The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of N-doped TiO2
2.3. Synthesis of Ag/N Co-Doped TiO2
2.4. Characterizations
2.5. Photocatalytic Experiment
2.6. Analytical Procedure
2.7. Antibacterial Study
3. Result and Discussion
3.1. Silver Deposition Analysis
3.2. Structural and Elemental Analyses
3.3. Band-Gap Energy
3.4. Evaluation of Photoactivity by Application of Coumarin
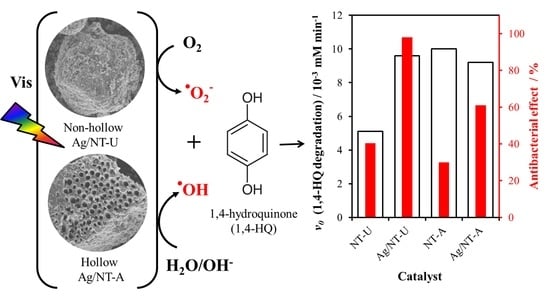
3.5. Evaluation of Photoactivity by Application of 1,4-hydroquinone
3.6. Antibacterial Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Łabuz, P.; Dubin, G.; Dąbrowski, J.M. Surface modification of nanocrystalline TiO2 materials with sulfonated porphyrins for visible light antimicrobial therapy. Catalysts 2019, 9, 821. [Google Scholar] [CrossRef] [Green Version]
- Murcia, J.J.; Ávila-Martínez, E.G.; Rojas, H.; Cubillos, J.; Ivanova, S.; Penkova, A.; Laguna, O.H. Powder and nanotubes titania modified by dye sensitization as photocatalysts for the organic pollutants elimination. Nanomaterials 2019, 9, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- González-Torres, J.C.; Poulain, E.; Domínguez-Soria, V.; García-Cruz, R.; Olvera-Neria, O. C-, N-, S-, and F-doped anatase TiO2 (101) with oxygen vacancies: Photocatalysts active in the visible region. Int. J. Photoenergy 2018, 2018, 7506151. [Google Scholar] [CrossRef] [Green Version]
- Ravindranath, R.; Roy, P.; Periasamy, A.P.; Chang, H.T. Effects of deposited ions on the photocatalytic activity of TiO2-Au nanospheres. RSC Adv. 2014, 4, 57290–57296. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Jalil, A.A.; Triwahyono, S.; Shamsuddin, N. New insights into self-modification of mesoporous titania nanoparticles for enhanced photoactivity: Effect of microwave power density on formation of oxygen vacancies and Ti3+ defects. RSC Adv. 2015, 5, 90991–91000. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Hupka, J. Preparation and characterization of Pt/Pd-modified titanium dioxide nanoparticles for visible light irradiation. Catal. Today 2014, 230, 181–187. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, P.; Chen, F.; Liu, Y.; Liu, Z.; Wang, D.; Dai, Y. Enhancement of stability of N-doped TiO2 photocatalysts with Ag loading. Appl. Surf. Sci. 2013, 265, 796–801. [Google Scholar] [CrossRef]
- Morikawa, T.; Ohwaki, T.; Suzuki, K.I.; Moribe, S.; Tero-Kubota, S. Visible-light-induced photocatalytic oxidation of carboxylic acids and aldehydes over N-doped TiO2 loaded with Fe, Cu or Pt. Appl. Catal. B Environ. 2008, 83, 56–62. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Li, W.; Pan, H. Photocatalytic reduction of CO2 over noble metal-loaded and nitrogen-doped mesoporous TiO2. Appl. Catal. A Gen. 2012, 429–430, 31–38. [Google Scholar] [CrossRef]
- Harikishore, M.; Sandhyarani, M.; Venkateswarlu, K.; Nellaippan, T.A.; Rameshbabu, N. Effect of Ag Doping on Antibacterial and Photocatalytic Activity of Nanocrystalline TiO2. Procedia Mater. Sci. 2014, 6, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Gaidau, C.; Petica, A.; Ignat, M.; Iordache, O.; Ditu, L.M.; Ionescu, M. Enhanced photocatalysts based on Ag-TiO2 and Ag-N-TiO2 nanoparticles for multifunctional leather surface coating. Open Chem. 2016, 14, 383–392. [Google Scholar] [CrossRef]
- Yang, G.; Yin, H.; Liu, W.; Yang, Y.; Zou, Q.; Luo, L.; Li, H.; Huo, Y.; Li, H. Synergistic Ag/TiO2-N photocatalytic system and its enhanced antibacterial activity towards Acinetobacter baumannii. Appl. Catal. B Environ. 2018, 224, 175–182. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Sun, S.; Swart, H.C.; Gupta, R.K. Noble metals-TiO2 nanocomposites: From fundamental mechanisms to photocatalysis, surface enhanced Raman scattering and antibacterial applications. Appl. Mater. Today 2018, 11, 82–135. [Google Scholar] [CrossRef]
- Ye, T.; Wei, Z.; Spinney, R.; Tang, C.J.; Luo, S.; Xiao, R.; Dionysiou, D.D. Chemical structure-based predictive model for the oxidation of trace organic contaminants by sulfate radical. Water Res. 2017, 116, 106–115. [Google Scholar] [CrossRef]
- Cornish, B.J.P.A.; Lawton, L.A.; Robertson, P.K.J. Hydrogen peroxide enhanced photocatalytic oxidation of microcystin-lR using titanium dioxide. Appl. Catal. B Environ. 2000, 25, 59–67. [Google Scholar] [CrossRef]
- Wang, T.; Wei, J.; Shi, H.; Zhou, M.; Zhang, Y.; Chen, Q.; Zhang, Z. Preparation of electrospun Ag/TiO2 nanotubes with enhanced photocatalytic activity based on water/oil phase separation. Phys. E Low-Dimens. Syst. Nanostructures 2017, 86, 103–110. [Google Scholar] [CrossRef]
- Sun, M.; Fang, Y.; Sun, S.; Wang, Y. Surface co-modification of TiO2 with N doping and Ag loading for enhanced visible-light photoactivity. RSC Adv. 2016, 6, 12272–12279. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Kristóf-Makó, É.; Jakab, M.; Zsirka, B. Coumarin-based quantification of hydroxyl radicals and other reactive species generated on excited nitrogen-doped TiO2. J. Photochem. Photobiol. A Chem. 2020, 112913. [Google Scholar] [CrossRef]
- Enguita, F.J.; Leitão, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. Biomed Res. Int. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Chen, Z.; Jing, J.; Hou, J. The photocatalytic phenol degradation mechanism of Ag-modified ZnO nanorods. J. Mater. Chem. C 2020, 8, 3000–3009. [Google Scholar] [CrossRef]
- Baniamerian, H.; Tsapekos, P.; Alvarado-Morales, M.; Shokrollahzadeh, S.; Safavi, M.; Angelidaki, I. Effect of surfactants on photocatalytic toxicity of TiO2-based nanoparticles toward Vibrio fischeri marine bacteria. Inorg. Chem. Commun. 2020, 116. [Google Scholar] [CrossRef]
- He, J.; Zeng, X.; Lan, S.; Lo, I.M.C. Reusable magnetic Ag/Fe, N-TiO2/Fe3O4@SiO2 composite for simultaneous photocatalytic disinfection of E. coli and degradation of bisphenol A in sewage under visible light. Chemosphere 2019, 217, 869–878. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Yu, H.; Zhang, S.; Fang, Y.; Zhang, S.; Peng, F. A facile fabrication of hierarchical Ag nanoparticles-decorated N-TiO2 with enhanced photocatalytic hydrogen production under solar light. Int. J. Hydrogen Energy 2016, 41, 3446–3455. [Google Scholar] [CrossRef]
- Szabó-Bárdos, E.; Kulcsár, P.; Kováts, N.; Békéssy, Z.; Eck-Varanka, B.; Horváth, O. Assessment of the potential bactericide effect of self-cleaning floors: A proposed protocol. Luminescence 2020, 35, 434–436. [Google Scholar] [CrossRef]
- Szabó-Bárdos, E.; Czili, H.; Horváth, A. Photocatalytic oxidation of oxalic acid enhanced by silver deposition on a TiO2 surface. J. Photochem. Photobiol. A Chem. 2003, 154, 195–201. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kidkhunthod, P.; Chanlek, N.; Soontaranon, S.; Wantala, K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019, 478, 1–14. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Shokri, M.; Rad, B. Enhancement of photocatalytic activity of TiO2 nanoparticles by Silver doping: Photodeposition versus liquid impregnation methods. Glob. Nest J. 2008, 10, 1–7. [Google Scholar] [CrossRef]
- Abbad, S.; Guergouri, K.; Gazaout, S.; Djebabra, S.; Zertal, A.; Barille, R.; Zaabat, M. Effect of silver doping on the photocatalytic activity of TiO2 nanopowders synthesized by the sol–gel route. J. Environ. Chem. Eng. 2020, 8, 103718. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Cao, J.; Xu, M.; Wang, Z. Simple fabrication of rod-like N-doped TiO2/Ag with enhanced visible-light photocatalytic activity. Ceram. Int. 2014, 40, 3975–3979. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Li, M.; Xing, Z.; Jiang, J.; Li, Z.; Yin, J.; Kuang, J.; Tan, S.; Zhu, Q.; Zhou, W. Surface plasmon resonance-enhanced visible-light-driven photocatalysis by Ag nanoparticles decorated S-TiO2−x nanorods. J. Taiwan Inst. Chem. Eng. 2018, 82, 198–204. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, Y.; Li, P.; Tu, W.; Li, P.; Tang, L.; Ye, J.; Zou, Z. Photocatalytic reduction of CO2 over Ag/TiO2 nanocomposites prepared with a simple and rapid silver mirror method. Nanoscale 2016, 8, 11870–11874. [Google Scholar] [CrossRef]
- Louit, G.; Foley, S.; Cabillic, J.; Coffigny, H.; Taran, F.; Valleix, A.; Renault, J.P.; Pin, S. The reaction of coumarin with the OH radical revisited: Hydroxylation product analysis determined by fluorescence and chromatography. Radiat. Phys. Chem. 2005, 72, 119–124. [Google Scholar] [CrossRef]
- Liu, G.; Wang, X.; Wang, L.; Chen, Z.; Li, F.; (Max) Lu, G.Q.; Cheng, H.M. Drastically enhanced photocatalytic activity in nitrogen doped mesoporous TiO2 with abundant surface states. J. Colloid Interface Sci. 2009, 334, 171–175. [Google Scholar] [CrossRef]
- Bamwenda, G.R.; Tsubota, S.; Nakamura, T.; Haruta, M. Photoassisted hydrogen production from a water-ethanol solution: A comparison of activities of AuTiO2 and PtTiO2. J. Photochem. Photobiol. A Chem. 1995, 89, 177–189. [Google Scholar] [CrossRef]
- Gallo, A.; Montini, T.; Marelli, M.; Minguzzi, A.; Gombac, V.; Psaro, R.; Fornasiero, P.; Dal Santo, V. H2 production by renewables photoreforming on Pt-Au/TiO2 catalysts activated by reduction. ChemSusChem 2012, 5, 1800–1811. [Google Scholar] [CrossRef]
- Sobana, N.; Muruganadham, M.; Swaminathan, M. Nano-Ag particles doped TiO2 for efficient photodegradation of Direct azo dyes. J. Mol. Catal. A Chem. 2006, 258, 124–132. [Google Scholar] [CrossRef]
- Pham, T.D.; Lee, B.K. Effects of Ag doping on the photocatalytic disinfection of E. coli in bioaerosol by Ag-TiO2/GF under visible light. J. Colloid Interface Sci. 2014, 428, 24–31. [Google Scholar] [CrossRef]
- Rengaraj, S.; Li, X.Z. Enhanced photocatalytic activity of TiO2 by doping with Ag for degradation of 2,4,6-trichlorophenol in aqueous suspension. J. Mol. Catal. A Chem. 2006, 243, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Demirci, S.; Dikici, T.; Yurddaskal, M.; Gultekin, S.; Toparli, M.; Celik, E. Synthesis and characterization of Ag doped TiO2 heterojunction films and their photocatalytic performances. Appl. Surf. Sci. 2016, 390, 591–601. [Google Scholar] [CrossRef]
- Szabó-Bárdos, E.; Markovics, O.; Horváth, O.; Toro, N.; Kiss, G. Photocatalytic degradation of benzenesulfonate on colloidal titanium dioxide. Water Res. 2011, 45, 1617–1628. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzciński, K.; Łapiński, M.; Nowaczyk, G.; Zielińska-Jurek, A. UV–Vis-induced degradation of phenol over magnetic photocatalysts modified with Pt, Pd, Cu and Au nanoparticles. Nanomaterials 2018, 8, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Mahrouqi, H.K.N.; Nawi, M.A.; Nawawi, W.I. Photodegradation of 4-Chlorophenol using Carbon Coated TiO2 under Solar Irradiation. Int. J. Sci. Res. Publ. 2014, 4, 1–7. [Google Scholar]
- Azizi, A.; Abouseoud, M.; Amrane, A. Phenol removal by a sequential combined fenton-enzymatic process. Nat. Environ. Pollut. Technol. 2017, 16, 321–330. Available online: http://neptjournal.com/upload-images/NL-59-50-(48)D-551.pdf (accessed on 15 November 2020).
- Tao, Y.; Cheng, Z.L.; Ting, K.E.; Yin, X.J. Photocatalytic Degradation of Phenol Using a Nanocatalyst: The Mechanism and Kinetics. J. Catal. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Raza, M.A.; Kanwal, Z.; Rauf, A.; Sabri, A.N.; Riaz, S.; Naseem, S. Size- and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 2016, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The inhibitory effects of silver nanoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]













| Catalyst | SBET Values/m2 g−1 | Pore Volume V1.7–100 nm/cm3 g−1 | Crystallite Size/nm | Band-Gap Energy/eV |
|---|---|---|---|---|
| NT-U | 32 | 0.08456 | 25.2 | 3.11 |
| Ag/NT-U 10−5 | 47 | 0.10149 | 24.8 | 3.01 |
| NT-A | 61 | 0.14307 | 19.0 | 3.13 |
| Ag/NT-A 10−5 | 62 | 0.14563 | 18.8 | 3.01 |
| Catalyst | v0 (1,4-HQ) /10−3 mM min−1 | Ratio | v0 (Other Reactive Species) /10−3 mM min−1 | Ratio |
|---|---|---|---|---|
| NT-U | 5.10 | 1.88 | 2.33 | 2.00 |
| Ag/NT-U 10−6 | 9.60 | 4.68 | ||
| NT-A | 10.00 | 0.92 | 3.79 | 0.91 |
| Ag/NT-A 10−6 | 9.20 | 3.44 |
| Ag-Loading/mol g−1 | Ag/NT-U/% | Ag/NT-A/% |
|---|---|---|
| 0 | 40.4 | 30.0 |
| 10−6 | 98.0 | 61.2 |
| 10−5 | 70.0 | 52.2 |
| 10−4 | 46.8 | 40.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Pósfai, M.; Makó, É.; Juzsakova, T.; Fónagy, O. The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect. Nanomaterials 2020, 10, 2261. https://doi.org/10.3390/nano10112261
Wafi A, Szabó-Bárdos E, Horváth O, Pósfai M, Makó É, Juzsakova T, Fónagy O. The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect. Nanomaterials. 2020; 10(11):2261. https://doi.org/10.3390/nano10112261
Chicago/Turabian StyleWafi, Abdul, Erzsébet Szabó-Bárdos, Ottó Horváth, Mihály Pósfai, Éva Makó, Tatjána Juzsakova, and Orsolya Fónagy. 2020. "The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect" Nanomaterials 10, no. 11: 2261. https://doi.org/10.3390/nano10112261