Magnetically Targeted Stem Cell Delivery for Regenerative Medicine
Abstract
:1. Introduction
| Imaging Modality | Contrast Agent | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|
| MRI | Superparamagnetic iron oxide nanoparticles (SPIONs) | Painless, full body 3D scanning, no ionizing radiation is used, difficult but possible quantification of cells, manipulation of cells using external magnetic field | Tracer dilutes when cell divides, possible transfer of agent to other cells, not usable in patients with implants, imaging process can be claustrophobic | [17,18] |
| MRI | Gadolinium, fluorescent agents, and perfluorocarbon | Full body 3D scanning, no ionizing radiation, detection of individual cell is possible | Tracer dilutes when cell divides, possible transfer of agent to other cells, not usable in patients with implants, imaging process can be claustrophobic | [17,18] |
| Optical | Protein fluorescent markers, fluorescent dyes, luciferase substrates, and near infrared fluorophores | Extended palette of fluorophores permits simultaneous analysis of different cell types and lineages, can be combined with other imaging modalities, does not use ionizing radiation | Dye cytotoxicity, tracer dilutes when cell divides, tissue penetration depth is limited | [17,19,20] |
| PET & SPECT | SPECT: High-energy gamma emitters; PET: High-energy positron emitters | High detail, full body 3D scanning, transgenic approaches translate to no cell division dilutions in tracer signal, quantification is possible with SPECT | Ionizing radiation, quantification can be difficult in PET, genetic modification of stem cells, intravenous injection of contrast agent, radioactive tracer can cause allergic reaction | [17,21] |
| Ultra-Sound | Microbubbles | No ionizing radiation, possible to detect single cells, fast, relatively inexpensive, can image soft tissues | Low resolution, restricted to specific parts of the body, quantification is difficult, contrast agent dilutes when cell divides and can transfer to other cells | [17,22] |
| X-ray & CT | High density iodine or gadolinium | X-ray is fast and relatively inexpensive, CT permits full body, 3D scanning | High contrast agent concentrations can be toxic, ionizing radiation in dyes, X-ray data is hard to quantify, increase in possibility of cancer development at later age | [17,23] |
2. Magnetic Targeting: Basic Principles, Components, Production, and Coatings
2.1. Magnetic Nanoparticle
2.2. Synthesis of Magnetic Nanoparticles
2.2.1. Co-Precipitation
2.2.2. Thermal Decomposition
2.2.3. Microemulsion
2.2.4. Hydrothermal Synthesis
2.3. Magnetic Field Gradient
2.4. Protection and Stabilization of Nanoparticles
2.4.1. Polyethylene Glycol
2.4.2. Dextran
2.4.3. Starch
2.4.4. Citrate
3. Tagging Stem Cells with Iron Oxide Nanoparticles
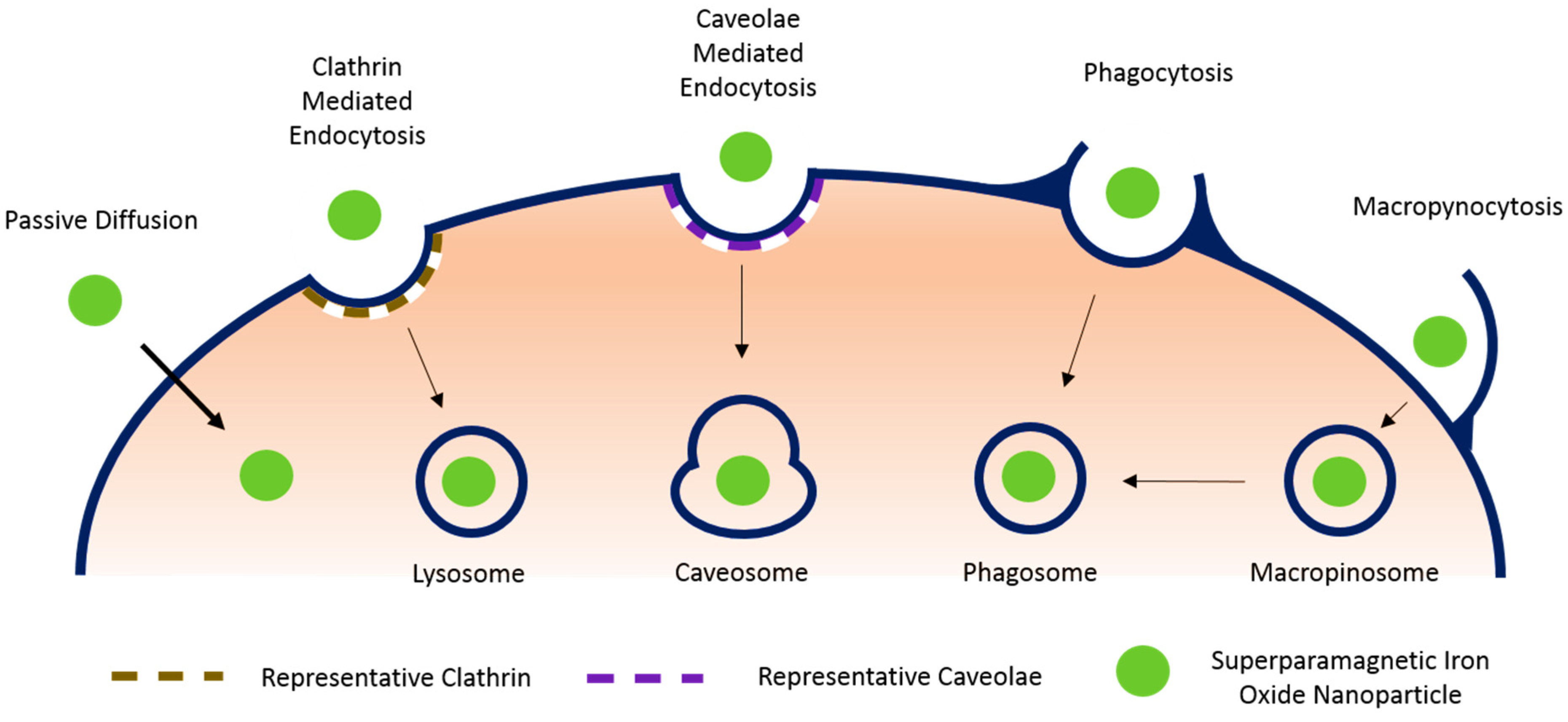
3.1. Passive Diffusion
3.2. Endocytosis
3.2.1. Clathrin Mediated Endocytosis
3.2.2. Caveolae Mediated Endocytosis
3.2.3. Phagocytosis
3.2.4. Macropynocytosis
3.3. External Binding (Antibody Mediated Targeting)
4. Application of SPIONs in Pathological Models
| Cell Type | Organ | Condition | Model | SPION Type | Ref. |
|---|---|---|---|---|---|
| Mesenchymal stem cell | Spine | Spinal cord injury | Unspecified rat | poly-L-lysine-coated SPIONs | [7] |
| Spinal cord injury | Sprague–dawley rat | poly-L-lysine-coated SPIONs | [49] | ||
| Heart | Myocardial infarction | Sprague–dawley rat | SPIO plus poly-L-lysine | [47] | |
| Vasculature | Coronary embolization | Sprague–dawley rat | Resovist | [38] | |
| Myocardial infarction & Heart failure | 4 mm Quarts Tube | Resovist | [37] | ||
| Eye | Retinal degeneration | S334ter-4 heterozygous transgenic rats | FluidMAG-nanoparticles | [6] | |
| Liver | No specific condition, Proof of concept | Nude Rats | Ferumoxide PLL complexes | [14] | |
| Knee | Cartilage injury | In vitro model | Positively charged ferric oxide nano-composites | [50] | |
| Cartilage injury | Mini pig | Ferucarbotran | [51] | ||
| Bone marrow stromal cell | Spine | Spinal cord injury | Sprague–dawley rat | poly-L-lysine-coated SPIONs | [52] |
| Neural progenitor cell | Spine | Spinal cord injury | Sprague–dawley rat | RGDS peptide magnetic bead complex | [53] |
| Cardiosphere-derived tem cell | Heart | Myocardial infarction | Wistar kyoto rats | Ferumoxytol | [11] |
| Exogenous bone marrow-derived stem cell (CD45-positive) | Heart | Myocardial infarction | Wistar kyoto rats | Ferumoxytol | [54] |
| Endogenous stem cell (CD34-positive) | Heart | Myocardial infarction | Wistar kyoto rats | Ferumoxytol | [54] |
| Endothelial progenitor cell | Vasculature | Common carotid artery injury | Sprague–dawley rat | Feridex | [16] |
| Bovine aortic endothelial cell | Vasculature | Reendothelialization deficiency after common carotid artery angioplasty | Sprague–dawley rat | Polylactide MNP | [25] |
| Retinal pigment epithelial cells | Eye | Choroidal neovascularization | In vitro model | Magnetite cationic liposomes | [55] |
4.1. Delivery of Mesenchymal Stem Cells for Spinal Cord Injury
4.2. Delivery of Circulating Progenitors for Vascular Injury
4.3. Delivery of Mesenchymal Stem Cells for Retinal Degenerations
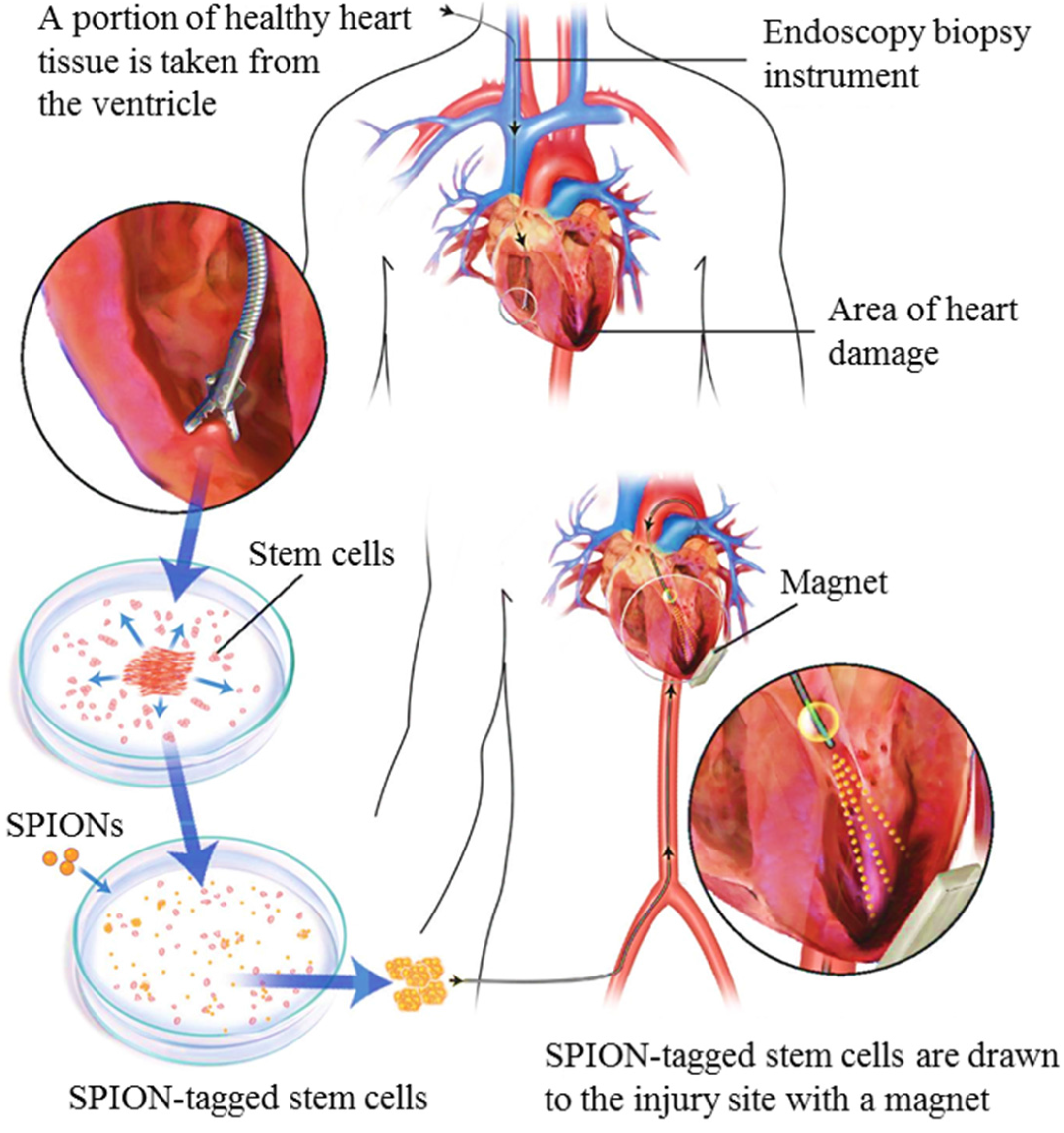
4.4. Delivery of Stem Cells for Heart Regeneration
4.5. Delivery of Stem Cells for Cartilage Regeneration
5. New Technology: Magnetic Bifunctional Cell Engagers (MagBICE)
6. Discussion
7. Conclusion
Acknowledgements
Conflicts of Interest
References
- Rudolph, K. Adult Stem Cells in Aging, Diseases, and Cancer; Karger AG: Basel, Switzerland, 2015. [Google Scholar]
- Stem Cell Information. National Institutes of Health, U.S. Department of Health and Human Services Website. Available online: http://stemcells.nih.gov/info/basics/pages/basics1.aspx (accessed on 5 June 2015).
- Krishna, K.A.; Krishna, K.S.; Berrocal, R.; Rao, K.S.; Sambasiva Rao, K.R.S. Myocardial infarction and stem cells. J. Pharm. Bioallied Sci. 2011, 3, 182–188. [Google Scholar] [PubMed]
- Wu, X.; Wang, G.; Tang, C.; Zhang, D.; Li, Z.; Du, D.; Zhang, Z. Mesenchymal stem cell seeding promotes reendothelialization of the endovascular stent. J. Biomed. Mater. Res. 2011, 98A, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Leeper, N.J.; Hunter, A.L.; Cooke, J.P. Stem cell therapy for vascular regeneration: Adult, embryonic, and induced pluripotent stem cells. Circulation 2010, 122, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Yanai, A.; Häfeli, U.O.; Metcalfe, A.L.; Soema, P.; Addo, L.; Gregory-Evans, C.Y.; Po, K.; Shan, X.; Moritz, O.L.; Gregory-Evans, K. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012, 21, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Tukmachev, D.; Lunov, O.; Zablotskii, V.; Dejneka, A.; Babic, M.; Syková, E.; Kubinová, Š. An effective strategy of magnetic stem cell delivery for spinal cord injury therapy. Nanoscale 2015, 7, 3954–3958. [Google Scholar] [CrossRef] [PubMed]
- Shinozuka, K.; Dailey, T.; Tajiri, N.; Ishikawa, H.; Kaneko, Y.; Borlongan, C.V. Stem cell transplantation for neuroprotection in stroke. Brain Sci. 2013, 3, 239–261. [Google Scholar] [CrossRef] [PubMed]
- United Networks for Organs Sharing. Available online: http://www.unos.org (accessed on 23 March 2015).
- Verfaillie, C.M. Optimizing hematopoietic stem cell engraftment: A novel role for thrombopoietin. J. Clin. Invest. 2002, 110, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.C.; Hensley, T.M.; Henry, E.T.; Shen, D.; Anthony, S.; Zhang, J.; Cheng, K. Magnetic targeting of cardiosphere-derived stem cells with ferumoxytol nanoparticles for treating rats with myocardial infarction. Biomaterials 2014, 35, 8528–8539. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, N.; Ma, B.; Maitz, M.F.; Wang, J.; Li, J.; Li, Q.; Zhao, Y.; Xiong, K.; Liu, X. Guidance of stem cells to a target destination in vivo by magnetic nanoparticles in a magnetic field. ACS Appl. Mater. Interfaces 2013, 5, 5976–5985. [Google Scholar] [CrossRef] [PubMed]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine (Lond) 2012, 7, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.L.; Jordan, E.K.; Wilson, L.B.; Yocum, G.T.; Lewis, B.K.; Frank, J.A. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum. Gene Ther. 2004, 360, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chemie Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Kyrtatos, P.G.; Lehtolainen, P.; Junemann-Ramirez, M.; Garcia-Prieto, A.; Price, A.N.; Martin, J.F.; Gadian, D.G.; Pankhurst, Q.A.; Lythgoe, M.F. Magnetic tagging increases delivery of circulating progenitors in vascular injury. JACC Cardiovasc. Interv. 2009, 2, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Frangioni, J.V.; Hajjar, R.J. In vivo tracking of stem cells for clinical trials in cardiovascular disease. Circulation 2004, 110, 3378–3383. [Google Scholar] [CrossRef] [PubMed]
- What Is an MRI Scan? WebMD Boots Website. Available online: http://www.webmd.boots.com/a-to-z-guides/what-is-an-mri-scan?page=2 (accessed on 7 June 2015).
- Progatzky, F.; Dallman, M.J.; Lo Celso, C. From seeing to believing: Labelling strategies for in vivo cell-tracking experiments. Interface Focus 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Fee, C.A.; Pettigrew, R.I. National institute of biomedical imaging and bioengineering: Poised for the future. Radiology 2003, 229, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Tests and procedures. Positron Emission Tomography (PET) Scan. Mayo Clinic Website. Available online: http://www.mayoclinic.org/tests-procedures/pet-scan/basics/risks/prc-20014301 (accessed on 7 June 2015).
- What Is an Ultrasound? Benefits of Ultrasound. WebMD Boots Website. Available online: http://www.webmd.com/a-to-z-guides/what-is-an-ultrasound?page=2#2 (accessed on 7 June 2015).
- U.S. Food and Drug Administration. Medical X-ray Imaging. Available online: http://www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandprocedures/medicalimaging/medicalx-rays/default.htm#risks (accessed on 7 June 2015).
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 13–15. [Google Scholar] [CrossRef]
- Polyak, B.; Fishbein, I.; Chorny, M.; Alferiev, I.; Williams, D.; Yellen, B.; Friedman, G.; Levy, R.J. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl. Acad. Sci. USA 2008, 105, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.S.; Bashaw, L.A.; Miller, B.R.; Jordan, E.K.; Bulte, J.W.; Frank, J.A. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: Methods and techniques. Transplantation 2003, 76, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Thu, M.S.; Bryant, L.H.; Coppola, T.; Jordan, E.K.; Budde, M.D.; Lewis, B.K.; Chaudhry, A.; Ren, J.; Varma, N.R.; Arbab, A.S.; et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nat. Med. 2012, 18, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-coated iron oxide nanoparticles: A versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Cortajarena, A.L.; Ortega, D.; Ocampo, S.M.; Gonzalez-García, A.; Couleaud, P.; Miranda, R.; Belda-Iniesta, C.; Ayuso-Sacido, A. Engineering iron oxide nanoparticles for clinical settings. Nanobiomedicine 2014, 1. [Google Scholar] [CrossRef]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sosnovik, D.E.; Nahrendorf, M.; Weissleder, R. Magnetic nanoparticles for MR imaging: Agents, techniques and cardiovascular applications. Basic Res. Cardiol. 2008, 103, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Manuscript, A. In vivo MRI cell tracking: Clinical studies. AJR Am. J. Roentgenol. 2010, 193, 314–325. [Google Scholar]
- Sheng-nan, S.; Chao, W.; Zan-zan, Z. Magnetic iron oxide nanoparticles : Synthesis and surface coating techniques for biomedical applications. Chin. Phys. B 2014, 23, 1–19. [Google Scholar]
- Saiz, S.; Nanozarah, P.; Kaedah, M.; Kimia, K. Size-controlled synthesis and characterization of Fe3O4 nanoparticles by chemical coprecipitation method. Sains Malays. 2008, 37, 389–394. [Google Scholar]
- Pei, N.; Huang, Z.; Ma, W.; Ge, J.; Zheng, W. In vitro study of deep capture of paramagnetic particle for targeting therapeutics. J. Magn. Magn. Mater. 2009, 321, 2911–2915. [Google Scholar] [CrossRef]
- Huang, Z.; Pei, N.; Wang, Y.; Xie, X.; Sun, A.; Shen, L.; Zhang, S.; Liu, X.; Zou, Y.; Qian, J.; et al. Deep magnetic capture of magnetically loaded cells for spatially targeted therapeutics. Biomaterials 2010, 31, 2130–2140. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shen, Y.; Pei, N.; Sun, A.; Xu, J.; Song, Y.; Huang, G.; Sun, X.; Zhang, S.; Qin, Q.; et al. The effect of nonuniform magnetic targeting of intracoronary-delivering mesenchymal stem cells on coronary embolisation. Biomaterials 2013, 34, 9905–9916. [Google Scholar] [CrossRef] [PubMed]
- Umut, E. Surface modification of nanoparticles used in biomedical applications. Mod. Surf. Eng. Treat. 2013, 5, 185–208. [Google Scholar]
- National Institute of Diabetes and Digestive and Kidney Diseases: Hemochromatosis. NIH. Available online: http://www.niddk.nih.gov/health-information/health-topics/liver-disease/hemochromatosis/Pages/facts (accessed on 15 April 2015).
- Huong, N.T.; Giang, L.T.K.; Binh, N.T.; Minh, L.Q. Surface modification of iron oxide nanoparticles and their conjuntion with water soluble polymers for biomedical application. J. Phys. Conf. Ser. 2009, 187. [Google Scholar] [CrossRef]
- Usher, T.C.; Walls, S.H. Process of Making Carboxylated Dextran. U.S. Patent 6,703,499 B1, 9 March 2004. [Google Scholar]
- Andreas, K.; Georgieva, R.; Ladwig, M.; Mueller, S.; Notter, M.; Sittinger, M.; Ringe, J. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials 2012, 33, 4515–4525. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Li, S.; Wang, S.; Moore, R. Nanomedicine; Springer: New York, NY, USA, 2014. [Google Scholar]
- Gutova, M.; Frank, J.A.; D’Apuzzo, M.; Khankaldyyan, V.; Gilchrist, M.M.; Annala, A.J.; Metz, M.Z.; Abramyants, Y.; Herrmann, K.A.; Ghoda, L.Y.; et al. Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: studies leading to clinical use. Stem Cells Transl. Med. 2013, 2, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Shen, Y.; Sun, A.; Huang, G.; Zhu, H.; Huang, B.; Xu, J.; Song, Y.; Pei, N.; Ma, J.; et al. Magnetic targeting enhances retrograde cell retention in a rat model of myocardial infarction. Stem Cell Res. Ther. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Kirchhausen, T. Clathrin. Annu. Rev. Biochem. 2000, 69, 699–727. [Google Scholar] [CrossRef] [PubMed]
- Vaněček, V.; Zablotskii, V.; Forostyak, S.; Růžička, J.; Herynek, V.; Babič, M.; Jendelová, P.; Kubinová, S.; Dejneka, A.; Syková, E. Highly efficient magnetic targeting of mesenchymal stem cells in spinal cord injury. Int. J. Nanomed. 2012, 7, 3719–3730. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Jin, X.; Dai, G.; Liu, J.; Chen, J.; Yang, L. In vitro targeted magnetic delivery and tracking of superparamagnetic iron oxide particles labeled stem cells for articular cartilage defect repair. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Kamei, G.; Kobayashi, T.; Ohkawa, S.; Kongcharoensombat, W.; Adachi, N.; Takazawa, K.; Shibuya, H.; Deie, M.; Hattori, K.; Goldberg, J.L.; et al. Articular cartilage repair with magnetic mesenchymal stem cells. Am. J. Sports Med. 2013, 41, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Hamasaki, T.; Yanada, S.; Ochi, M. Magnetic targeting of bone marrow stromal cells into spinal cord: Through cerebrospinal fluid. Neuroreport 2006, 17, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Tanaka, N.; Kamei, N.; Ishida, O.; Yanada, S.; Nakanishi, K.; Nishida, K.; Oishi, Y.; Kawamata, S.; Sakai, N.; et al. Magnetically labeled neural progenitor cells, which are localized by magnetic force, promote axon growth in organotypic cocultures. Spine 2007, 32, 2300–2305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Shen, D.; Hensley, T.M.; Middleton, R.; Sun, B.; Liu, W.; De Couto, G.; Marbán, E. Magnetic antibody-linked nanomatchmakers for therapeutic cell targeting. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Hibino, E.; Kobayashi, C.; Terasaki, H.; Kagami, H.; Ueda, M.; Kobayashi, T.; Honda, H. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.; Kuepper, F. Restenosis: Repeat narrowing of a coronary artery—Prevention and treatment. Circulation 2002, 105, 2586–2587. [Google Scholar] [CrossRef] [PubMed]
- Luma, L.G.; Davola, P.A.; Leed, R.J. The new face of bispecific antibodies: Targeting cancer and much more. Exp. Hematol. 2006, 34, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shen, D.; Zhang, J.; Ligler, F.S.; Cheng, K. Bispecific antibodies, nanoparticles and cells: Bringing the right cells to get the job done. Expert Opin. Biol. Ther. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kean, T.J.; Lin, P.; Caplan, A.I.; Dennis, J.E. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, V.R.; Huang, J.; Liu, D.; Jina, P.; Liua, B.; Lia, L.; Desharnaisa, J.; Hagena, C.; Levina, N.J.; Shields, M.J.; et al. Chemical generation of bispecific antibodies. Proc. Natl. Acad. Sci. USA 2010, 107, 22611–22616. [Google Scholar] [CrossRef] [PubMed]
- Electromagnetic Fields and Public Health. World Health Organization. Available online: http://www.who.int/peh-emf/publications/facts/fs299/en/ (accessed on 15 April 2015).
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cores, J.; Caranasos, T.G.; Cheng, K. Magnetically Targeted Stem Cell Delivery for Regenerative Medicine. J. Funct. Biomater. 2015, 6, 526-546. https://doi.org/10.3390/jfb6030526
Cores J, Caranasos TG, Cheng K. Magnetically Targeted Stem Cell Delivery for Regenerative Medicine. Journal of Functional Biomaterials. 2015; 6(3):526-546. https://doi.org/10.3390/jfb6030526
Chicago/Turabian StyleCores, Jhon, Thomas G. Caranasos, and Ke Cheng. 2015. "Magnetically Targeted Stem Cell Delivery for Regenerative Medicine" Journal of Functional Biomaterials 6, no. 3: 526-546. https://doi.org/10.3390/jfb6030526





