1. Introduction
Field observations have shown that different coral species exhibit different susceptibilities to bleaching stress [
1]. Laboratory experiments have also shown different susceptibilities of
in hospite Symbiodinium to thermal or light stress among coral species [
2]. Susceptibility to bleaching is well studied in adult corals, but, until recently, few studies have investigated the susceptibility of larval stages [
3,
4,
5,
6]. Recently, Cumbo
et al. [
7,
8] reported that respiration of
P. damicornis larvae increased at high temperatures. Rivest and Hofmann [
9] demonstrated that the oxygen consumption rate of the coral
P. damicornis larvae was higher at elevated temperatures. On the other hand, Putnam
et al. [
10] observed reductions in the rates of larval metabolism and key photosynthetic enzymes under continuous high-temperature exposure. They suggested that such reduced metabolism could lead to energetic debt, which is critical for survivorship. As planulae generally remain for at least 1 day on the ocean surface [
11] or in shallow water [
12], they tend to be exposed to high light and temperature stress during the planktonic stage. Graham
et al. [
13] reported that coral larvae enter a state of low metabolism after they become competent to metamorphose and that such a decline in metabolic rate extends the duration of the pelagic larval stage more than predicted based on larval energy reserves. Understanding coral susceptibility to environmental stress at various life history stages, particularly at the larval stage, is important to predict the response of coral populations to environmental stressors, such as increasing seawater temperature.
Algal symbionts of reef-building corals can become a source of reactive oxygen species (ROS) under stress [
14]. Stress-exposure experiments with
Acropora planulae inoculated with symbionts show that symbiotic planulae are more sensitive to high temperature and strong light stress than that of non-symbiotic planulae [
15,
16].
Acropora planulae inoculated with homologous
Symbiodinium cells suffer more severe oxidative damage than that of non-symbiotic planulae [
15,
16]. However, it is unknown whether symbiotic coral planulae with a vertical mode of symbiont transmission are more vulnerable to oxidative damage than non-symbiotic coral planulae with a horizontal mode of symbiont transmission. Our previous study showed that zooxanthellae-containing planulae of
Pocillopora damicornis, a vertical transmitter, exhibit higher tolerance to thermal stress than that of zooxanthella-free planula of
Acropora tenuis, a horizontal transmitter [
17,
18]. It is likely that zooxanthellate coral planulae, with a vertical mode of symbiont transmission similar to
Pocillopora damicornis, have the capacity to acclimate to high temperature and regulate metabolic rate or defense mechanisms to manage the oxidative stress caused by the symbiotic association at an early developmental stage.
As a first step to understand the mechanisms by which P. damicornis planulae become highly stress tolerant, we compared the temperature dependence of the respiration rates between P. damicornis and A. tenuis planulae. We examined how coral larval metabolism responds to increasing temperature. If the respiration rate of A. tenuis larvae increases more sharply with increasing temperature compared to that of P. damicornis larvae, A. tenuis larvae may suffer energy deficiencies at lower temperatures and, hence, exhibit higher susceptibility to thermal stress. We also compared the temperature dependency of respiration between larvae and adult branches to investigate whether larvae have particular metabolic characteristics to endure the stressful conditions on the ocean surface during the planktonic stage.
2. Materials and Methods
2.1. Sample Collection
Five colonies of A. tenuis were collected from the Sesoko Island north patch reef (26°38′53.6″ N, 127°51′13.2″ E), Okinawa, in May 2013. Five colonies of P. damicornis were collected from a tidal pool at Bise, which is 6.7 km north of Sesoko Island, in May and July 2013.
To measure respiration of planula larvae, planulae of A. tenuis (spawner) and P. damicornis (brooder) were obtained as follows. Gametes released by four A. tenuis colonies were collected and mixed to induce fertilization. The resulting planulae were kept in 2-L chambers containing filtered seawater, which was changed daily. For P. damicornis, planulae released from each of five colonies were collected and kept separately in 2-L chambers. Larvae of both species were kept at room temperature (25–26 °C).
To measure the respiration and photosynthetic rates of adult colonies, a branch approximately 3 cm long was cut from each of the five adult colonies using pliers. The branches were allowed to recover in an outdoor flowing-seawater tank system at Sesoko Station for 2–7 days until use. Branches from five colonies were used as replicates for both A. tenuis and P. damicornis.
2.2. Temperature Dependence of Respiration in Planula Larvae
Ten planulae from each species, which had been kept at 25 °C, were placed in a well of a 16-well chamber slide (Lab-Tek, Scotts Valley, CA, USA). The well was filled with 400 μL of 0.22-μm-filtered seawater and a ring-shaped piece of Parafilm (PECHINEY PLASTIC PACKAGING, Chicago, IL, USA) was placed between the well and the lid to make the well airtight. Then, the chamber slide was immersed in a water bath (E-Thermobucket; TAITEC, Saitama, Japan) maintained at 25 °C. The planulae were allowed to acclimate to the temperature for 15 min in a glass bottle containing 100 mL of filtered seawater before measuring respiration. After measurement at 25 °C, planulae were put into a glass bottle containing 100 mL of filtered seawater and allowed to acclimate to a new temperature (27 °C) for about 15 min. When the water bath temperature increased to the desired temperature, planulae were again put into the well to measure of respiration at the new temperature. Thus the same planulae were used for respiration measurements at different temperatures, first at 25 °C, then at 27 °C, 30 °C, and finally at 32 °C. The planulae experienced a 2–3 °C temperature increase in about 15 min.
The oxygen concentration of the seawater containing planulae was measured every minute for 20–40 min in the dark using a fiber-optic oxygen meter (FIBOX3; PreSens, Regensburg, Germany) and a fluorescent oxygen sensor plate (SP-PST3-NAU-D5-YOP; PreSens, Regensburg, Germany) glued to the lid of the well. The oxygen concentration during measurements was not less than 80%. The chamber was shaken by hand every 5 min to equilibrate the oxygen concentration.
The respiration measurements were repeated six times for
A. tenuis planulae and five times for
P. damicornis planulae using different planulae. For
A. tenuis, planulae (28–35 days after fertilization) were sampled repeatedly (six times) from a single batch of larvae for respiration measurements. Planulae derived from each of five
P. damicornis colonies were used for replicated measurements of larval respiration. The number of replicates was five, but the age of planulae among the replicates varied; four measurements were made using planulae 5–9 days after release and one measurement used planulae 42 days after release. Although Cumbo
et al. [
8] reported that larvae differed in respiration rate and protein biomass during the first 5 day after release, the respiration rates obtained using the 42-day-old planulae were within the range of data obtained using 5–9-day-old planulae. Therefore, the data from five experiments were pooled.
Dry weight of the planulae used for the respiration measurements was estimated by filtering 20–50 planulae (derived from the same batch but not the same planulae used for the measurements) through a glass-fiber filter (GF/A 25 mm diameter; Whatman, Maidstone, UK) that had been dried (80 °C, 24 h) and weighed using a microbalance (Mettler-Toledo, Columbus, OH, USA) before use. The filter paper with planulae was quickly rinsed with distilled water to remove salts and then dried at 80 °C for 24 h. The dry weight of each of the five larvae batches derived from different P. damicornis colonies was measured, as well as the dry weight of five A. tenuis planulae replicates derived from the same gamete cross.
Respiration rate was calculated from the regression line of the oxygen concentration against time (min) in the dark. Planulae respiration rates were expressed as μg oxygen per hour per mg dry weight of each planula sample (μgO
2·h
−1·mgDW
−1). The logR values were plotted against temperature, and the temperature quotient (Q
10) was calculated from the slope of the regression line to obtain the temperature dependence of respiration. Q
10 indicates the increase in the respiration rate caused by a 10 °C increase in temperature. Q
10 values were calculated using the following equation, where R
2 and R
1 are relative respiration rates at two temperatures, T
1 and T
2:
2.3. Temperature Dependence of Dark Respiration and Net Photosynthesis of Adult Branches
The temperature dependence of dark respiration (DR) and net photosynthesis (NP) of adult branches of P. damicornis and A. tenuis was also investigated. Respiration and net photosynthetic rates of coral branches were measured using a luminescent dissolved oxygen probe (HQ40d; HACH, Loveland, CO, USA), which was also equipped with a temperature sensor. The coral branches were placed in a 440-mL chamber containing 0.22-μm-filtered seawater. The chamber was placed in a water bath (E-Thermobucket; TAITEC) with temperatures of 25, 27, 30, 32, or 33 °C. The seawater in the chamber was stirred using a magnetic stirrer. The oxygen concentration in the seawater was measured every minute initially in the dark for 30 min to measure the respiration rate. Then, the coral branches were illuminated from four directions by two fiber light sources, each with two light guides at a light intensity of 350–400 μmol photons m−2·s−1 for 30 min to measure net photosynthetic rate. The light sources (PICL-NEX; NPI cold light system and PCS-NHF150; Moritex, Cambridge, UK) were equipped with a 15-V, 150-W halogen lamp (Type 6423, Philips, Littlestown, PA, USA). The respiration and photosynthesis measurements were conducted from low to high temperature using the same branch samples. It took 15–30 min for the seawater temperature in the chamber to reach the new set temperature. Thus, the coral branches were adapted to each new temperature for at least 15 min before the experiment was started and were illuminated to maintain the oxygen concentration in the chamber above 80%. The experiment was repeated using five different branches of A. tenuis and P. damicornis, respectively.
After the measurements, the coral branches were fixed in 10% formaldehyde in fresh seawater and decalcified in a 1:1 mixture of 10% formaldehyde and 10% acetic acid in tap water. The decalcified samples were rinsed in tap water, dried (80 °C, 24 h), and weighed (FX-300 electronic balance; A& D, Milpitas, CA, USA) to obtain tissue dry weight.
Respiration and net photosynthesis rates were calculated from the regression line of the oxygen concentration against time (min) in the dark and in light, respectively. Respiration and net photosynthesis rates are expressed as μgO2·mgDW−1·h−1. The temperature dependence of respiration of the adult branches was analyzed as described above. Mean Q10 values were obtained from five replicates for A. tenuis and P. damicornis.
2.4. Statistical Analyses
All statistical analyses were conducted using STATISTICA ver. 6.0 (Dell, Inc., Austin, TX, USA). Respiration rates were compared at each temperature between planula larvae and adult branches or between the two species using a t-test. The relationships between the respiration rate or net photosynthesis rate and temperature were analyzed using regression analysis. Mean temperature coefficients (Q10) of the respiration rates were compared between the two species or between planulae and adult branches of the same species using analysis of variance (ANOVA) when the data met the assumptions for parametric analysis (Shapiro–Wilk test for normality and Levene’s test for homoscedasticity). The Kolmogorov–Smirnov test was used when the data did not meet parametric assumptions. A p-value < 0.05 was considered significant.
4. Discussion
While algal symbionts are generally believed to become a source of ROS and cause oxidative damage in the host coral under stressful conditions (e.g., [
14]), our previous study showed that symbiotic larvae of
P. damicornis are more thermally stress tolerant than
A. tenuis zooxanthella-free larvae [
17,
18]. We hypothesized that different thermal tolerance between the two species may be due to different temperature dependencies of their metabolic rates. However, the present results show that
P. damicornis and
A. tenuis larvae had similar temperature dependencies of their respiration rates with temperature quotient (Q
10) values around 2. This is consistent with recently reported values of Q
10 [
8,
9]. Thus, the present results do not support our hypothesis that stress-sensitive
A. tenuis larvae suffer greater increases in respiration rate than do
P. damicornis larvae at elevated temperatures.
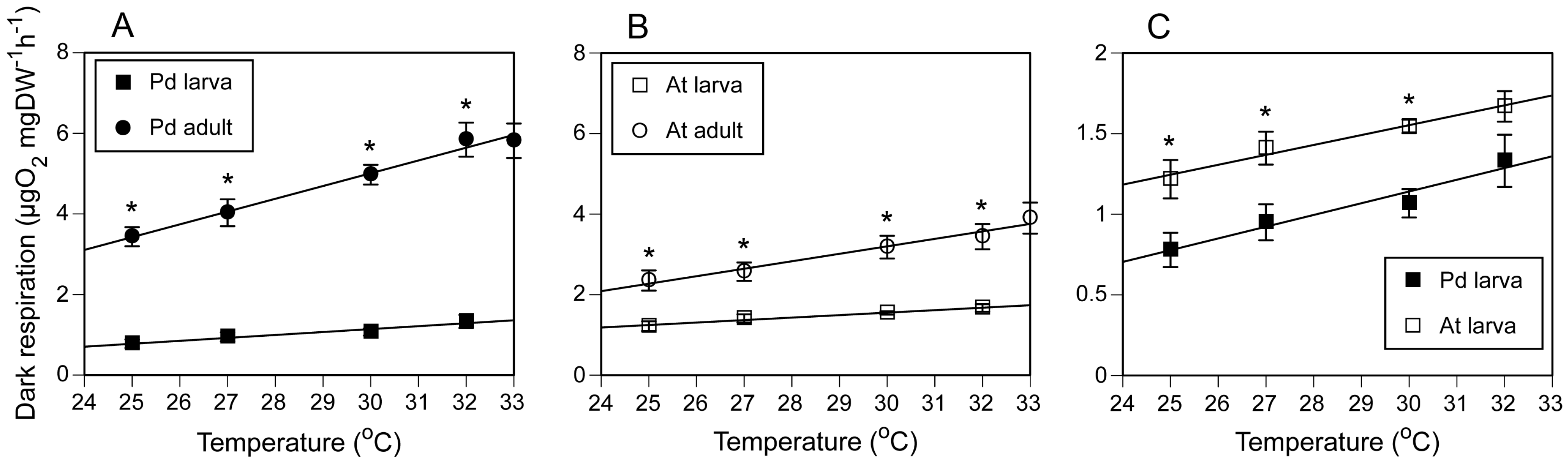
Respiration rates were lower in larvae than in adult branches in both species. Therefore, we hypothesized that larvae tolerate and survive under stressful conditions by maintaining a lower metabolic rate than adult colonies.
P. damicornis larvae had a significantly lower respiration rate than
A. tenuis larvae at 25, 27, and 30 °C. The same tendency was observed at 32 °C, although the difference was not significant. In contrast, adult branches of
P. damicornis had higher respiration rates than those of
A. tenuis branches, indicating that
P. damicornis larvae have a greater capacity to reduce their metabolic rate than
A. tenuis larvae. This finding may, in part, contribute to the higher thermal tolerance of
P. damicornis larvae compared to
A. tenuis larvae. The lower larval respiration rates are consistent with the observation that coral larvae can maintain a low metabolic rate after becoming competent to metamorphose [
13,
19]. Putnam
et al. [
20] suggested that larvae of
P. damicornis are physiologically well suited to fluctuating temperatures. Graham
et al. [
13] suggested that a decline in metabolic rate enables non-feeding coral larvae to survive for a longer period than predicted based on larval energy reserves.
The lower respiration rates of
P. damicornis larvae may not be the sole explanation for higher tolerance as other potential mechanisms were not investigated. Other factors may also be involved in the high thermal tolerance of
P. damicornis larvae.
P. damicornis larvae could receive photosynthetically derived nutrients from algal symbionts [
21,
22]. Thus,
P. damicornis larvae may enjoy a favorable energy budget and longer longevity compared to
A. tenuis larvae. Survivorship of
P. damicornis larvae may be determined by a trade-off between beneficial
versus harmful influences of the symbionts, such as energy supplied by and oxidative stress caused by symbionts. Our previous study showed that colonies of
P. damicornis in Okinawa might harbor multiple symbiont types such as A1, C1, C3 and C71 [
17], while
A. tenuis colonies are associated with the subclade C3 [
23]. An alternative possibility is that
P. damicornis larvae have a more effective antioxidant system than that of
A. tenuis larvae. Further studies on antioxidant systems and other physiological characteristics of
P. damicornis larvae may provide information to clarify the high stress tolerance mechanisms of
P. damicornis larvae compared with those of
A. tenuis larvae.
The respiration rates of adult branches of
P. damicornis and
A. tenuis increased almost linearly in the range of 25–32 °C, as in other tropical corals [
24,
25,
26]. The Q
10 values of the respiration rates of the adult branches of
P. damicornis and
A. tenuis were about 2.0, which are similar to the Q
10 values for planulae of the two species. The Q
10 values are in the same range reported for other tropical corals [
26] or similar to the recently reported values for larvae of
P. damicornis [
8,
9]. However, Howe and Marshall [
27] reported that the respiration rate of the temperate zooxanthellate coral
Plesiastrea versipora increases in the range of 12.8–18.3 °C, with Q
10 values of 2.2–2.3, but that it leveled out in the range of 18.3–21 °C, with Q
10 values of 0.4–1.1. This shows that temperate coral might lose normal temperature response around the upper limit of its habitat temperature.
The relationship between net photosynthetic rate and temperature was hyperbolic with highest net photosynthetic rate at 27–30 °C in
P. damicornis. A similar tendency was observed in
A. tenuis, though regression using a quadratic function did not yield significant results. Corals show a significant decrease in the production/respiration ratio under high temperature [
3,
28], which may lead to an energy shortage. When the net photosynthetic and dark respiration curves were plotted in the same figure, the two curves crossed at about 32 °C for the
P. damicornis branches and around 34 °C for the
A. tenuis branches. In the future studies, it may be possible to use this relationship to compare the threshold temperature causing energy deficits among different coral species, after first developing a protocol using normal light irradiance for corals.
This study showed that dry weight-specific respiration rate differed among planulae of P. damicornis and A. tenuis and between planulae and adult branches of the same species, whereas Q10 values for dark respiration were very similar for planulae and adult branches of both species. It should be noted that the protein biomass-specific respiration rate might be a more suitable parameter for comparing respiration rate between organisms with differing lipid contents.