Marine Microphytobenthic Assemblage Shift along a Natural Shallow-Water CO2 Gradient Subjected to Multiple Environmental Stressors
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Site and Sampling Stations
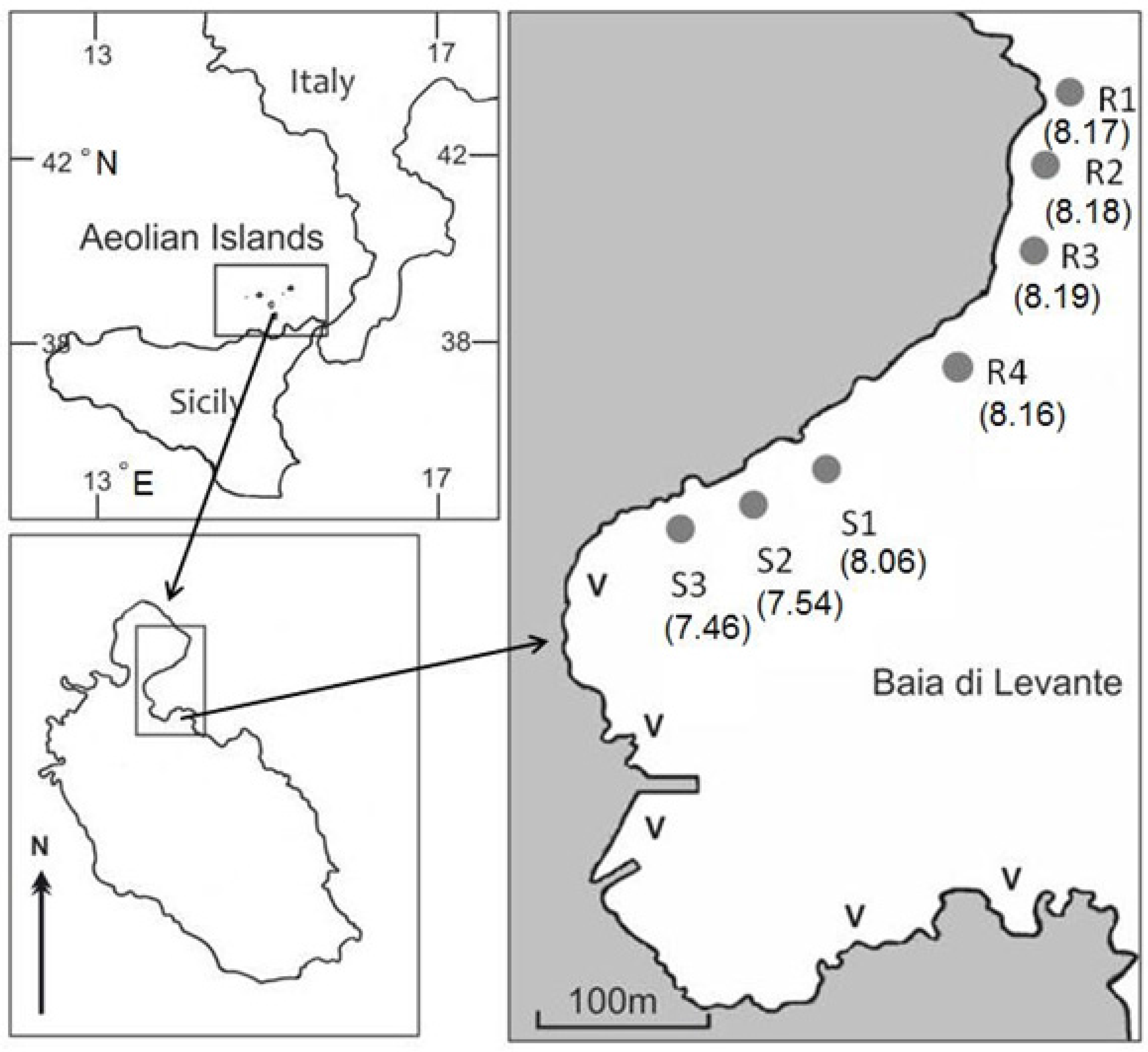
| Station | pH (NBS Scale) | TA mmol kg−1 | pCO2 (μatm) | HCO3− mmol kg−1 | |
|---|---|---|---|---|---|
| max | 8.35 | 241 | 2.206 | ||
| R1 | med | 8.17 | 2.682 | 388 | 2.341 |
| min | 8.06 | (±0.12) | 513 | 2.405 | |
| max | 8.29 | 274 | 2.177 | ||
| R2 | med | 8.18 | 2.591 | 365 | 2.251 |
| min | 8.08 | (±0.03) | 471 | 2.311 | |
| max | 8.29 | 272 | 2.165 | ||
| R3 | med | 8.18 | 2.579 | 364 | 2.247 |
| min | 8.10 | (±0.04) | 446 | 2.288 | |
| max | 8.26 | 295 | 2.189 | ||
| R4 | med | 8.12 | 2.582 | 424 | 2.28 |
| min | 8.08 | (±0.05) | 470 | 2.307 | |
| max | 8.22 | 355 | 2.401 | ||
| S1 | med | 8.08 | 2.79 | 510 | 2.499 |
| min | 7.76 | (±0.08) | 1119 | 2.627 | |
| max | 8.10 | 474 | 2.436 | ||
| S2 | med | 7.71 | 2.742 | 1244 | 2.601 |
| min | 7.07 | (±0.07) | 5628 | 2.697 | |
| max | 8.24 | 337 | 2.392 | ||
| S3 | med | 7.66 | 2.796 | 1428 | 2.662 |
| min | 6.80 | (±0.12) | 10,730 | 2.762 | |
2.2. In Situ Microphytobenthic Sampling
2.3. Chlorophyll-a Extraction
2.4. Diatom Abundance
2.5. Epi-Fluorescence Microscopy
2.6. SEM Analysis
2.7. Statistical Analysis
3. Results
3.1. Dissolved Nutrient Concentrations and Sediment Properties
| Station | Nitrite (μM) | Nitrate (μM) | Silicate (μM) |
|---|---|---|---|
| R1 | 0.01 ± 0.001 | 0.24 ± 0.05 | 3.43 ± 0.05 |
| S1 | 0.01 ± 0.005 | 0.13 ± 0.03 | 8.34 ± 2.73 |
| S2 | 0.02 ± 0.003 | 0.16 ± 0.06 | 15.12 ± 2.45 |
| S3 | 0.02 ± 0.001 | 0.33 ± 0.1 | 19.39 ± 1.24 |
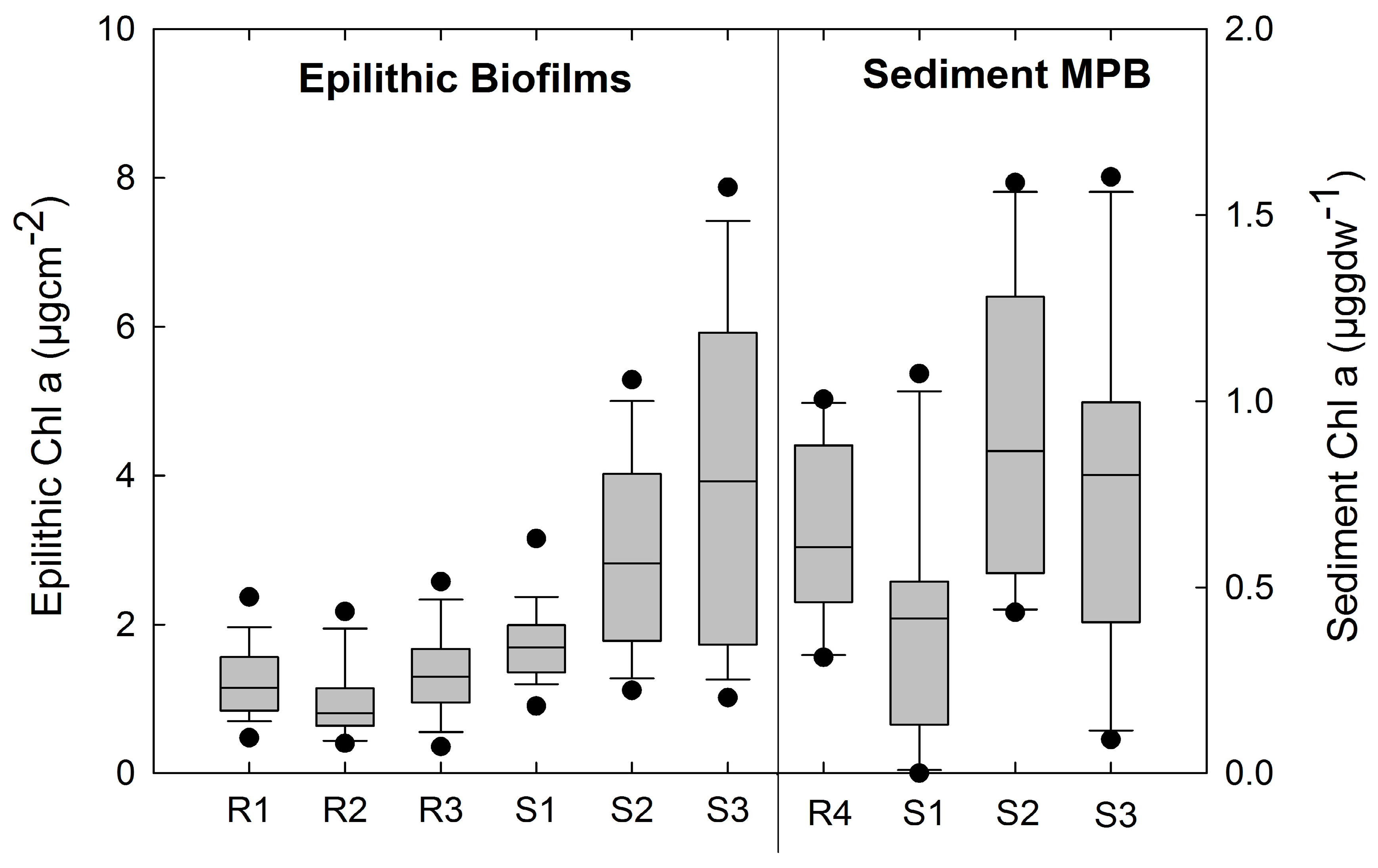
3.2. Photosynthetic Standing Crop (Chla)
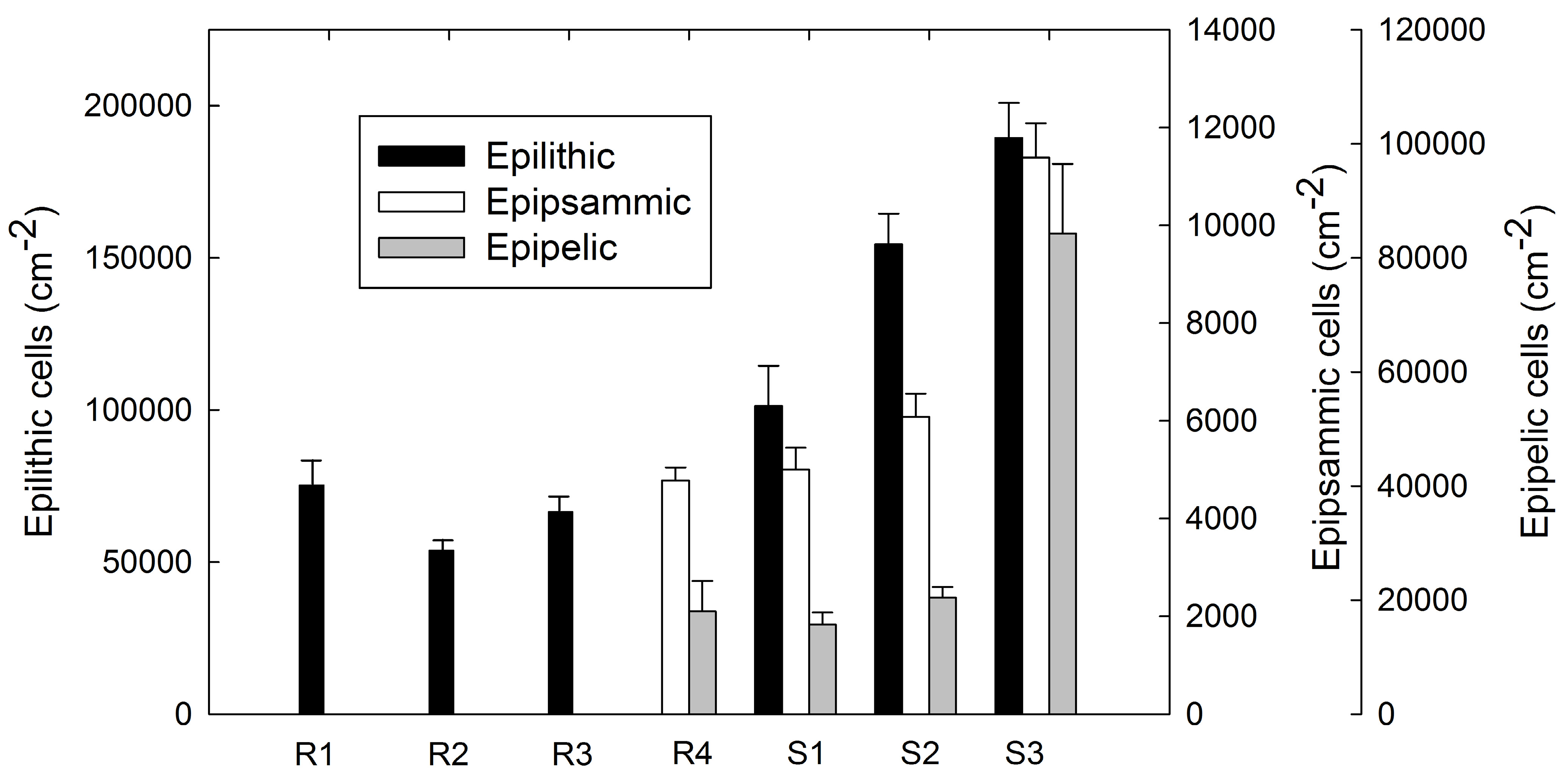
3.3 Benthic Diatom Abundance
3.4. Epi-Fluorescence Analysis

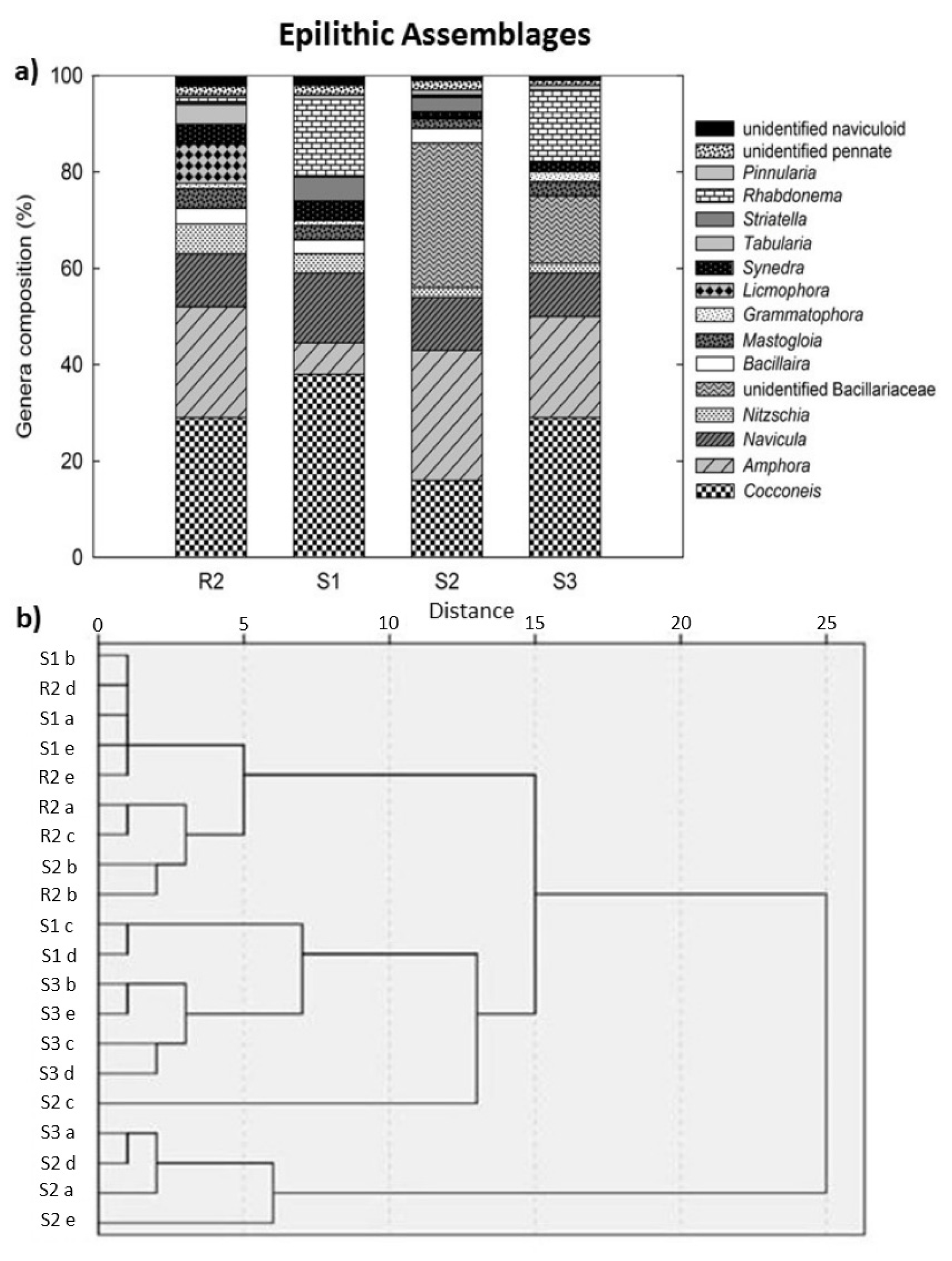
3.5. Benthic Diatom Assemblage Composition

4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hönisch, B.; Ridgwell, A.; Schmidt, D.N.; Thomas, E.; Gibbs, S.J.; Sluijs, A.; Zeebe, R.; Kump, L.; Martindale, R.C.; Greene, S.E.; et al. The geological record of ocean acidification. Science 2012, 335, 1058–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, S.D.; Kroeker, K.J.; Fabricius, K.E.; Kline, D.I.; Russell, B.D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos. Trans. R. Soc. B 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.; Beardall, J.; Raven, J.A. CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005, 56, 99–131. [Google Scholar] [CrossRef] [PubMed]
- Beardall, J.; Giordano, M. Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms and their regulation. Funct. Plant Biol. 2002, 29, 335–347. [Google Scholar] [CrossRef]
- Collins, S.; Bell, G. Phenotypic consequences of 1000 generations of selection at elevated CO2 in a green alga. Nature 2004, 431, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Trimborn, S.; Wolf-Gladrow, D.; Ritcher, K-L.; Rost, B. The effect of pCO2 on carbon acquisition and intracellular assimilation in four marine diatoms. J. Exp. Mar. Biol. Ecol. 2009, 376, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Rost, B.; Zondervan, I.; Wolf-Gladrow, D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: Current knowledge, contradictions and research directions. Mar. Ecol. Prog. Ser. 2008, 373, 227–237. [Google Scholar] [CrossRef]
- Rossoll, D.; Bermúdez, R.; Hauss, H.; Schulz, K.G.; Riebesell, U.; Sommer, U.; Winder, M. Ocean acidification-induced food quality deterioration constrains trophic transfer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinutok, S.; Hill, R.; Doblin, M.A.; Wuhrer, R.; Ralph, P.J. Warmer more acidic conditions cause decreased productivity and calcification in subtropical coral reef sediment-dwelling calcifiers. Limnol. Oceangr. 2011, 56, 1200–1212. [Google Scholar] [CrossRef]
- Connell, S.D.; Russell, B.D. The direct effects of increasing CO2 and temperature on non-calcifying organisms: Increasing the potential for phase shifts in kelp forests. Proc. R. Soc. Lond. B 2010, 277, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Xu, J.; Gao, G.; Li, Y.; Hutchins, D.A.; Huang, B.; Wang, L.; Zheng, Y.; Jin, P.; Cai, X.; et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Clim. Chang. 2012, 2, 519–523. [Google Scholar] [CrossRef]
- Lefebvre, S.C.; Benner, E.; Stillman, J.H.; Parker, A.E.; Drake, M.K.; Rossignol, P. Nitrogen source and pCO2 synergisitically affect carbon allocation, growth and morphology of the coccolithophore Emiliania huxleyi: Potential implications of ocean acidification for the carbon cycle. Glob. Chang. Biol. 2012, 18, 493–503. [Google Scholar]
- Roberts, D.A.; Birchenough, S.N.R.; Lewis, C.; Sanders, M.B.; Bolam, T.; Sheahan, D. Ocean acidification increases the toxicity of contaminated sediments. Glob. Chang. Biol. 2013, 19, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Chen, X.; Zhuang, J. The positive relationship between ocean acidification and pollution. Mar. Pollut. Bull. 2015, 91, 14–21. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, H.L.; Geider, R.J.; Miller, D.C. Microphytobenthos: The ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. 1. Distribution, abundance and primary production. Estuaries 1996, 19, 186–201. [Google Scholar] [CrossRef]
- Magalhães, C.M.; Bordalo, A.A.; Wiebe, W.J. Intertidal biofilms on rocky substratum can play a major role in estuarine carbon and nutrient dynamics. Mar. Ecol. Prog. Ser. 2003, 258, 275–281. [Google Scholar] [CrossRef]
- Underwood, G.J.C.; Paterson, D.M. The importance of extracellular carbohydrate production by marine epipelic diatoms. Adv. Bot. Res. 2003, 40, 184–240. [Google Scholar]
- Brodie, J.; Williamson, C.J.; Smale, D. The future of the northeast Atlantic benthic flora in a high CO2 world. Ecol. Evol. 2014, 4, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Bowes, G.; Ross, C.; Zhang, X.H. Climate change and ocean acidification effects on seagrassess and marine macroalgae. Glob. Chang. Biol. 2013, 19, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Lidbury, I.; Johnson, V.R.; Hall-Spencer, J.M.; Munn, C.B.; Cunliffe, M. Community-level response of coastal microbial biofilms to ocean acidification in a natural carbon dioxide vent system. Mar. Pollut. Bull. 2012, 64, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.R.; Brownlee, C.; Rickaby, R.E.M.; Graziano, M.; Milazzo, M.; Hall-Spencer, J.M. Responses of marine benthic microalgae to elevated CO2. Mar. Biol. 2013, 160, 1813–1824. [Google Scholar] [CrossRef]
- Russell, B.D.; Connell, S.D.; Findlay, H.S.; Tait, K.; Widdicombe, S.; Mieszkowska, N. Ocean acidification and rising temperatures may increase biofilm primary productivity but decrease grazer consumption. Philos. Trans. R. Soc. B 2013, 368. [Google Scholar] [CrossRef] [PubMed]
- Snoeijs, P. Monitoring pollution effects by diatom community composition. A comparison of sampling methods. Arch. Hydrobiol. 1991, 121, 497–510. [Google Scholar]
- Kroeker, K.J.; Micheli, F.; Gambi, M.C. Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Clim. Chang. 2012, 3, 156–159. [Google Scholar] [CrossRef]
- Kerfahi, D.; Hall-Spencer, J.M.; Tripathi, B.M.; Milazzo, M.; Lee, J.; Adams, J. Shallow water marine sediment bacterial community shifts along a natural CO2 gradient in the Mediterranean Sea off Vulcano, Italy. Microb. Ecol. 2014, 67, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Hall-Spencer, J.M.; Rodolfo-Metalpa, R.; Martin, S.; Ransome, E.; Fine, M.; Turner, S.M.; Rowley, S.J.; Tedesco, D.; Buia, M-C. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 2008, 454, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; De’ath, G.; Noonan, S.; Uthike, S. Ecological effects of ocean acidification and habitat complexity on reef-associated macroinvertebrate communities. Proc. R. Soc. B 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Vikas, M.; Dwarakish, G.S. Coastal pollution: A review. Aquat. Procedia 2015, 4, 381–388. [Google Scholar] [CrossRef]
- Vasilakopoulos, P.; Maravelias, C.D.; Tserpes, G. The alarming decline of Mediterranean fish stocks. Curr. Biol. 2014, 24, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, S.; Leonardo, R.D.; Costa, V.; Tramati, C.D.; Luzzu, F.; Mazzola, A. Trace element bias in the use of CO2 vents as analogues for low pH environments: Implications for contamination levels in acidified oceans. Estuar. Coast. Shelf Sci. 2013, 134, 19–30. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Gentill, B.; Duarte, C.M.; Kleypas, J.A.; Middelburg, J.J.; Antione, D. Light availability in the coastal ocean: Impact on the distribution of benthic photosynthetic organisms and contribution to primary production. Biogeosciences 2006, 3, 489–513. [Google Scholar] [CrossRef]
- Johnson, V.R. Using volcanic CO2 Gradients to Investigate the Responses of Marine Benthic Algae to Ocean Acidification. PhD Thesis, School of Biomedical and Biological Sciences, Marine Biology and Ecology Research Centre, Plymouth University, Plymouth, UK, 2012. [Google Scholar]
- Lewis, E.; Wallace, W.R. Program Developed for CO2 System Calculations; Carbon dioxide information analysis center, Oak Ridge National Laboratory, U.S. Department of Energy: Oak Ridge, TN, USA, 1998. [Google Scholar]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Kerrison, P.; Hall-Spencer, J.M.; Suggett, D.; Hepburn, L.J.; Steinke, M. Assessment of pH variability at a coastal CO2 vent for ocean acidification studies. Estuar. Coast. Shelf Sci. 2011, 94, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Boatta, F.; D’Alessandro, W.; Gagliano, A.; Liotta, M.; Milazzo, M.; Rodolfo-Metalpa, R.; Hall-Spencer, J.M.; Parello, F. Geochemical survey of Levante Bay, Vulcano Island (Italy) and its suitability as a natural laboratory for the study of ocean acidification. Mar. Pollult. Bull. 2013, 73, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Hansson, L.-A. Chlorophyll-a determination of periphyton on sediments: Identification of problems and recommendation of method. Freshw. Biol. 1998, 20, 347–352. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–1126. [Google Scholar] [CrossRef]
- Hickman, M.; Round, F.E. Primary production and standing crops of epipsammic and epipelic algae. Br. Phycol. J. 1970, 5, 247–255. [Google Scholar] [CrossRef]
- Hickman, M. Methods for determining the primary productivity of epipelic and epipsammic algal associations. Limnol. Oceanogr. 1969, 14, 936–941. [Google Scholar] [CrossRef]
- McAlice, B.J. Phytoplankton sampling with the Sedgewick Rafter Cell. Limnol. Oceanogr. 1971, 16, 19–28. [Google Scholar] [CrossRef]
- Eaton, J.W.; Moss, B. The estimation of numbers and pigment content in epipelic algal populations. Limnol. Oceanogr. 1996, 11, 584–595. [Google Scholar] [CrossRef]
- Nagarkar, S.; Williams, G.A. Comparative techniques to quantify cyanobacteria dominated epilithic biofilms on tropical rocky shores. Mar. Ecol. Prog. Ser. 1997, 154, 281–291. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.; Tobin, M.L.; Hawkins, S.J.; Norton, T.A. Problems in extraction and spectrophotometric determination of chlorophyll from epilithic microbial biofilms: Towards a standard method. J. Mar. Biol. Assoc. UK 1999, 79, 551–558. [Google Scholar] [CrossRef]
- Celis-Plá1, P.S.M.; Hall-Spencer, J.M.; Antunes Horta, P. Macroalgal responses to ocean acidification depend on nutrient and light levels. Front. Mar. Sci. 2015, 2. [Google Scholar] [CrossRef]
- Hinga, K.R. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002, 238, 281–300. [Google Scholar] [CrossRef]
- Hama, T.; Kawahima, S.; Shimotori, K.; Satoh, Y.; Omori, Y.; Wada, S.; Adachi, T.; Hasegawa, S.; Midorikawa, T.; Ishii, M.; et al. Effect of ocean acidification on coastal phytoplankton composition and accompanying organic nitrogen production. J. Oceanogr. 2012, 68, 183–194. [Google Scholar] [CrossRef]
- Burkhardt, S.; Zondervan, I.; Riebesell, U. Effect of CO2 concentration on C:N:P ratio in marine phytoplankton: A species comparison. Limnol. Oceanogr. 1999, 44, 683–690. [Google Scholar] [CrossRef]
- Crawfurd, K.J.; Raven, J.A.; Wheeler, G.L.; Baxter, E.J.; Joint, I. The response of Thalassiosira pseudonana to long-term exposure to increased CO2 and decreased pH. PLoS ONE 2011, 6, e26695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torstensson, A.; Chierici, M.; Wulff, A. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa. Polar Biol. 2011, 35, 205–214. [Google Scholar] [CrossRef]
- Tortell, P.D.; DiTullio, G.R.; Sigman, D.M.; Morel, F.M.M. CO2 effects on taxonomic composition and nutrient utilisation in an Equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 2002, 236, 37–43. [Google Scholar] [CrossRef]
- Tortell, P.D.; Payne, C.D.; Li, Y.; Trimborn, S.; Rost, B.; Smith, W.O.; Riesselman, C.; Dunbar, R.B.; Sedwick, P.; DiTullio, G.R. CO2 sensitivity of southern ocean phytoplankton. Geophysi. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Feng, Y.; Hare, C.E.; Leblanc, K.; Rose, J.M.; Zhang, Y.; DiTullio, G.R.; Lee, P.A.; Wilhelm, S.W.; Rowe, J.M.; Nemcek, N.; et al. Effects of increased pCO2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Mar. Ecol. Prog. Ser. 2009, 388, 13–25. [Google Scholar] [CrossRef]
- Sun, J.; Hutchins, D.A.; Feng, Y.; Seubert, E.L.; Caron, D.A.; Fu, F-X. Effects of changing pCO2 and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011, 56, 829–840. [Google Scholar] [CrossRef]
- Gao, K.; Xu, J.; Gao, G.; Li, Y.; Hutchins, D.A.; Huang, B.; Wang, L.; Zheng, Y.; Jin, P.; Cai, X.; et al. Rising CO2 and increased light exposure synergistically reduce marine primary productivity. Nat. Clim. Chang. 2012, 2, 519–523. [Google Scholar]
- Hopkinson, B.M.; Dupont, C.L.; Allen, A.E.; Morel, F.M.M. Efficiency of the CO2-concentrating mechanism of diatoms. Proc. Nat. Acad. Sci. USA 2011, 108, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Hicks, N.; Bulling, M.T.; Solan, M.; Raffaelli, D.; White, P.C.L.; Paterson, M.P. Impact of biodiversity-climate futures on primary production and metabolism in a model benthic estuarine system. BMC Ecol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsterberg, C.; Eklöf, J.S.; Gamfeldt, L.; Havenhand, J.N.; Sundbäck, K. 2013. Consumers mediate the effects of experimental ocean acidification and warming on primary producers. Proc. Nat. Acad. Sci. USA 2013, 110, 8603–8608. [Google Scholar] [CrossRef] [PubMed]
- Underwood, G.J.C.; Barnett, M. What Determines Species Composition in Microphytobenthic Biofilms? In Functioning of Microphytobenthos in Estuaries. Proceedings of the Microphytobenthos symposium, Amsterdam, The Netherlands, Royal Netherlands Academy of Arts and Sciences, 21–23 August 2006; Kromkamp, J., Ed.; pp. 121–138.
- Jesus, B.; Brotas, V.; Ribeiro, L.; Mendes, C.R.; Cartaxana, P.; Paterson, D.M. Adaptations of microphytobenthos assemblages to sediment type and tidal position. Cont. Shelf Res. 2009, 29, 1624–1634. [Google Scholar] [CrossRef]
- Cook, P.L.M.; Roy, H. Advective relief of CO2 limitation in microphytobenthos in highly productive sandy sediments. Limnol. Oceanogr. 2006, 51, 1594–1601. [Google Scholar] [CrossRef]
- Kitidis, V.; Laverock, B.; McNeill, L.C.; Beesley, A.; Cummings, D.; Tait, K. Impact of ocean acidification on benthic and water column ammonia oxidation. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Dando, P.R.; Stüben, D.; Varnavas, S.P. Hydrothermalism in the Mediterranean Sea. Progr. Oceanogr. 1999, 44, 333–367. [Google Scholar] [CrossRef]
- Egge, J.K.; Aksnes, D.L. Silicate as a regulating nutrient in phytoplankton competition. Mar. Ecol. Prog. Ser. 1992, 83, 281–289. [Google Scholar] [CrossRef]
- Martin-Jézéquel, V.; Hildebrand, M.; Brzezinski, M.A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 2000, 36, 821–840. [Google Scholar] [CrossRef]
- Claquin, P.; Leynaert, A.; Sferratore, A.; Garnier, J.; Ragueneau, O. Physiological Ecology of Diatoms Along the Land-Sea Continuum. In Land-Ocean Nutrient Fluxes: Silica Cycle; Ittekot, V., Humborg, C., Garnier, J., Eds.; Island Press: Washington, DC, USA, 2006. [Google Scholar]
- Thamatrakoln, K.; Hildebrand, M. Silicon uptake in diatoms revisited: A model for saturable and nonsaturable uptake kinetics and the role of silicon transporters. Plant Physiol. 2008, 146, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U. Acid test for marine biodiversity. Nature 2008, 454, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Gazeau, F.; Martin, S.; Hansson, L.; Gattuso, J.-P. Ocean acidification in the coastal zone. Available online: http://www.loicz.org/imperia/md/content/loicz/print/newsletter/Inprint_2011_3_online72.pdf (accessed on 24 November 2015).
- Middelboe, A.L.; Hansen, P.J. High pH in shallow-water macroalgal habitats. Mar. Ecol. Prog. Ser. 2007, 338, 107–117. [Google Scholar] [CrossRef]
- Joint, I.; Doney, S.C.; Karl, D.M. Will ocean acidification affect marine microbes? ISME J. 2011, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wooton, J.T.; Pfister, C.A.; Forester, J.D. Dyanamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Nat. Acad. Sci. USA 2008, 105, 18848–18853. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequent dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Breitbarth, E.; Bellerby, R.J.; Neill, C.C.; Ardelan, M.V.; Meyerhofer, M.; Zollner, E.; Croot, P.L.; Riebesell, U. Ocean acidification affects iron speciation during a coastal seawater mesocosm experiment. Biogeosciences 2010, 7, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Richards, R.; Chaloupka, M.; Sanò, M.; Tomlinson, R. Modelling the effects of “coastal” acidification on copper speciation. Ecol. Model. 2011, 22, 3559–3567. [Google Scholar] [CrossRef]
- Millero, F.J.; Woosley, R.; DiTrolio, B.; Waters, J. Effect of ocean acidification on the speciation of metals in seawater. Oceanography 2009, 22, 72–85. [Google Scholar] [CrossRef]
- Dyson, K.E.; Bulling, M.T.; Solan, M.; Hernandez-Milian, G.; Raffaelli, D.G.; White, P.C.L.; Paterson, D.M. Influence of macrofaunal assemblages and environmental heterogeneity on microphytobenthic production in experimental systems. Proc. R. Soc. B 2007, 274, 2547–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cigliano, M.; Gambi, M.C.; Rodolfo-Metalpa, R.; Patti, F.P.; Hall-Spencer, J.M. Effects of ocean acidification on invertebrate settlement at volcanic CO2 vents. Mar. Biol. 2010, 157, 2489–2502. [Google Scholar] [CrossRef]
- Arnold, T.; Mealey, C.; Leahey, H.; Miller, A.W.; Hall-Spencer, J.M.; Milazzo, M.; Maers, K. Ocean acidification and the loss of protective phenolics in seagrasses. PLoS ONE 2012, 7, e35107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kübler, J.E.; Johnston, A.M.; Raven, J.A. The effects of reduced and elevated CO2 and O2 on the seaweed Lomentaria articulate. Plant Cell Environ. 1991, 22, 1303–1310. [Google Scholar] [CrossRef]
- Porzio, L.; Buia, M.C.; Hall-Spencer, J.M. Effects of ocean acidification on macroalgal communities. J. Exp. Mar. Biol. Ecol. 2011, 400, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Tribollet, A.; Atkinson, M.J.; Langdon, C. Effects of elevated pCO2 on epilithic and endolithic metabolism on reef carbonates. Glob. Chang. Biol. 2006, 12, 2200–2208. [Google Scholar]
- Badger, M.R.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Yellowlees, D.C.; Leggat, W.; Price, G.D. The diversity and co-evolution of Rubisco, plastids, pyrenoids and chloroplast-based CCMs in the algae. Can. J. Bot. 1998, 76, 1052–1071. [Google Scholar]
- Kaplan, A.; Reinhold, L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 539–559. [Google Scholar] [CrossRef] [PubMed]
- Badger, M.R.; Price, G.D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J. Exp. Bot. 2002, 54, 609–622. [Google Scholar] [CrossRef]
- Carrasco, M.; Mercado, J.M.; Niel, F.X. Diversity of inorganic carbon acquisition mechanisms by intact microbial mats of Microcoleus chthonoplastes (Cyanobacteriae, Oscillatoriaceae). Physiol. Plant. 2008, 133, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, D.A.; Fu, F-X.; Zhang, Y.; Warner, M.E.; Feng, Y.; Portune, K.; Bernhardt, P.W.; Mullholland, M.R. CO2 control of Trichodesmium N2 fixation, photosynthesis, growth rates and elemental ratios: Implications for past, present and future ocean biogeochemistry. Limnol. Oceanogr. 2007, 52, 1293–1304. [Google Scholar]
- Levitan, O.; Rosenberg, G.; Setlik, I.; Setlikova, E.; Grigel, J.; Klepetar, J.; Prasil, O.; Berman-Frank, I. Elevated CO2 enhances nitrogen fixation and growth in the marine cyanobacterium Trichodesmium. Glob. Chang. Biol. 2007, 13, 531–538. [Google Scholar] [CrossRef]
- Kranz, S.A.; Sültemeyer, D.; Richter, K.U.; Rost, B. Carbon acquisition in Trichodesmium: Diurnal variation and effect of pCO2. Limnol. Oceanogr. 2009, 54, 548–559. [Google Scholar] [CrossRef]
- Kletou, D.; Hall-Spencer, J.M. Threats to Ultraoligotrophic Marine Ecosystems. In Marine Ecosystems; Cruzado, A., Ed.; InTech—Open Access Publisher: Rijeka, Croatia, 2012. [Google Scholar]
- Wu, Y.; Campbell, D.D.; Irwin, A.J.; Suggett, D.J.; Finkel, Z.V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 2014, 59, 1027–1034. [Google Scholar] [CrossRef]
- Flynn, K.J.; Blackford, C.J.; Baird, M.E.; Raven, J.A.; Clark, D.R.; Beardall, J.; Brownlee, C.; Fabian, H.; Wheeler, G.L. Changes in pH at the exterior surface of plankton with ocean acidification. Nat. Clim. Chang. 2012, 2, 510–513. [Google Scholar] [CrossRef]
- Hervé, V.; Derr, J.; Douady, S.; Quinet, M.; Moisan, L.; Lopez, P.J. Multiparametric analyses reveal the pH-dependence of silicon biomineralization in diatoms. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Boney, A.D. Growth interactions between littoral diatoms and juvenile marine algae. J. Exp. Mar. Biol. Ecol. 1984, 81, 21–45. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, V.R.; Brownlee, C.; Milazzo, M.; Hall-Spencer, J.M. Marine Microphytobenthic Assemblage Shift along a Natural Shallow-Water CO2 Gradient Subjected to Multiple Environmental Stressors. J. Mar. Sci. Eng. 2015, 3, 1425-1447. https://doi.org/10.3390/jmse3041425
Johnson VR, Brownlee C, Milazzo M, Hall-Spencer JM. Marine Microphytobenthic Assemblage Shift along a Natural Shallow-Water CO2 Gradient Subjected to Multiple Environmental Stressors. Journal of Marine Science and Engineering. 2015; 3(4):1425-1447. https://doi.org/10.3390/jmse3041425
Chicago/Turabian StyleJohnson, Vivienne R., Colin Brownlee, Marco Milazzo, and Jason M. Hall-Spencer. 2015. "Marine Microphytobenthic Assemblage Shift along a Natural Shallow-Water CO2 Gradient Subjected to Multiple Environmental Stressors" Journal of Marine Science and Engineering 3, no. 4: 1425-1447. https://doi.org/10.3390/jmse3041425







