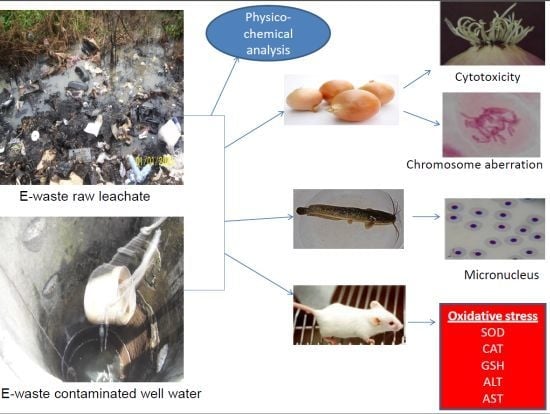
In Vivo Cytogenotoxicity and Oxidative Stress Induced by Electronic Waste Leachate and Contaminated Well Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site

2.2. Sample Collection
2.3. Physico-Chemical and Heavy Metal Analyses
2.4. Biological Materials Used for the Study
2.5. Allium Cepa Assay
2.6. Micronucleus and Nuclear Abnormality Assay
2.7. Biochemical Assays in Mice
2.8. Statistical Analysis
3. Results
3.1. Physico-Chemical and Heavy Metal Analyses
| Parameter | TW | IWW | AWW1 | AWW2 | AWW3 | ARL | USEPA27 | NESREA26 |
|---|---|---|---|---|---|---|---|---|
| pH | 7.1 | 7.4 | 7.2 | 7.1 | 6.2 | 7.8 | 6.5–8.5 | 6–9 |
| EC | 640 | 300 | 970 | 810 | 650 | 990 | - | - |
| COD | 1.5 | 7.4 | 21.6 | 79.6 | 2.6 | 547.8 | 410 | 90 |
| BOD | 0.3 | 2.3 | 13.8 | 44.3 | 0.8 | 324.2 | 250 | 50 |
| TDS | 56.3 | 81.6 | 41.2 | 36.2 | 49.5 | 200.01 | 500 | 500 |
| Alkalinity | 11.6 | 18.4 | 60.8 | 50 | 4 | 72 | 20 | 150 |
| Acidity | 3.6 | 1.8 | 13.6 | 13 | 1.3 | 19 | - | - |
| Chloride | 518.4 | 136.8 | 457.2 | 676.8 | 604.8 | 3762 | 250 | 250 |
| Ammonia | 24.6 | 17.79 | 37.2 | 33.9 | 31.8 | 471.3 | 0.03 | 1 |
| Phosphates | ND | ND | 0.24 | 0.51 | ND | 0.78 | 5 | 2 |
| Nitrates | ND | ND | 0.12 | 0.23 | ND | 285.6 | 10 | 10 |
| Sulphate | ND | ND | 0.16 | 0.25 | ND | 5.69 | - | - |
| Lead | ND | ND | 0.19 | 0.11 | 0.21 | 1.6 | 0.02 | 0.05 |
| Cadmium | ND | ND | 1.10 | 1.42 | 0.61 | 44.48 | 0.01 | 0.2 |
| Chromium | ND | ND | ND | ND | ND | 18.64 | 0.1 | 0.05 |
| Copper | ND | 0.04 | 0.12 | ND | 0.16 | 42.15 | 1.3 | 0.5 |
| Iron | 4.85 | 5.05 | 5.65 | 1 | 5 | 134.01 | 0.3 | - |
| Manganese | 0.05 | 0.03 | 0.23 | 0.2 | 0.25 | 30.1 | 0.05 | 0.2 |
| Nickel | ND | ND | ND | ND | ND | 11.42 | - | - |
| Zinc | 0.63 | 0.96 | 1.13 | 0.25 | 0.26 | 54.62 | 5 | - |
| Silver | ND | ND | ND | ND | ND | 17.29 | 0.1 | - |
| Arsenic | ND | ND | ND | ND | ND | 4.82 | - | - |
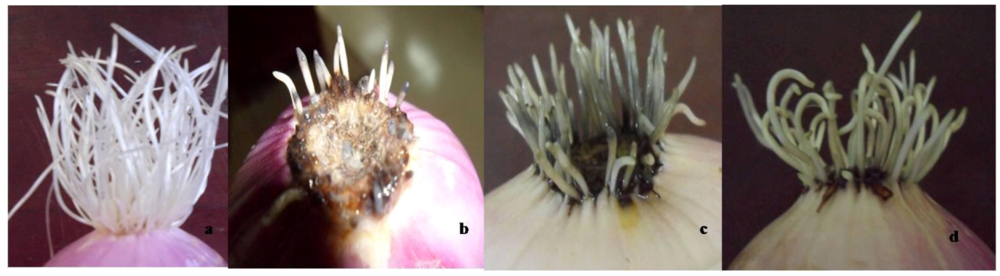
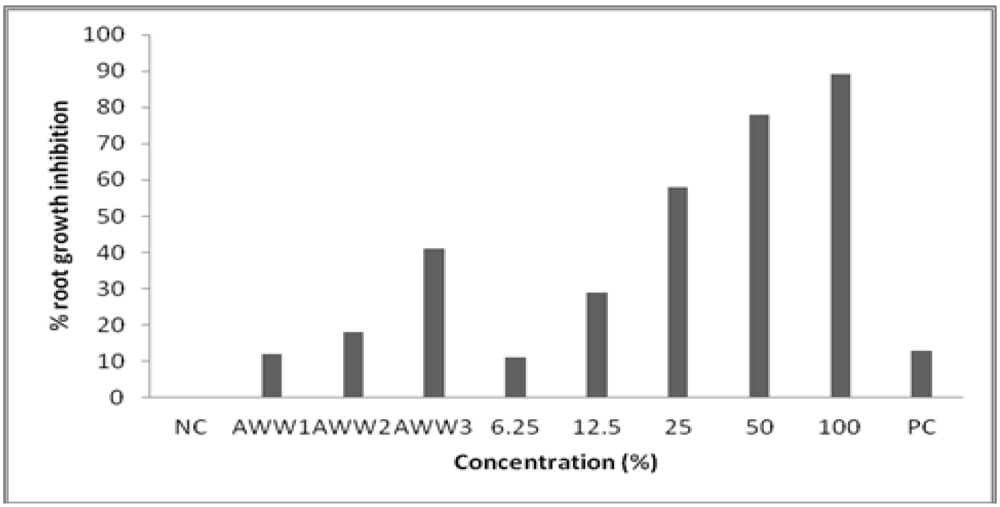
3.2. Toxicity to Root Growth in A. cepa
3.3. Mitotic Inhibition and Chromosomal Aberration in A. cepa
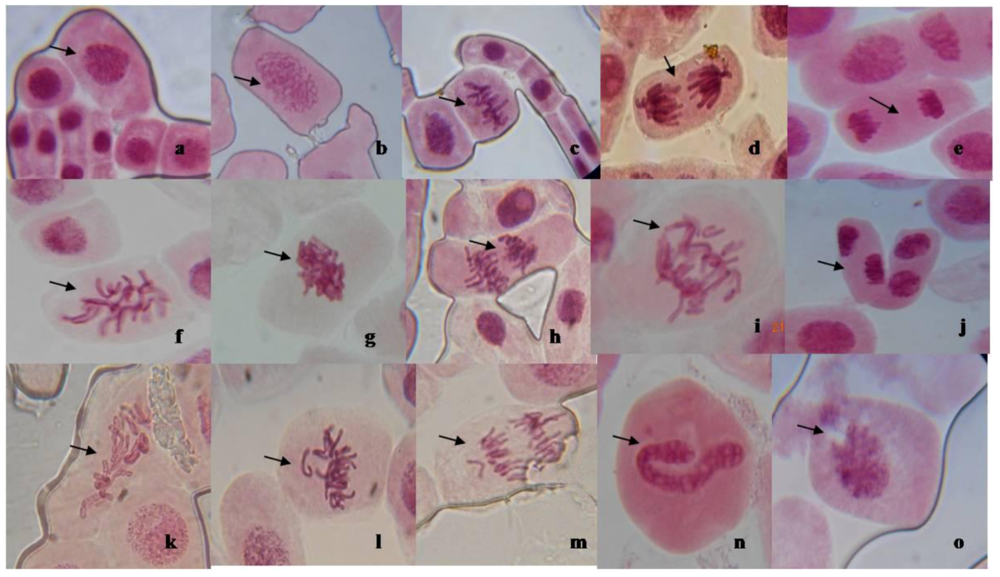
| Mitotic indices and chromosomal aberration | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Test sample | Conc. (%) | Number of dividing cells | Mitotic index (%) | Mitotic inhibition (%) | No of cells at metaphase | No of cells at anaphase | No of cells at telophase | Total aberrant cells | Frequency of aberrant cells (%) based on | |
| Total cells scored | No of dividing cells | |||||||||
| Control | NC | 318 | 7.95 | 0 | 49 | 58 | 45 | 0a | - | - |
| PC | 271 | 6.78 | 14.78 | 5 | 6 | 69 | 36bd | 0.90 | 13.28 | |
| Well watersamples | AWW1 | 201 | 5.03 | 36.79 | 12 | 16 | 66 | 34b | 0.85 | 16.92 |
| AWW2 | 239 | 5.98 | 24.84 | 15 | 19 | 71 | 41b | 1.03 | 17.16 | |
| AWW3 | 222 | 5.55 | 30.19 | 13 | 21 | 68 | 29b | 0.73 | 13.06 | |
| ARL(%) | 6.25 | 211 | 5.28 | 33.65 | 18 | 26 | 44 | 11c | 0.28 | 5.21 |
| 12.5 | 255 | 6.38 | 19.81 | 18 | 25 | 65 | 30d | 0.75 | 11.77 | |
| 25 | 179 | 4.47 | 43.71 | 9 | 20 | 39 | 34d | 0.85 | 18.99 | |
| 50 | 168 | 4.20 | 47.17 | 11 | 11 | 30 | 33d | 0.83 | 19.64 | |
| 100 | 124 | 3.10 | 61.01 | 8 | 12 | 31 | 38d | 0.95 | 30.65 | |
3.4. Micronucleus and Nuclear Abnormality Assay in Fish
| Treatment | Exposure period (days) | |||||
|---|---|---|---|---|---|---|
| MN† (Mean ± SE) | NA‡ (Mean ± SE) | |||||
| 7 | 14 | 28 | 7 | 14 | 28 | |
| Tap water | 3.73 ± 0.62 | 4.10 ± 0.91 | 1.30 ± 0.22 | 0.00 | 0.00 | 0.00 |
| 50% AWW | 4.93 ± 0.31 | 4.73 ± 0.41 | 3.37 ± 0.19 | 0.00 | 0.00 | 0.00 |
| 100% AWW | 4.60 ± 0.23 | 6.53 ± 0.52 | 3.93 ± 0.81 * | 0.00 | 0.00 | 0.00 |
| 12.5% ARL | 6.37 ± 0.41 * | 9.67 ± 0.66 * | 10.90 ± 1.03 * | 1.33 ± 0.58 | 0.90 ± 0.36 | 0.00 |
| 25% ARL | 7.87 ± 1.02 * | 9.40 ± 1.18 * | 10.10 ±0.94 * | 3.47 ± 1.57 * | 4.17 ± 1.52 * | 2.20 ± 0.97 |
| 50% ARL | 9.47 ± 0.43 * | 10.03 ± 1.00 * | 8.77 ± 0.91 * | 12.6 ± 1.92 * | 9.47 ± 1.61 * | 4.80 ± 1.64 * |
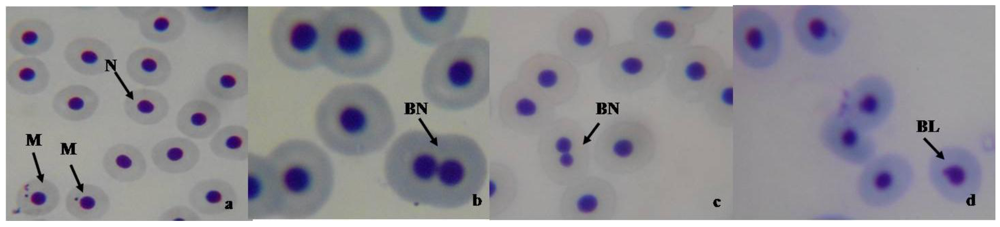
3.5. Biochemical Assay in Mice
| E-waste MDA CAT SOD GSH ALT AST leachate (µmol/mL) (µm/mg) (U/mL/Min) (µm/g tissue) (U/mL) (U/mL) | ||||||
| DW | 5.0 ± 0.18 | 76.25 ± 0.96 | 4.65 ± 0.09 | 8.60 ± 0.08 | 19.75 ± 0.96 | 41.75 ± 0.5 |
| 1% | 5.4 ± 0.14 | 76.25 ± 0.96 | 4.58 ± 0.05 | 8.65 ± 0.09 | 21.00 ± 0.82 | 42.50 ± 0.58 |
| 5% | 5.85 ± 0.06 | 78.50 ± 0.58 | 3.9 ± 0.08 | 9.55 ± 0.10 | 26.25 ± 0.50 * | 45.25 ± 0.96 |
| 10% | 8.0 ± 0.16 * | 84.25 ± 1.71 * | 2.33 ± 0.09 * | 10.85 ± 0.19 * | 29.75 ± 0.96 * | 49.25 ± 0.96 * |
| 25% | 11.98 ± 0.15 * | 97.25 ± 1.71 * | 1.88 ± 0.10 * | 12.50 ± 0.08 * | 40.75 ± 1.26 * | 54.75 ± 0.96 * |
| 50% | 18.8 ± 0.18 * | 134.0 ± 1.41 * | 1.48 ± 0.05 * | 14.15 ± 0.13 * | 47.0 ± 0.82 * | 61.0 ± 0.82 * |
| Well water | ||||||
| NC | 4.99 ± 0.14 | 76.60 ± 0.06 | 4.66 ± 0.08 | 8.60 ± 0.09 | 19.74 ± 0.80 | 41.74 ± 0.9 |
| 1 week | 5.08 ± 0.13 | 78.00 ± 0.82 | 4.45 ± 0.06 | 8.71 ± 0.06 | 20.00 ± 0.82 | 41.25 ± 0.5 |
| 2 weeks | 5.38 ± 0.13 | 81.75 ± 0.96 | 4.08 ± 0.05 | 8.95 ± 0.06 | 25.00 ± 1.41 | 43.50 ± 0.58 |
| 3 weeks | 5.78 ± 0.09 | 83.02 ± 0.96 | 3.55 ± 0.13 * | 10.05 ± 0.06 * | 28.75 ± 0.50 * | 48.25 ± 0.50 |
| 4 weeks | 7.68 ± 0.17 | 89.0 ± 0.82 * | 3.58 ± 0.15 * | 11.48 ± 0.05 * | 32.00 ± 1.41 * | 51.25 ± 1.50 * |
| 5 weeks | 8.35 ± 0.10 * | 96.0 ± 0.82 * | 2.20 ± 0.08 * | 12.38 ± 0.09 * | 36.75 ± 0.50 * | 56.25 ± 0.50 * |
4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Osibanjo, O.; Nnorom, I.C. The challenges of electronic waste (e-waste) management in developing countries. Waste Manag. Res. 2007, 25, 489–501. [Google Scholar] [CrossRef]
- Nnorom, I.C.; Osibanjo, O. Electronic waste (e-waste): Material flows and management practices in Nigeria. Waste Manag. 2008, 28, 1472–1479. [Google Scholar] [CrossRef]
- Bridgen, K.; Labunska, I.; Santillo, D.; Johnston, P. (Eds.) Greenpeace. Chemical Contamination at E-waste Recycling and Disposal Sites in Accra and Korforidua, Ghana. August 2008. Available online: http://www.greenpeace.org/ghanacontamination (accessed on 5 February 2010).
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- Chen, A.; Dietrich, K.N.; Huo, X.; Ho, S. Development of Neurotoxicants in E-waste: An emerging health concern. Environ. Health Perspect. 2011, 119, 431–438. [Google Scholar]
- Puckett, J. (Ed.) Basel Action Network. The digital dump: Exporting re-use and abuse to Africa. Basel Action Network, 24 October 2005. Available online: http://www.ban.org (accessed on 18 February 2010).
- Alabi, O.A.; Bakare, A.A. Genotoxicity and mutagenicity of electronic waste leachates using animal bioassays. Toxicol. Environ. Chem. 2011, 93, 1073–1088. [Google Scholar] [CrossRef]
- Manhart, A.; Osibanjo, O.; Aderinto, A.; Prakash, S. Informal e-waste management in Lagos, Nigeria—Socio-economic impacts and feasibility of international recycling co-operations. Final Report of Component 3 of the UNEP SBC E-waste Africa Project. Öko-Institut e.V.: Lagos & Freiburg, Germany, 2011. [Google Scholar]
- Osibanjo, O.; Nnorom, I.O.; Bakare, A.A.; Alabi, O.A. Environmental and public health consequences of adopting crude recovery techniques in e-waste management in developing countries: An emerging global crisis. In Advances in Environmental Research Vol. 17; Daniels, J.A., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; ISBN: 978-1-61209-965-1. [Google Scholar]
- Alabi, O.A.; Bakare, A.A.; Xu, X.; Li, B.; Zhang, Y.; Huo, X. Comparative evaluation of environmental contamination and DNA damage induced by electronic waste in Nigeria and China. Sci. Total Environ. 2012, 423, 62–72. [Google Scholar] [CrossRef]
- American Public Health Association (APHA), Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA: Washington, DC, USA, 1988.
- United State Environmental Protection Agency (USEPA). Method 8270C revision 3, semi volatile organic compounds by gas chromatography/mass spectrometry (GC/MS). 1996. Available online: www.epa.gov/epaoswer/hazwaste/test/main.htm (accessed on 15 January 2010).
- Fiskesjo, G. The Allium test as a standard in environmental monitoring. Hereditas 1985, 102, 99–112. [Google Scholar] [CrossRef]
- CIOMS. International guiding principles for biomedical research involving animals. 1985. Available online: http://www.cioms.ch/publications/guidelines/1985_texts_of_guidelines.htm (accessed on 07 April 2010).
- Grant, W.F. Chromosome aberration assay in Allium. A report of the U.S. environmental protection agency gene-tox program. Mutat. Res. 1982, 99, 273–291. [Google Scholar] [CrossRef]
- Carrasco, K.R.; Tilbury, K.L.; Mayers, M.S. Assessment of the piscine micronuclei test as an in situ biological indicator of chemical contaminants effects. Can. J. Fish Aquat. Sci. 1990, 47, 2123–2136. [Google Scholar] [CrossRef]
- Cavas, T.; Ergene-Gozukara, S. Evaluation of the genotoxic potential of lambda–cyhalothrin using nuclear and nucleolar biomarkers on fish cells. Mutat. Res. 2003, 534, 93–99. [Google Scholar] [CrossRef]
- Cavas, T.; Ergene-Gozukara, S. Micronucleus test in fish cells: A bioassay for in situ monitoring of genotoxic pollution in the marine environment. Environ. Mol. Mutagen. 2005, 46, 64–70. [Google Scholar] [CrossRef]
- Agnihotri, N.; Kaur, H.; Kaur, N.; Sarotra, P. Role of oxidative stress in lansoprasole mediated gastric and hepatic protection in Wistar rats. Ind. J. Gastroenterol. 2007, 26, 118–121. [Google Scholar]
- Sinha, K.A. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar]
- Gornall, A.G.; Barawill, J.C.; David, M.M. Determination of serum protein by means of biuret reaction. J. Biol. Chem. 1949, 177, 751–761. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione-s-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar]
- Shokunbi, O.S.; Odetola, A.A. Gastroprotective and antioxidant activities of Phyllanthus amarus extracts on absolute ethanol induced ulcer in albino rats. J. Med. Plants Res. 2008, 2, 261–267. [Google Scholar]
- Reitman, S.; Frankel, S. A colorimetric method for determination of serum glucose oxaloacetate and glutamic pyruvate transaminases. Am. J. Clin. Pathol. 1957, 28, 53–56. [Google Scholar]
- National Environmental Standards and Regulation Enforcement Agency (NESREA), (Federal Republic of Nigeria Official Gazette), National Environmental (Sanitation and Waste Control). Federal Government of Nigeria Printer: Abuja, Nigeria, 2009; FGP 112/102009/L000 (OL54). No.60 (96); pp. 1057–1102.
- United State Environmental Protection Agency (USEPA). Drinking water contaminants. Washington, DC, USA, 2009. Available online: http://water.epa.gov/drink/contaminants/index.cfm#List (accessed on 5 February 2010).
- Sia Su, G. Impact on drinking water sources in close proximity to the Payatas dumpsite, Philippianes. J. Public Health 2007, 15, 51–55. [Google Scholar] [CrossRef]
- Okonko, I.O.; Adejoye, O.D.; Ogunnusi, T.A.; Fajobi, E.A.; Shittu, O.B. Microbiological and physicochemical analysis of different water samples used for domestic purposes in Abeokuta and Ojota, Lagos State, Nigeria. Afr. J. Biotech. 2008, 7, 617–621. [Google Scholar]
- Gupta, S.K.; Gupta, R.C.; Gupta, A.B. Recurrent diarrhea in children living in areas with high levels of nitrate in drinking water. Arch. Environ. Health 2001, 56, 369–373. [Google Scholar] [CrossRef]
- Sia Su, G. Water-borne illness from contaminated drinking water sources in close proximity to a dumpsite in Payatas, The Philippianes. J. Rural Trop. Public Health 2005, 4, 43–48. [Google Scholar]
- Shugart, L.R.; McCarthy, J.F.; Halbrook, R.S. Biological markers of environmental and ecological contamination: An overview. Risk Anal. 1992, 12, 353–360. [Google Scholar] [CrossRef]
- Russo, C.; Rocco, L.; Morescalchi, M.A.; Stingo, V. Assessment of environmental stress by the micronucleus test and comet assay on the genome of teleost populations from two natural environments. Ecotoxicol. Environ. Saf. 2004, 57, 168–174. [Google Scholar] [CrossRef]
- Gómez-Arroyo, S.; Armienta, M.A.; Cortés-Eslava, J.; Villalobos-Pietrini, R. Sister chromatid exchanges in Vicia faba induced by arsenic-contaminated drinking water from Zimapan, Hidalgo, Mexico. Mutat. Res. 1997, 394, 1–7. [Google Scholar] [CrossRef]
- Kong, M.S.; Ma, T.H. Genotoxicity of contaminated soil and shallow well water detected by plant bioassays. Mutat. Res. 1999, 426, 221–228. [Google Scholar] [CrossRef]
- Leme, D.M.; Marin-Morales, M.A. Allium cepa in environmental monitoring: A review on its application. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Sudhakar, R.; Gowda, N.; Venu, G. Mitotic abnormalities induced by silk Dyeing Industry Effluents in the cells of Allium cepa. Cytologia 2001, 66, 235–239. [Google Scholar] [CrossRef]
- Metin, M.; Burun, B. Effects of the high doses of Urginea maritima (L.) baker extract on chromosomes. Caryologia 2010, 63, 367–375. [Google Scholar]
- Glinska, S.; Bartezak, M.; Oleksiaka, S.; Wolska, A.; Gabara, B.; Posmyk, M.; Janas, K. Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals. Ecotoxicol. Environ. Saf. 2007, 68, 343–350. [Google Scholar] [CrossRef]
- Ahmed, M.; Grant, W.F. Cytological effects of the pesticides phosdrin and bladex in Tradescantia and Vicia faba. Can. J. Genet. Cytol. 1972, 14, 157–165. [Google Scholar]
- Klasterska, I.; Natarjan, A.T.; Ramel, C. An interpretation of the origin of subchromatid aberrations and chromosome stickiness as a category of chromatid aberration. Hereditas 1976, 83, 153–162. [Google Scholar] [CrossRef]
- Kaufman, B.P. Cytochemical studies of changes induced in cellular materials by ionizing radiations. Ann. N. Y. Acad. Sci. 1958, 59, 553–559. [Google Scholar] [CrossRef]
- Halıem, A.S. Cytological effects of the herbicide sencorer on mitosis of Allium cepa. Egypt. J. Bot. 1990, 33, 93–104. [Google Scholar]
- Shahın, S.A.; El-Amoodı, K.H.H. Induction of numerical chromosomal aberrations during DNA synthesis using the fungicides nimrod and rubigan-4 in root tips of Vicia faba L. Mutat. Res. 1991, 261, 169–176. [Google Scholar] [CrossRef]
- Leme, D.M.; Angelis, D.F.; Marin-Morales, M.A. Action mechanisms of petroleum hydrocarbons present in waters impacted by an oil spill on the genetic material of Allium cepa root cells. Aquat. Toxicol. 2008, 88, 214–219. [Google Scholar] [CrossRef]
- Ergene, S.; Cava, T.; Celik, A.; Koleli, N.; Aymak, C. Evaluation of river water genotoxicity using piscine micronucleus test. Environ. Mol. Mutag. 2007, 48, 421–429. [Google Scholar] [CrossRef]
- DeLemos, C.T.; Rodel, P.M.; Terra, N.R.; Erdtmann, B. Evaluation of basal micronucleus frequency and hexavalent chromium effects in fish erythrocytes. Environ. Toxicol. Chem. 2007, 20, 1320–1324. [Google Scholar]
- Alimba, C.G.; Saliu, J.K.; Adesanya, A.; Bakare, A.A. Evaluation of Genotoxicity of municipal landfill leachate by micronucleus test using Clarias gariepinus. Res. Environ. Life Sci. 2011, 4, 1–6. [Google Scholar]
- Barsiene, J.; Dedonyte, V.; Rybakovas, A.; Andreikenaite, L.; Andersen, O.K. Investigation of micronuclei and other nuclear abnormalities in peripheral blood and kidney of marine fish treated with crude oil. Aquat. Toxicol. 2006, 78, S99–S104. [Google Scholar] [CrossRef]
- Ozkan, F.; Gündüz, S.G.; Berköz, M.; Hunt, A.O. Induction of micronuclei and other nuclear abnormalities in peripheral erythrocytes of Nile tilapia, Oreochromis niloticus, following exposure to sublethal cadmium doses. Turkish J. Zool. 2011, 35, 585–592. [Google Scholar]
- Ventura, B.C.; Angelis, D.F.; Marin-Morales, M.A. Mutagenic and genotoxic effects of the atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic. Biochem. Phys. 2008, 90, 42–51. [Google Scholar] [CrossRef]
- Rodilla, V. Origin and evolution of binucleated cells and binucleated cell with micronuclei in cisplatin—Treated CHO cultures. Mutat. Res. 1993, 300, 281–291. [Google Scholar] [CrossRef]
- Cavas, T. In vivo genotoxicity of mercury chloride and lead acetate: Micronucleus test on acridine orange stained fish cells. Food Chem. Toxicol. 2008, 46, 352–358. [Google Scholar] [CrossRef]
- Li, H.; Han, M.; Hou, L.; Li, G.; Sang, N. Landfill leachate ingestions induced protein oxidation and DNA–protein crosslinks in mouse viscera. J. Hazard. Mat. 2010, 174, 54–58. [Google Scholar] [CrossRef]
- Bakare, A.A.; Patel, S.; Pandey, A.K.; Bajpayee, M.; Dhawan, A. DNA and oxidative damage induced in somatic organs and tissues of mouse by municipal sludge leachate. Toxicol. Ind. Health 2012, 28, 614–623. [Google Scholar] [CrossRef]
- Ferrari, B.; Radetski, C.M.; Veber, A.M.; Ferard, J.F. Ecotoxicological assessment of solid wastes: A combined liquid—And solid—Phase testing approach using a battery of bioassays and biomarkers. Environ. Toxicol. Chem. 1999, 18, 1195–1202. [Google Scholar]
- Radetski, C.M.; Ferrari, B.; Cotelle, S.; Masfaraud, J.F.; Ferard, J.F. Evaluation of the genotoxic, mutagenic and oxidant stress potentials of municipal solid waste incinerator bottom ash leachates. Sci. Total Environ. 2004, 333, 209–216. [Google Scholar] [CrossRef]
- Sang, N.; Han, M.; Li, G.; Mingzhu-Huang, M. Landfill leachate affects metabolic responses of Zea mays L. seedlings. Waste Manag. 2010, 30, 856–862. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwack, S.J.; Lee, B.M. Lipid peroxidation, antioxidant enzymes, and benzo[a]pyrene- quinones in the blood of rats treated with benzo[a]pyrene. Chem. Biol. Interact. 2000, 127, 139–150. [Google Scholar] [CrossRef]
- Dotan, Y.; Lichtenberg, D.; Pinchuk, I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Progress Lipid Res. 2004, 43, 200–227. [Google Scholar] [CrossRef]
- Guoyao, W.; Yun-Zhong, F.; Sheng, Y.; Joanne, R.; Nancy, D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar]
- Bray, T.M.; Taylor, C.G. Tissue glutathione, nutrition and oxidative stress. Can. J. Physiol. Pharmacol. 1993, 71, 746–751. [Google Scholar] [CrossRef]
- Dimitrova, M.S.T.; Tsinova, V.; Velcheva, V. Combined effect of zinc and lead on the hepatic superoxide dismutase-catalase system in carp (Cyprinus carpio). Comp. Biochem. Physiol. 1994, 108, 43–46. [Google Scholar]
- Molander, D.W.; Wroblewsk, F.; La Due, J.S. Transaminase compared with cholinesterase and alkaline phosphatase an index of hepatocellular integrity. Clin. Res. Proc. 1955, 3, 20–24. [Google Scholar]
- Friedman, L.S.; Martin, P.; Munoz, S.J. Liver function tests and the objective evaluation of the patient with liver disease. In Hepatology: A Textbook of Liver Disease, 3rd ed.; Zakin, D., Boyer, T.D., Eds.; WB Saunders: Philadelphia, 1996; pp. 791–833. [Google Scholar]
- Kaplan, M.M. Laboratory tests. In Diseases of the Liver, 7th ed.; Schiff, L., Schiff, E.R., Eds.; JB Lippinocott: Philadephia, PA, USA, 1993; pp. 108–144. [Google Scholar]
- Alimba, C.G.; Bakare, A.A.; Aina, O.O. Liver and kidney dysfunction in wistar rats exposed to municipal landfill leachate. Res. Environ. 2012, 2, 150–163. [Google Scholar]
- Bridgewater, L.; Manning, F.; Woo, E.; Patierno, S. DNA polymerase arrest by adducted trivalent chromium. Mol. Carcinog. 1994, 9, 122–133. [Google Scholar] [CrossRef]
- Resit, M.; Jenner, P.; Halliwell, B. Sulphite enhances peroxynitrite dependent a1- antiproteinase inactivation: A mechanism of lung injury by sulphur dioxide. FEBS Lett. 1998, 43, 231–234. [Google Scholar]
- Akutsu, K.; Takatori, S.; Nozawa, S.; Yoshiike, M.; Nakazawa, H.; Hayakawa, K.; Makino, T.; Iwamoto, T. Polybrominateddiphenyl ethers in human serum and sperm quality. Bull. Environ. Contam. Toxicol. 2008, 80, 345–350. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, J.; Li, K.Q.; Miao, X.H.; Li, G.; Fan, F.Y. Chromosomal aberrations and DNA damage in human populations exposed to the processing of electronics waste. Environ. Sci. Pollut. Res. Int. 2009, 16, 329–338. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Chen, A.; Zhou, Y.; Wu, K.; Liu, J.; Guo, Y.; Huo, X. Birth outcomes related to informal E-waste recycling in Guiyu, China. Reprod. Toxicol. 2012, 33, 94–98. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bakare, A.A.; Alabi, O.A.; Gbadebo, A.M.; Ogunsuyi, O.I.; Alimba, C.G. In Vivo Cytogenotoxicity and Oxidative Stress Induced by Electronic Waste Leachate and Contaminated Well Water. Challenges 2013, 4, 169-187. https://doi.org/10.3390/challe4020169
Bakare AA, Alabi OA, Gbadebo AM, Ogunsuyi OI, Alimba CG. In Vivo Cytogenotoxicity and Oxidative Stress Induced by Electronic Waste Leachate and Contaminated Well Water. Challenges. 2013; 4(2):169-187. https://doi.org/10.3390/challe4020169
Chicago/Turabian StyleBakare, Adekunle A., Okunola A. Alabi, Adeyinka M. Gbadebo, Olusegun I. Ogunsuyi, and Chibuisi G. Alimba. 2013. "In Vivo Cytogenotoxicity and Oxidative Stress Induced by Electronic Waste Leachate and Contaminated Well Water" Challenges 4, no. 2: 169-187. https://doi.org/10.3390/challe4020169
APA StyleBakare, A. A., Alabi, O. A., Gbadebo, A. M., Ogunsuyi, O. I., & Alimba, C. G. (2013). In Vivo Cytogenotoxicity and Oxidative Stress Induced by Electronic Waste Leachate and Contaminated Well Water. Challenges, 4(2), 169-187. https://doi.org/10.3390/challe4020169