Fabrication of Poly-l-lactic Acid/Dicalcium Phosphate Dihydrate Composite Scaffolds with High Mechanical Strength—Implications for Bone Tissue Engineering
Abstract
:1. Introduction
2. Results
2.1. Incorporation of PLLA on DCPD Disks
| P/L Ratio | Liquid Phase | PLLA Incorporation (mg/mm3) |
|---|---|---|
| 1.00 | Deionized Water | 0.06 ± 0.01 |
| Sodium Citrate | 0.05 ± 0.01 | |
| 1.25 | Deionized Water | 0.06 ± 0.01 |
| Sodium Citrate | 0.05 ± 0.01 | |
| 1.50 | Deionized Water | 0.06 ± 0.01 |
| Sodium Citrate | 0.05 ± 0.01 |
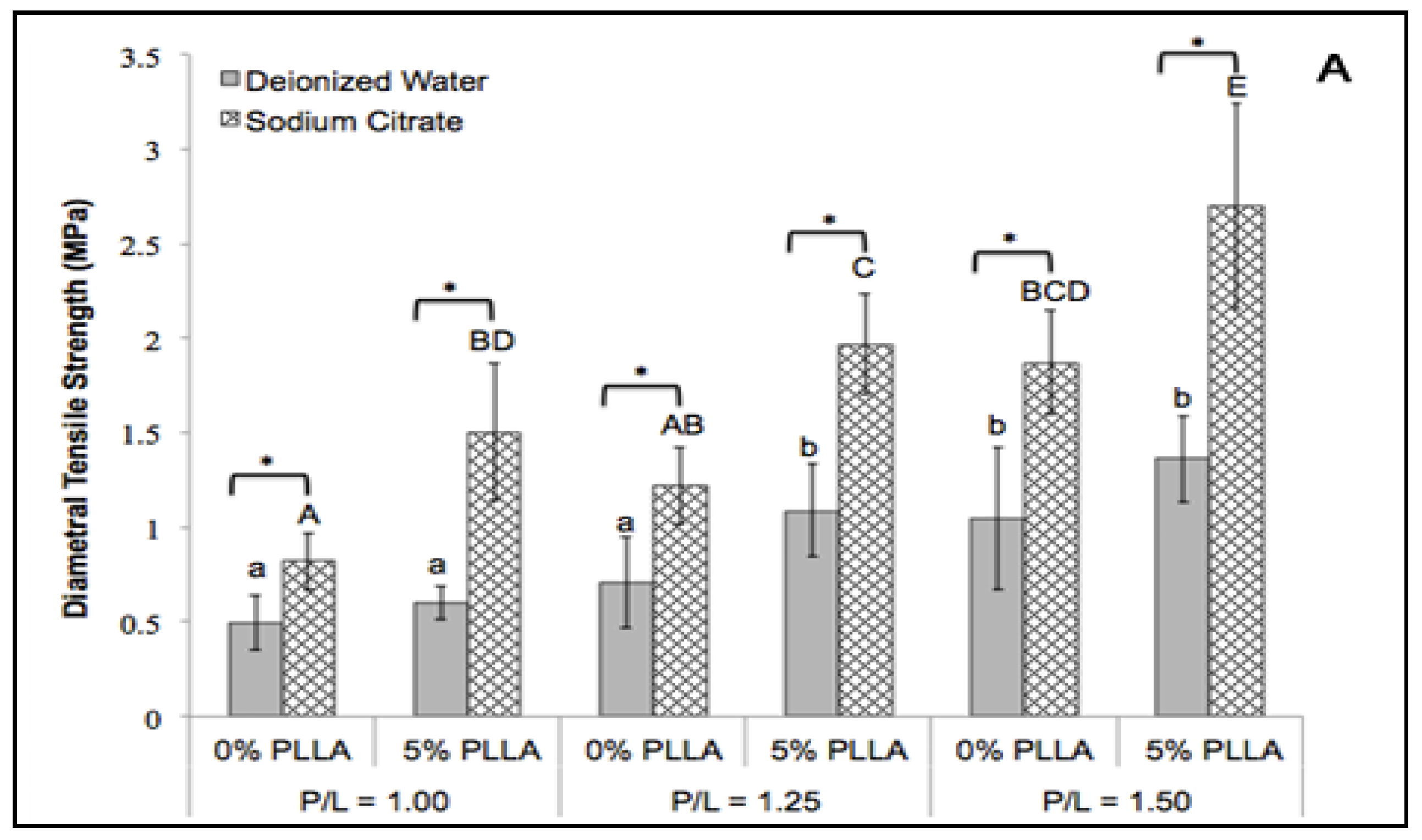
2.2. Diametral Tensile Strength
2.3. Fracture Energy
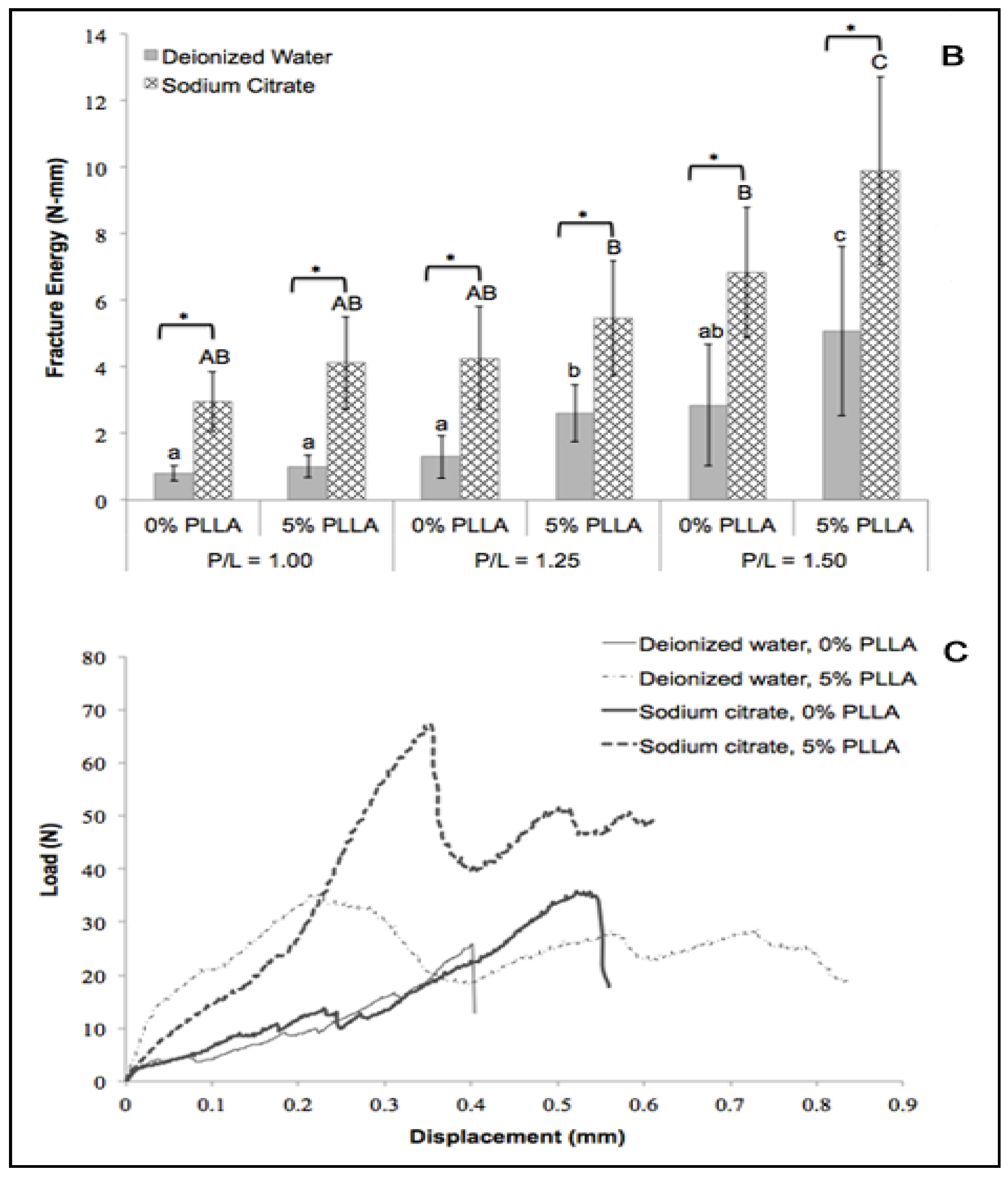
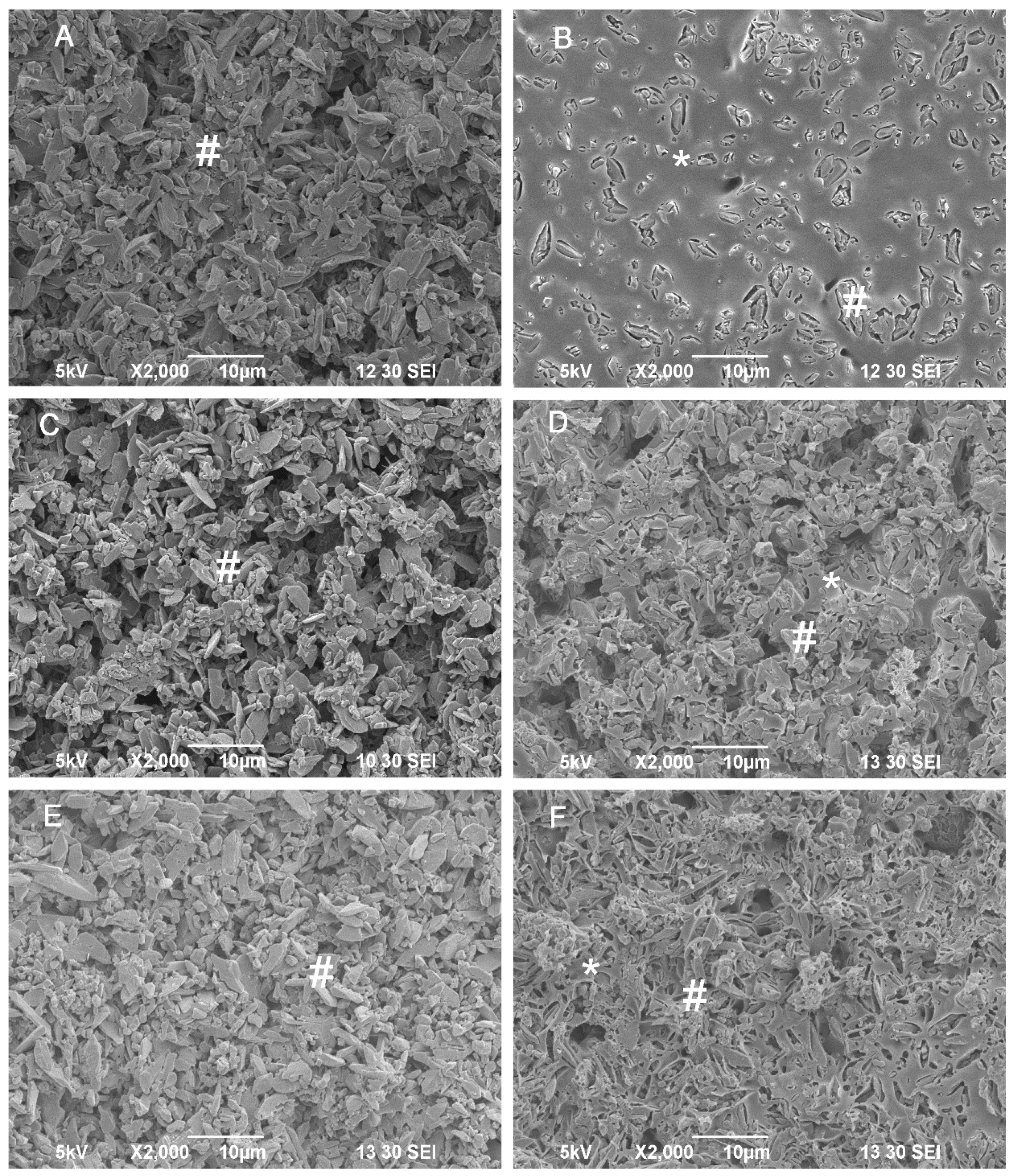
2.4. In Vitro Degradation Behavior
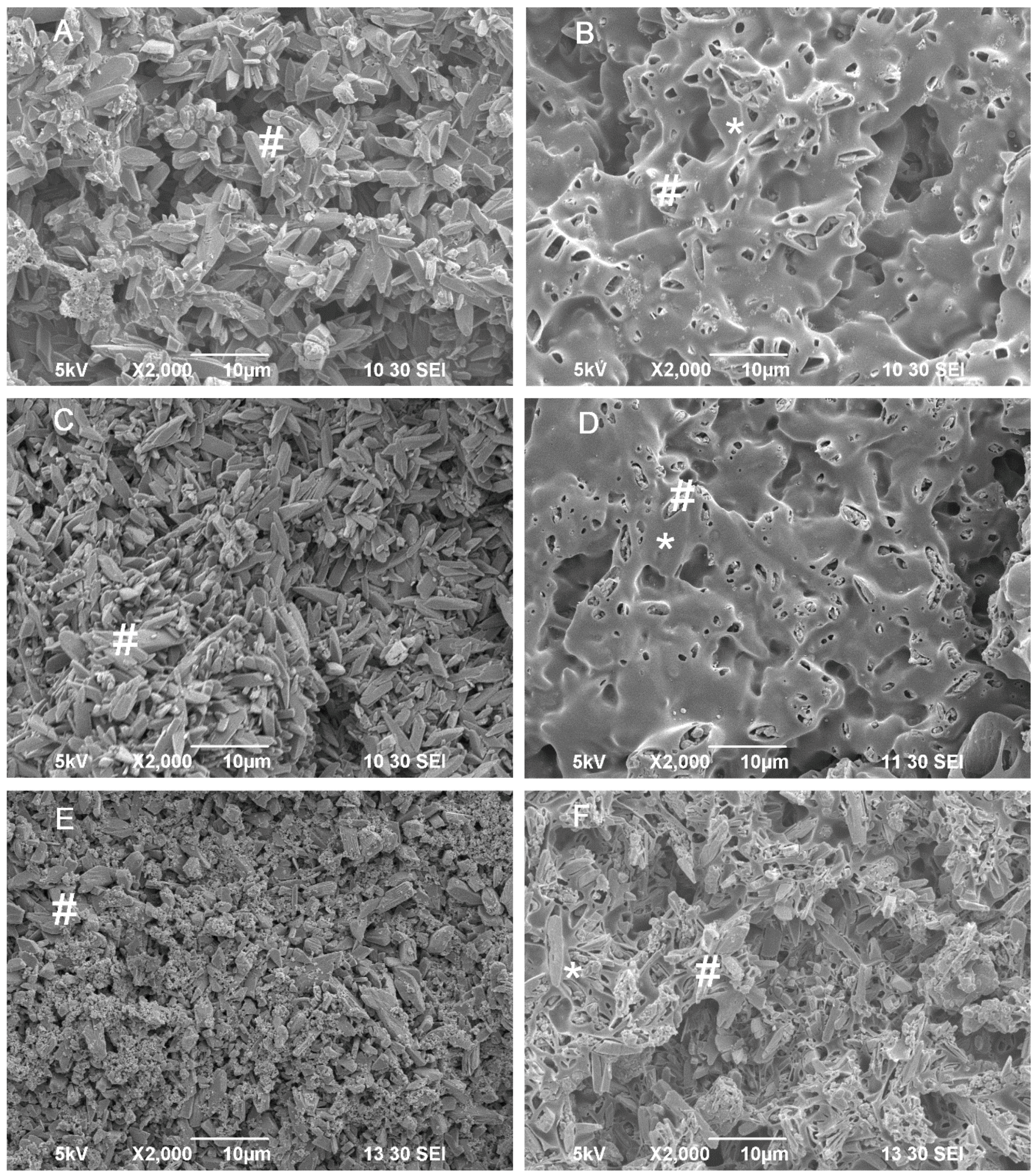
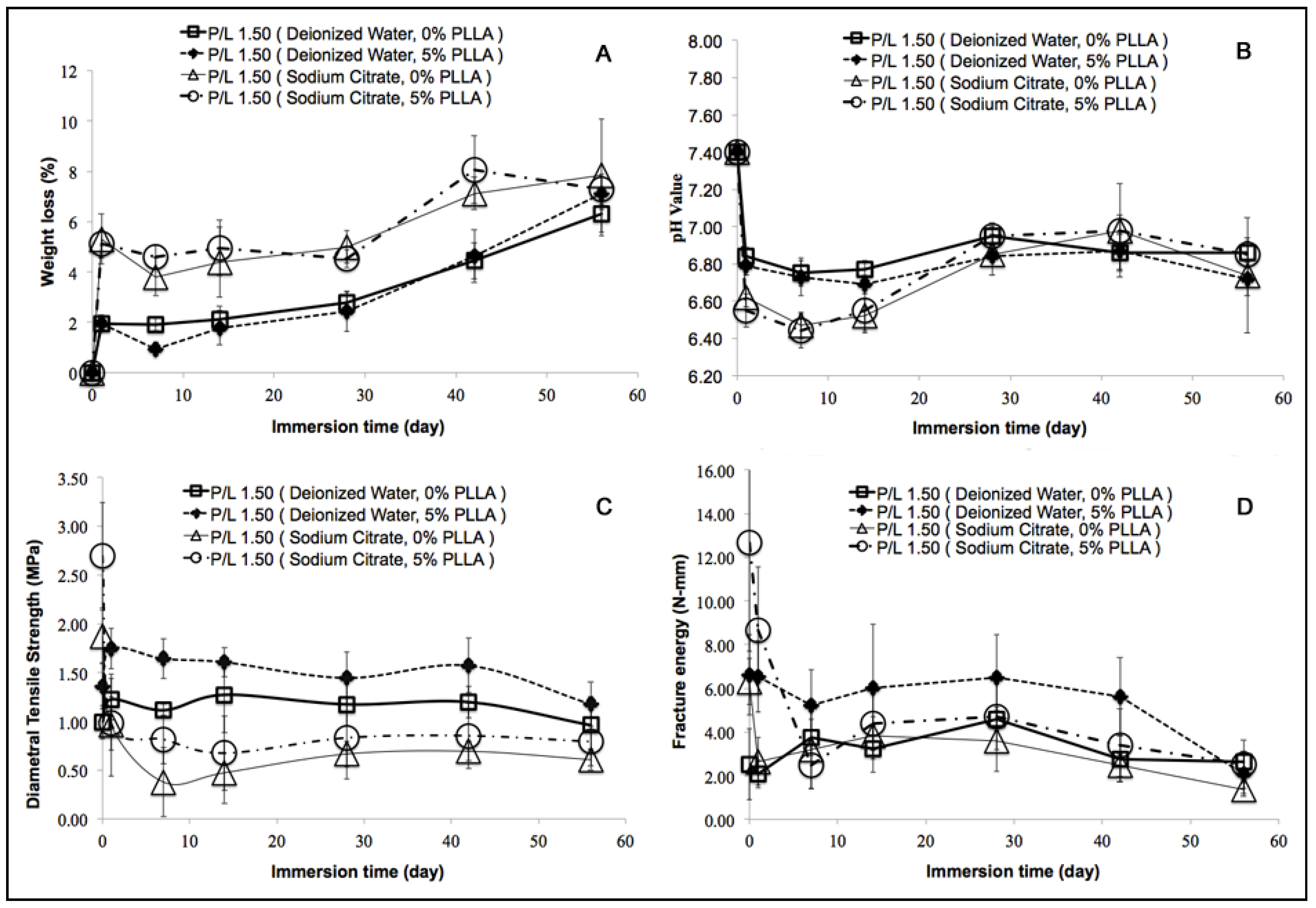
2.5. Mechanical Property of the Composite Scaffolds
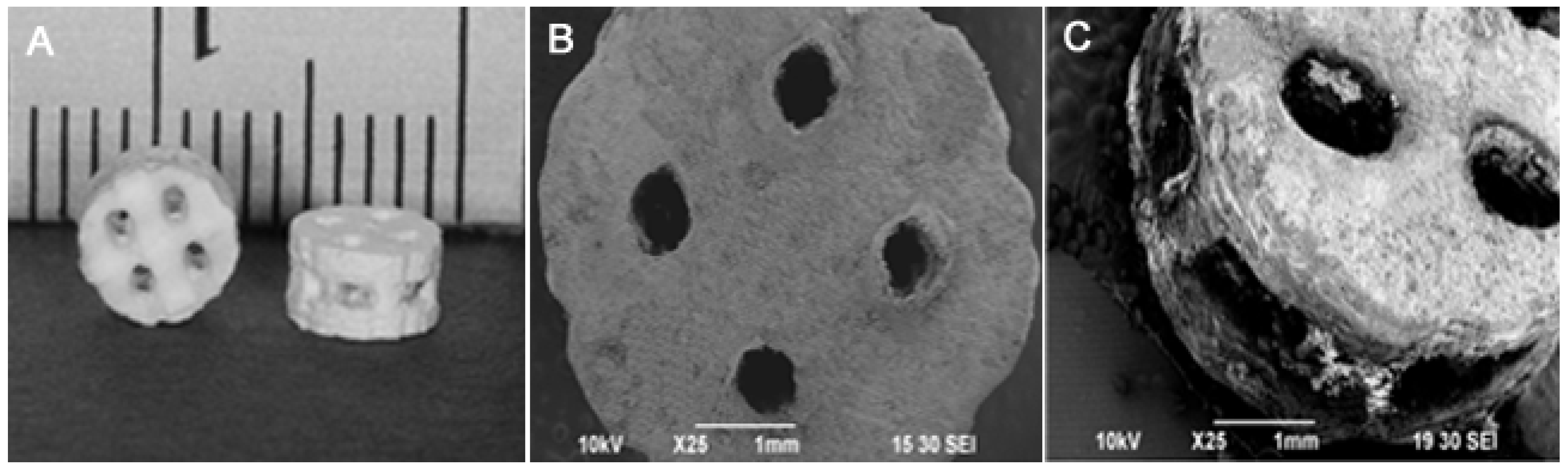
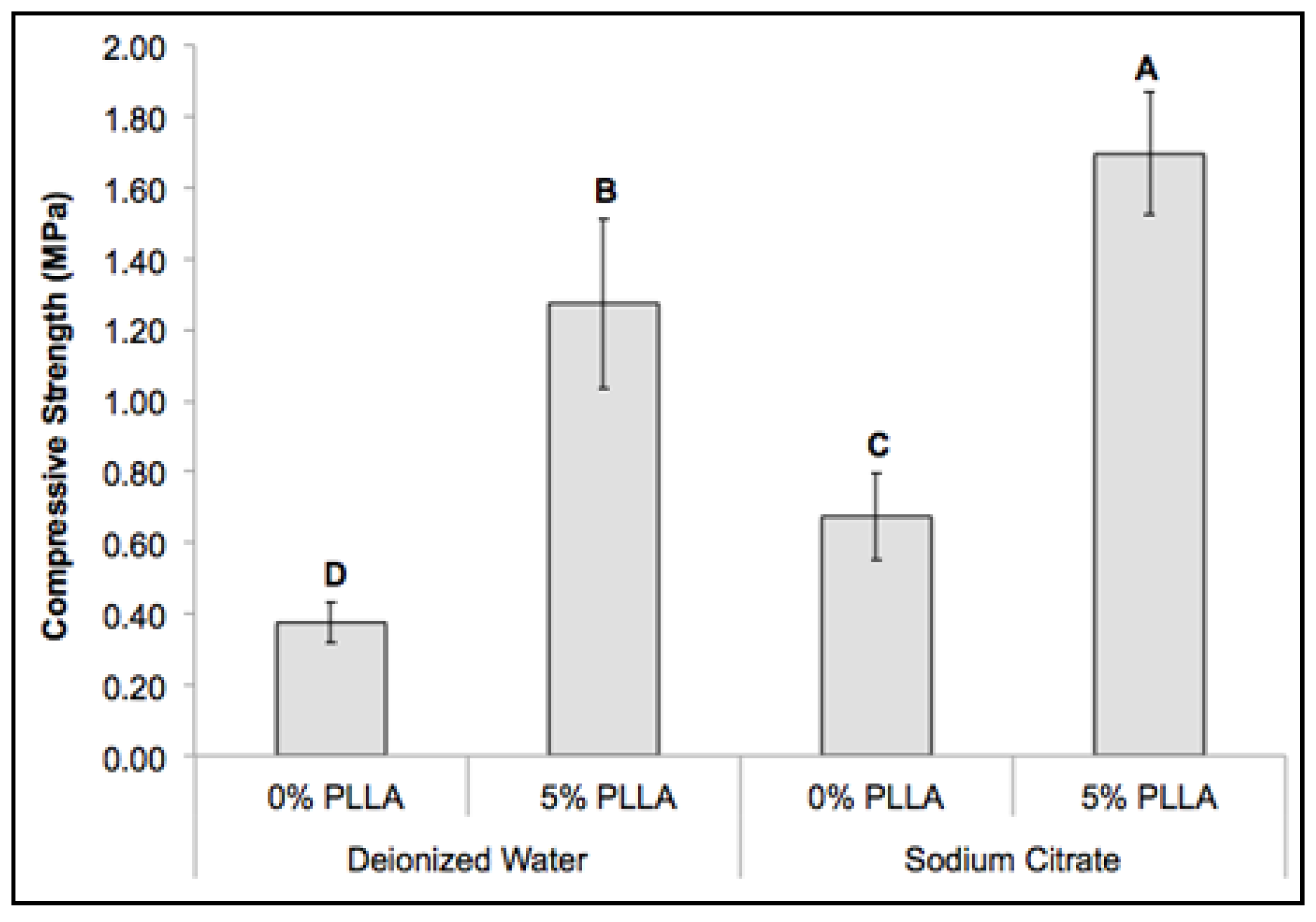
2.6. Cytocompatibility of the DCPD Cements and Cell Proliferation
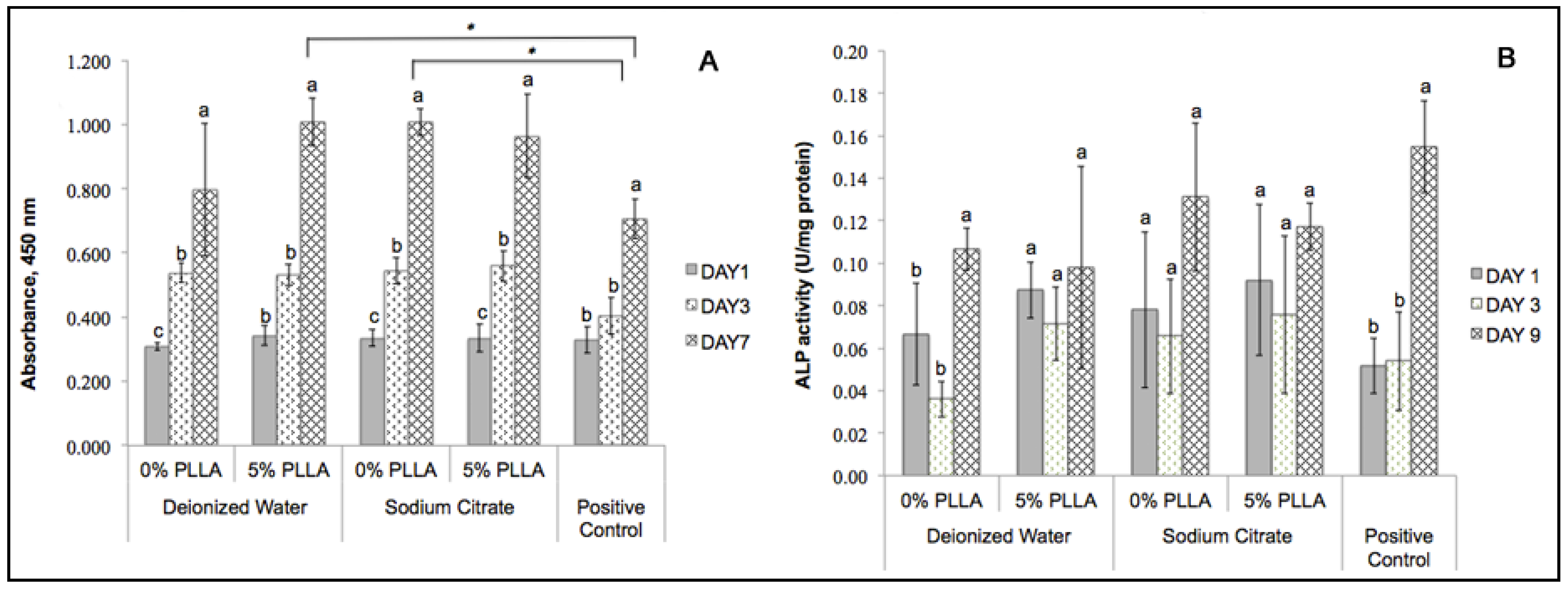
2.7. Determining Cell Differentiation by ALP Activity
2.8. Cell Attachment and Morphology
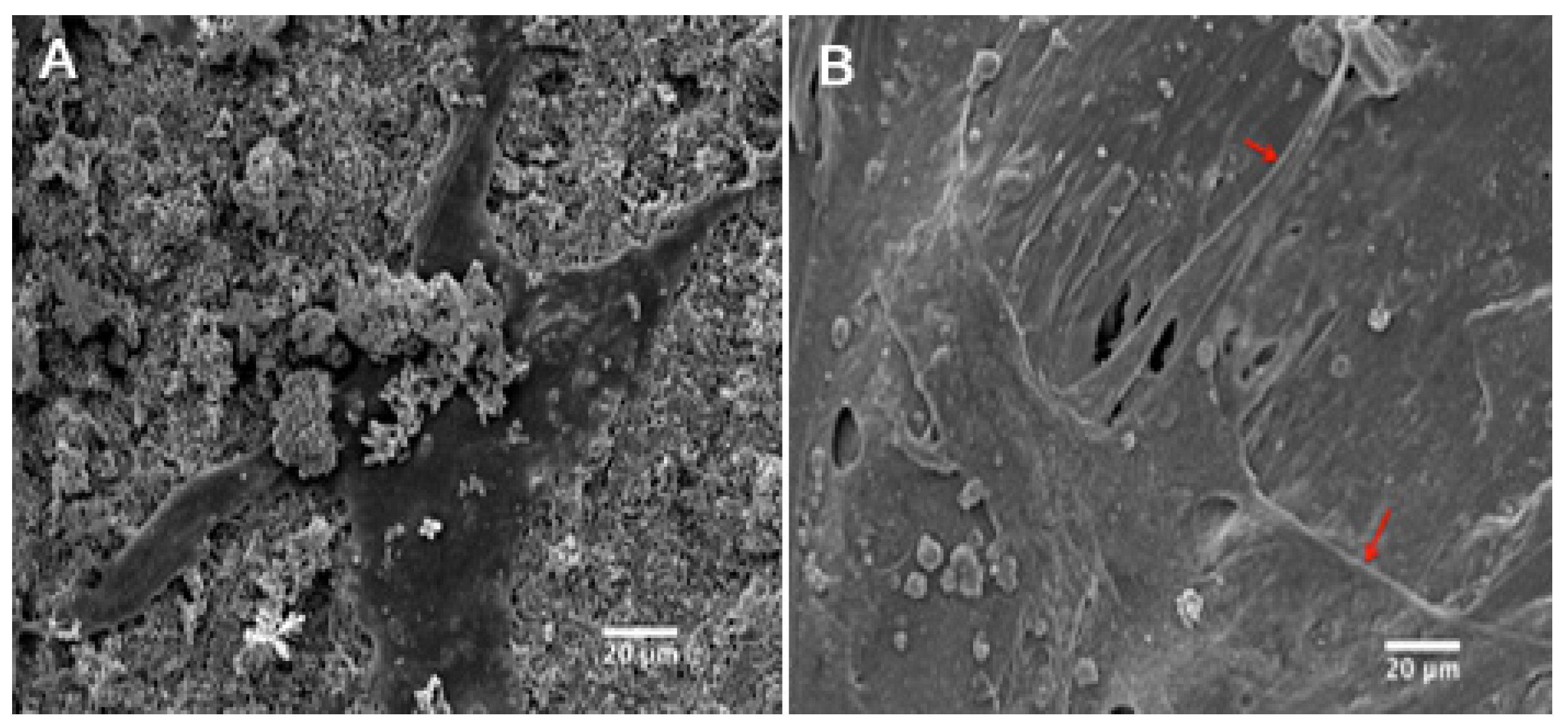
3. Discussion
4. Experimental Section
4.1. DCPD Disk Preparation
4.2. Mechanical Testing and Degradation of Disk Specimens
4.3. In Vitro Degradation of Disk Specimens
4.4. Scaffold Fabrication and Mechanical Testing
4.5. In Vitro Cellular Study
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Arner, J.W.; Santrock, R.D. A historical review of common bone graft materials in foot and ankle surgery. Foot Ankle Spec. 2014, 7, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Diez, G.F.; Fontao, F.N.; Bassi, A.P.; Gama, J.C.; Claudino, M. Tomographic follow-up of bone regeneration after bone block harvesting from the mandibular ramus. Int. J. Oral Maxillofac. Surg. 2014, 43, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.R.; Wachter, N.; Patka, P.; Kinzl, L. First histological observations on the incorporation of a novel calcium phosphate bone substitute material in human cancellous bone. J. Biomed. Mater. Res. 2001, 58, 329–334. [Google Scholar] [CrossRef]
- Alkhraisat, M.H.; Marino, F.T.; Retama, J.R.; Jerez, L.B.; Lopez-Cabarcos, E. Beta-tricalcium phosphate release from brushite cement surface. J. Biomed. Mater. Res. A 2008, 84, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Donati, D.; Zolezzi, C.; Tomba, P.; Vigano, A. Bone grafting: Historical and conceptual review, starting with an old manuscript by vittorio putti. Acta Orthop. 2007, 78, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ma, P.X. Porous poly(l-lactic acid)/apatite composites created by biomimetic process. J. Biomed. Mater. Res. 1999, 45, 285–293. [Google Scholar] [CrossRef]
- Bohner, M. Calcium orthophosphates in medicine: From ceramics to calcium phosphate cements. Injury 2000, 31, 37–47. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef]
- Rosen, H.M.; Ackerman, J.L. Porous block hydroxyapatite in orthognathic surgery. Angle Orthod. 1991, 61, 185–191. [Google Scholar] [PubMed]
- He, F.; Ye, J. In vitro degradation, biocompatibility, and in vivo osteogenesis of poly(lactic-co-glycolic acid)/calcium phosphate cement scaffold with unidirectional lamellar pore structure. J. Biomed. Mater. Res. A 2012, 100, 3239–3250. [Google Scholar] [CrossRef] [PubMed]
- Ooms, E.M.; Wolke, J.G.C.; van de Heuvel, M.T.; Jeschke, B.; Jansen, J.A. Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials 2003, 24, 989–1000. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Stokes, K.E.; Hoellrich, R.G.; Chamberland, D.L.; McLoughlin, S.W. Effect of bioactive glass particle size on osseous regeneration of cancellous defects. J. Biomed. Mater. Res. 1998, 41, 527–533. [Google Scholar] [CrossRef]
- Wheeler, D.L.; Stokes, K.E.; Park, H.M.; Hollinger, J.O. Evaluation of particulate bioglass in a rabbit radius ostectomy model. J. Biomed. Mater. Res. 1997, 35, 249–254. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Theiss, F.; Apelt, D.; Brand, B.; Kutter, A.; Zlinszky, K.; Bohner, M.; Matter, S.; Frei, C.; Auer, J.A.; von Rechenberg, B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005, 26, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Chu, T.M. Calcium phosphate cement reinforcement by polymer infiltration and in situ curing: A method for 3D scaffold reinforcement. J. Biomed. Mater. Res. A 2010, 94, 547–555. [Google Scholar] [PubMed]
- Peroglio, M.; Gremillard, L.; Chevalier, J.; Chazeau, L.; Gauthier, C.; Hamaide, T. Toughening of bio-ceramics scaffolds by polymer coating. J. Eur. Ceram. Soc. 2007, 27, 2679–2685. [Google Scholar] [CrossRef]
- Kang, Y.; Scully, A.; Young, D.A.; Kim, S.; Tsao, H.; Sen, M.; Yang, Y. Enhanced mechanical performance and biological evaluation of a PLGA coated β-TCP composite scaffold for load-bearing applications. Eur. Polym. J. 2011, 47, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Bennett, J.; Treasure, T.; Voytik-Harbin, S.; Goebel, W.S.; Chu, T.M. Poly(propylene fumarate) reinforced dicalcium phosphate dihydrate cement composites for bone tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.P.; Mohammed, A.R.; Perrie, Y.; Gbureck, U.; Barralet, J.E. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009, 5, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Merkle, H.P.; Landuyt, P.V.; Trophardy, G.; Lemaitre, J. Effect of several additives and their admixtures on the physico-chemical properties of a calcium phosphate cement. J. Mater. Sci. Mater. Med. 2000, 11, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.T.; Torres, J.; Hamdan, M.; Rodriguez, C.R.; Cabarcos, E.L. Advantages of using glycolic acid as a retardant in a brushite forming cement. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Alge, D.L.; Santa Cruz, G.; Goebel, W.S.; Chu, T.M. Characterization of dicalcium phosphate dihydrate cements prepared using a novel hydroxyapatite-based formulation. Biomed. Mater. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Anahi, P.; Aldo, R.B.; Claudia, F.; Dirk, W.S.; Judith, A.R. Toughening and functionalization of bioactive ceramic and glass bone scaffolds by biopolymer coatings and infiltration: A review of the last 5 years. Med. Devices 2015, 12. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.D.; Smeets, R. Current trends and future perspectives of bone substitute materials—From space holders to innovative biomaterials. J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Blaker, J.J.; Nazhat, S.N.; Maquet, V.; Boccaccini, A.R. Long-term in vitro degradation of pdlla/bioglass bone scaffolds in acellular simulated body fluid. Acta Biomater. 2011, 7, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhuang, X.; Tang, Z.; Chen, X. Polylactic acid (PLA): Research, development and industrialization. Biotechnol. J. 2010, 5, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Kullkarni, R.K.; Pani, K.C.; Neuman, C.; Leonard, F. Polylactic acid for surgical implants. Arch Surg. 1966, 93, 839–843. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Self-setting calcium orthophosphate formulations: Cements, concretes, pastes and putties. Int. J. Mater. Chem. 2012, 1, 1–48. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Hölzel, T.; Klammert, U.; Würzler, K.; Müller, F.A.; Barralet, J.E. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv. Funct. Mater. 2007, 17, 3940–3945. [Google Scholar] [CrossRef]
- Wagoner Johnson, A.J.; Herschler, B.A. A review of the mechanical behavior of cap and cap/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Chen, D.; Fu, J.; Yang, S.; Hou, R.; Suo, J. Macroporous biphasic calcium phosphate scaffolds reinforced by poly-l-lactic acid/hydroxyapatite nanocomposite coatings for bone regeneration. Biochem. Eng. J. 2015, 98, 29–37. [Google Scholar] [CrossRef]
- Martínez-Vázquez, F.; Pajares, A.; Guiberteau, F.; Miranda, P. Effect of polymer infiltration on the flexural behavior of β-tricalcium phosphate robocast scaffolds. Materials 2014, 7, 4001–4018. [Google Scholar] [CrossRef]
- Pezzotti, G.; Asmus, S.M.F. Fracture behavior of hydroxyapatite/polymer interpenetrating network composites prepared by in situ polymerization process. Mater. Sci. Eng. A 2001, 316, 231–237. [Google Scholar] [CrossRef]
- Grover, L. In vitro ageing of brushite calcium phosphate cement. Biomaterials 2003, 24, 4133–4141. [Google Scholar] [CrossRef]
- Alge, D.L.; Goebel, W.S.; Chu, T.M. Effects of dcpd cement chemistry on degradation properties and cytocompatibility: Comparison of MCPM/β-TCP and MCPM/HA formulations. Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Dasarathy, H.; Riley, C. Electrodeposition of brushite coatings and their transformation to hydroxyapatite in aqueous solutions. J. Biomed. Mater. Res. 1999, 45, 302–310. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Boughton, P.; Zreiqat, H. Improvement of mechanical and biological properties of porous CASIO3 scaffolds by poly(d,l-lactic acid) modification. Acta Biomater. 2008, 4, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Ivanovski, S.; Hamlet, S.M.; Hutmacher, D.W. Effect of culture conditions and calcium phosphate coating on ectopic bone formation. Biomaterials 2013, 34, 5538–5551. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanataweethum, N.; Liu, W.C.; Goebel, W.S.; Li, D.; Chu, T.M. Fabrication of Poly-l-lactic Acid/Dicalcium Phosphate Dihydrate Composite Scaffolds with High Mechanical Strength—Implications for Bone Tissue Engineering. J. Funct. Biomater. 2015, 6, 1036-1053. https://doi.org/10.3390/jfb6041036
Tanataweethum N, Liu WC, Goebel WS, Li D, Chu TM. Fabrication of Poly-l-lactic Acid/Dicalcium Phosphate Dihydrate Composite Scaffolds with High Mechanical Strength—Implications for Bone Tissue Engineering. Journal of Functional Biomaterials. 2015; 6(4):1036-1053. https://doi.org/10.3390/jfb6041036
Chicago/Turabian StyleTanataweethum, Nida, Wai Ching Liu, W. Scott Goebel, Ding Li, and Tien Min Chu. 2015. "Fabrication of Poly-l-lactic Acid/Dicalcium Phosphate Dihydrate Composite Scaffolds with High Mechanical Strength—Implications for Bone Tissue Engineering" Journal of Functional Biomaterials 6, no. 4: 1036-1053. https://doi.org/10.3390/jfb6041036





