosynthesis: Designing Ultrafast Photosensitized Electron Transfer into Multiheme Cytochrome Protein Nanowires. Nanomaterials 2020, 10, 2143. https://doi.org/10.3390/nano10112143
Marzolf DR, McKenzie AM, O’Malley MC, Ponomarenko NS, Swaim CM, Brittain TJ, Simmons NL, Pokkuluri PR, Mulfort KL, Tiede DM, et al. Mimicking Natural Photosynthesis: Designing Ultrafast Photosensitized Electron Transfer into Multiheme Cytochrome Protein Nanowires. Nanomaterials. 2020; 10(11):2143. https://doi.org/10.3390/nano10112143
Chicago/Turabian StyleMarzolf, Daniel R., Aidan M. McKenzie, Matthew C. O’Malley, Nina S. Ponomarenko, Coleman M. Swaim, Tyler J. Brittain, Natalie L. Simmons, Phani Raj Pokkuluri, Karen L. Mulfort, David M. Tiede, and et al. 2020. "Mimicking Natural Photosynthesis: Designing Ultrafast Photosensitized Electron Transfer into Multiheme Cytochrome Protein Nanowires" Nanomaterials 10, no. 11: 2143. https://doi.org/10.3390/nano10112143




