An Assessment of Cell Culture Plate Surface Chemistry for in Vitro Studies of Tissue Engineering Scaffolds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scaffold Fabrication and Biocompatibility

2.2. Comparison of Different Commercial Tissue Culture Surfaces on Scaffold Cell Seeding and Proliferation
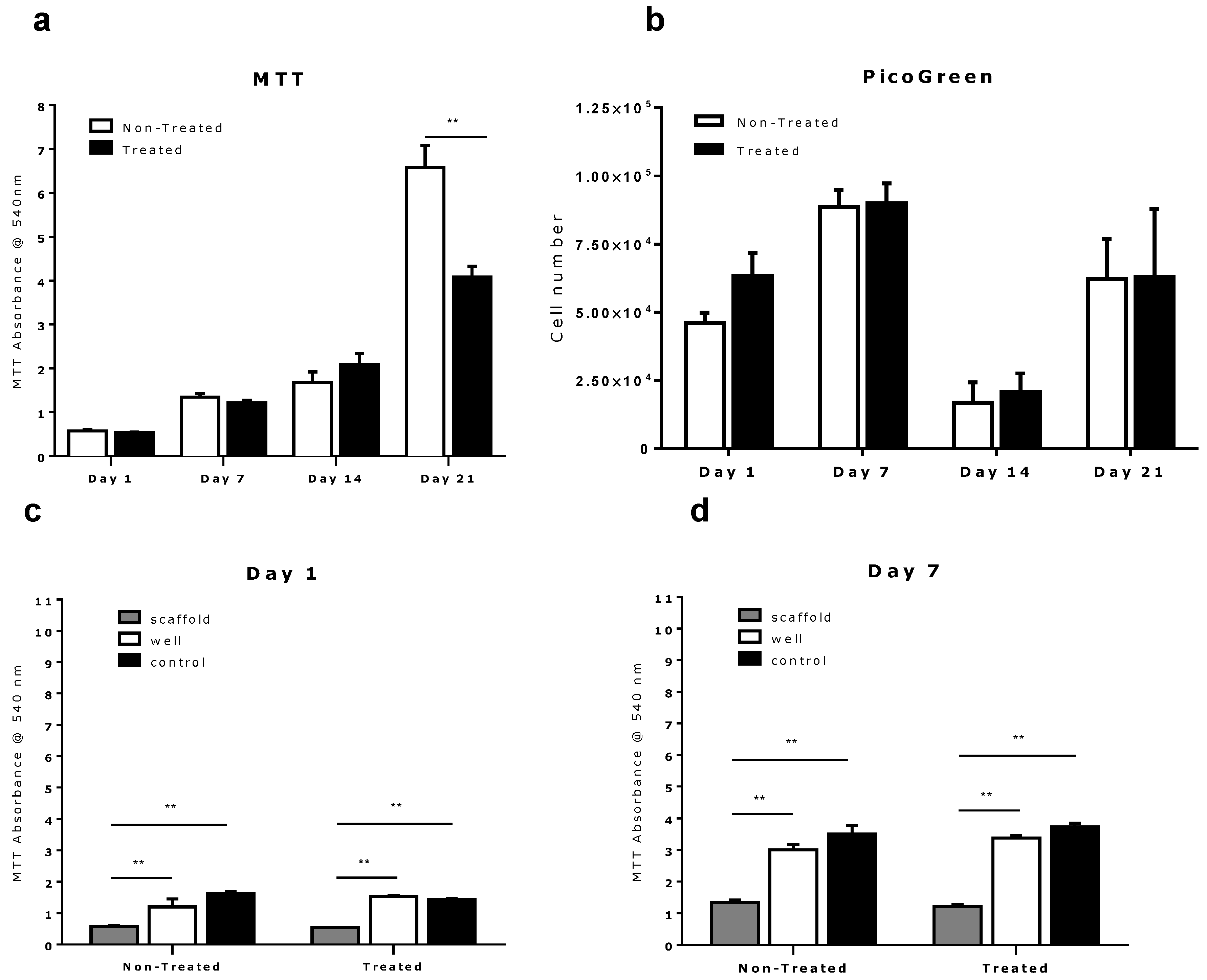
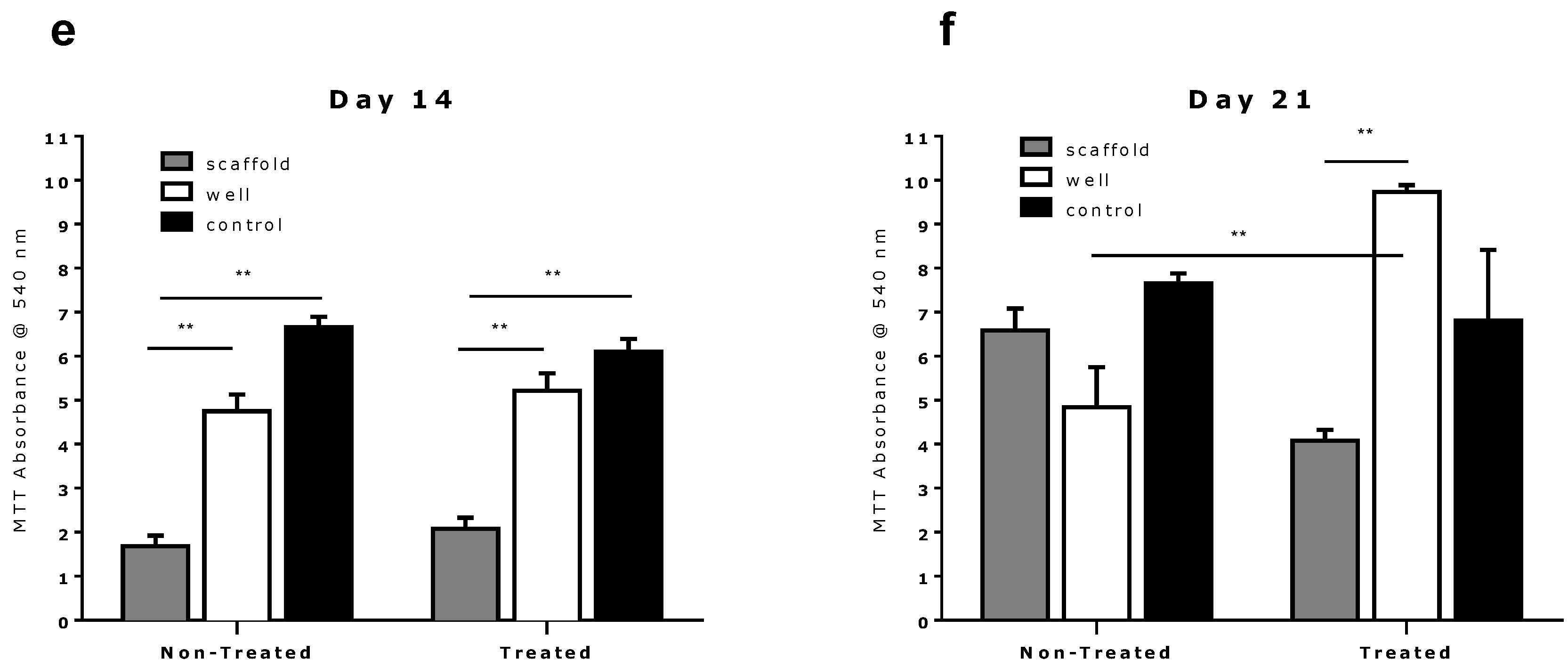
3. Experimental Section
3.1. Melt-Electrospun Scaffolds
3.2. Multi-Well Plates
3.3. Cell Culture
3.4. Cell Morphology and Attachment
3.5. Quantitative Analysis
3.5.1. Cell Metabolic Activity Assay-MTT
3.5.2. DNA Content—Picogreen
3.6. Statistics
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Maye, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Schantz, J.T.; Teoh, S.H.; Lim, T.C.; Endres, M.; Lam, C.X.F.; Hutmacher, D.W. Repair of calvarial defects with customized tissue-engineered bone grafts I. Evaluation of osteogenesis in a three-dimensional culture system. Tissue Eng. 2003, 9, S113–S126. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Electrospun-modified nanofibrous scaffolds for the mineralization of osteoblast cells. J. Biomed. Mater. Res. A 2008, 85, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.C.; Cipitria, A.; Epari, D.R.; Saifzadeh, S.; Krishnakanth, P.; Berner, A.; Woodruff, M.A.; Schell, H.; Mehta, M.; Schuetz, M.A.; et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Hesami, P.; Holzapfel, B.M.; Taubenberger, A.; Roudier, M.; Fazli, L.; Sieh, S.; Thibaudeau, L.; Gergory, L.S.; Hutmacher, D.W.; Clements, J.A. A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin. Exp. Metast. 2014, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef] [Green Version]
- Ristovski, N.; Bock, N.; Liao, S.; Powell, S.K.; Ren, J.; Kirby, G.T.S.; Blackwood, K.; Woodruff, M.A. Improved fabrication of melt electrospun tissue engineering scaffolds using direct writing and advanced electric field control. Biointerphases 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Wendt, D.; Marsano, A.; Jakob, M.; Heberer, M.; Martin, I. Oscillating perfusion of cell suspensions through three-dimensional scaffolds enhances cell seeding efficiency and uniformity. Biotechnol. Bioeng. 2003, 84, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Holy, C.E.; Shoichet, M.S.; Davies, J.E. Engineering three-dimensional bone tissue in vitro using biodegradable scaffolds: Investigating initial cell-seeding density and culture period. J. Biomed. Mater. Res. 2000, 51, 376–382. [Google Scholar] [CrossRef]
- Jones, G.; Cartmell, S.H. Optimization of cell seeding efficiencies on a three-dimensional gelatin scaffold for bone tissue engineering. J. Appl. Biomater. Biomech. 2006, 4, 172–180. [Google Scholar] [PubMed]
- Rubin, H. Altering bacteriological plastic petri dishes for tissue culture use. Public Health Rep. 1966, 8, 843–844. [Google Scholar]
- Kim, C.Y.; Goring, D.A.I. Surface morphology of polyethylene after treatment in a corona discharge. J. Appl. Polym. Sci. 1971, 15, 1357–1364. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Klein, T.J.; Melchels, F.P.W.; Woodruff, M.A. Effects of scaffold architecture on mechanical characteristics and osteoblast response to static and perfusion bioreactor cultures. Biotechnol. Bioeng. 2014, 111, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Holtorf, H.L.; Sheffield, T.L.; Ambrose, C.G.; Jansen, J.A.; Mikos, A.G. Flow perfusion culture of marrow stromal cells seeded on porous biphasic calcium phosphate ceramics. Ann. Biomed. Eng. 2005, 33, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Mygind, T.; Stiehler, M.; Baatrup, A.; Li, H.; Zou, X.; Flyvbjerg, A.; Kassem, M.; Bunger, C. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials 2007, 28, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Hua, J.; Rayan, F.; Blunn, G.W. Stem cell engineered bone with calcium-phosphate coated porous titanium scaffold or silicon hydroxyapatite granules for revision total joint arthroplasty. J. Mater. Sci. Mater. Med. 2014, 25, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Yeo, A.; Wong, W.J.; Khoo, H.H.; Teoh, S.H. Surface modification of PCL-TCP scaffolds improve interfacial mechanical interlock and enhance early bone formation: An in vitro and in vivo characterization. J. Biomed. Mater. Res. A. 2010, 92, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Blackwood, K.A.; Doustgani, A.; Poh, P.; Steck, R.; Stevens, M.M.; Woodruff, M.A. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J. Biomed. Mater. Res. A 2014, 102, 3140–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Sah, R.L.Y.; Doong, J.Y.H.; Grodzinsky, A.J. Fluorometric assay of DNA in cartilage explants using hoechst 33258. Anal. Biochem. 1988, 76, 168–176. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röder, A.; García-Gareta, E.; Theodoropoulos, C.; Ristovski, N.; Blackwood, K.A.; Woodruff, M.A. An Assessment of Cell Culture Plate Surface Chemistry for in Vitro Studies of Tissue Engineering Scaffolds. J. Funct. Biomater. 2015, 6, 1054-1063. https://doi.org/10.3390/jfb6041054
Röder A, García-Gareta E, Theodoropoulos C, Ristovski N, Blackwood KA, Woodruff MA. An Assessment of Cell Culture Plate Surface Chemistry for in Vitro Studies of Tissue Engineering Scaffolds. Journal of Functional Biomaterials. 2015; 6(4):1054-1063. https://doi.org/10.3390/jfb6041054
Chicago/Turabian StyleRöder, Alexander, Elena García-Gareta, Christina Theodoropoulos, Nikola Ristovski, Keith A. Blackwood, and Maria A. Woodruff. 2015. "An Assessment of Cell Culture Plate Surface Chemistry for in Vitro Studies of Tissue Engineering Scaffolds" Journal of Functional Biomaterials 6, no. 4: 1054-1063. https://doi.org/10.3390/jfb6041054





