Harvesting Method Affects Water Dynamics and Yield of Sweet Orange with Huanglongbing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Measurements
2.2. Citrus Sap Flow Measurements
2.3. Stem Water Potential (Ψ)
2.4. Harvesting Methods and Fruit Yield
2.5. Statistical Analysis
3. Results and Discussion
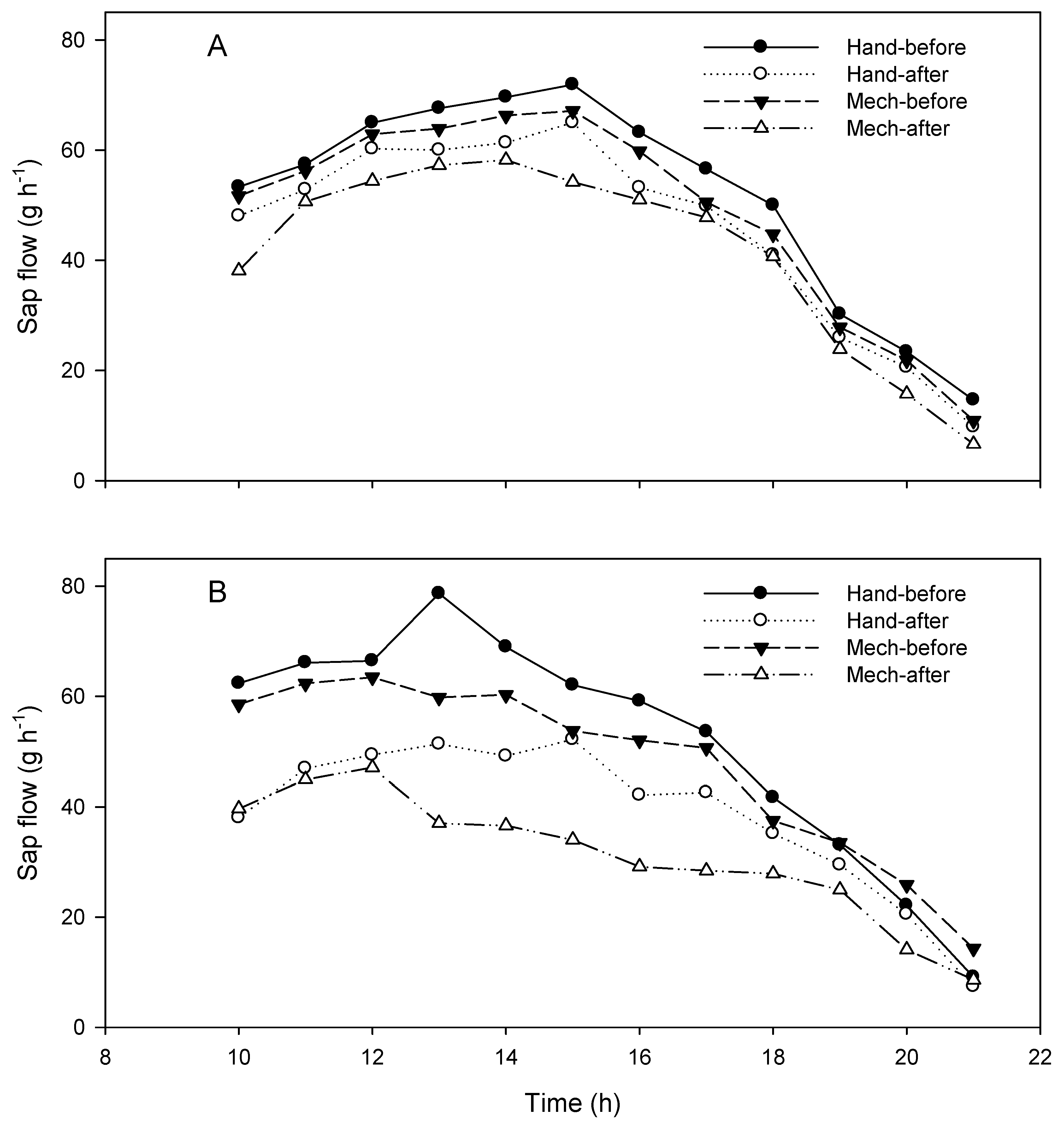
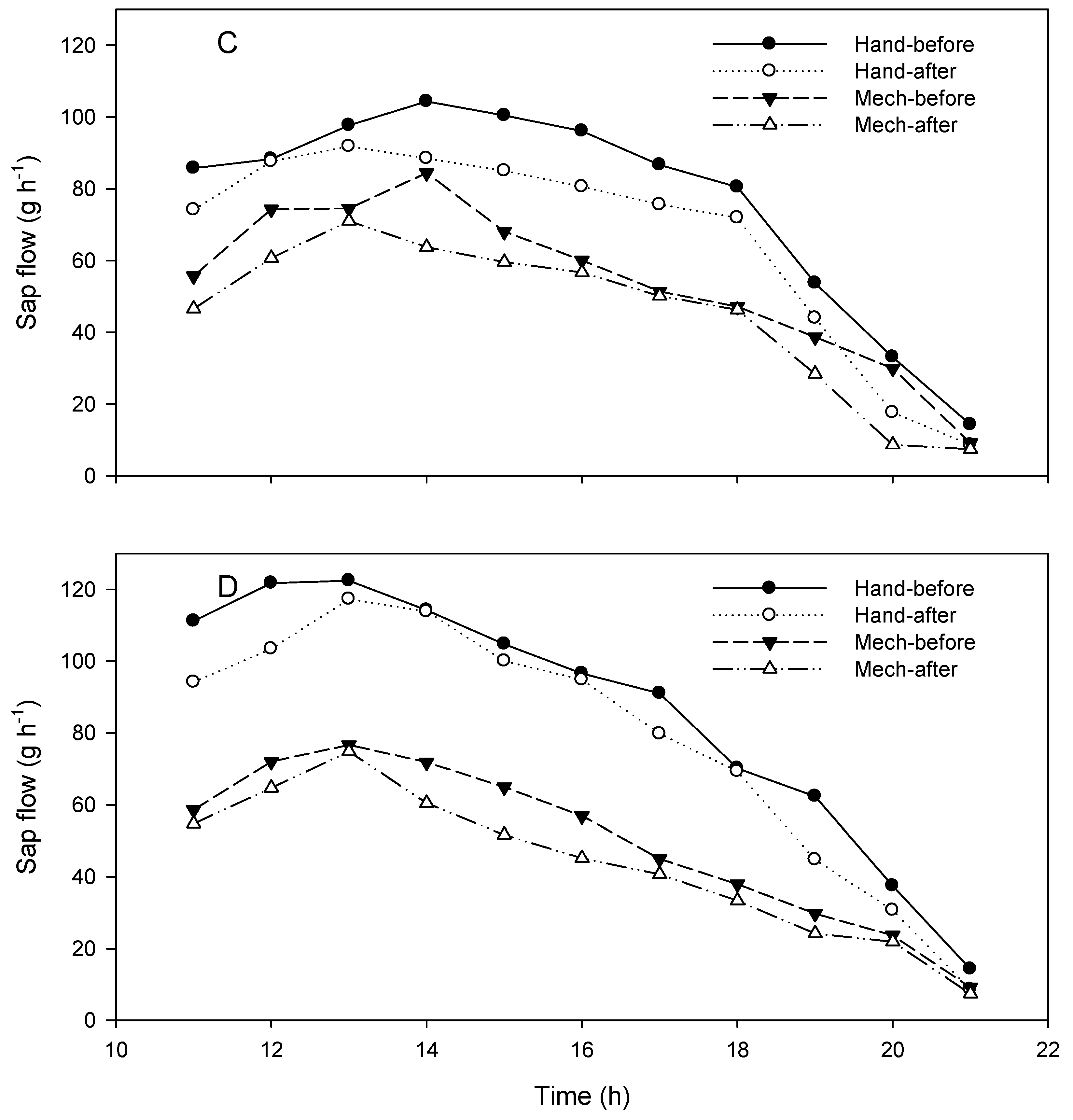
3.1. Sap Flow Measurements
3.2. Stem Water Potential (Ψstem)
3.3. Fruit Yields
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- USDA-NASS. Citrus Production by Type and State. 2016. Available online: https://quickstats.nass.usda.gov/results/0D73291D-33CC-3916-84E0-D4A1DC62C16C?pivot=short_desc (accessed on 15 May 2017).
- Roka, F.M.; Ehsani, R.J.; Futch, S.H.; Hyman, B.R. Citrus Mechanical Harvesting Systems—Continuous Canopy Shakers. Gainesville: University of Florida Institute of Food and Agricultural Sciences. 2014. Available online: http://edis.ifas.ufl.edu/pdffiles/FE/FE95100.pdf (accessed on 12 August 2017).
- Whitney, J.D. A review of citrus harvesting in Florida. In Proceedings of the Citrus Engineering Conference, Lakeland, FL, USA, 23 March 1995; ASME: New York, NY, USA, 1995; Volume 41, pp. 33–59. [Google Scholar]
- Hedden, S.L.; Churchill, D.B.; Whiteny, J.D. Trunk shakers for citrus harvestin—Part II: Tree growth, fruit yield and removal. Appl. Eng. Agric. 1988, 4, 102–106. [Google Scholar] [CrossRef]
- Spann, T.M.; Danyluk, M.D. Mechanical harvesting increases leaf and stem debris in loads of mechanically harvested citrus fruit. HortScience 2010, 45, 1297–1300. [Google Scholar]
- Li, K.T.; Syversten, J.P. Mechanical harvesting has little effect on water status and leaf gas exchange in citrus trees. J. Am. Soc. Hortic. Sci. 2005, 130, 661–666. [Google Scholar]
- Whitney, J.D. Trunk shaker and abscission chemical effects on yields, fruit removal, and growth of orange trees. Proc. Fla. State Hortic. Soc. 2003, 116, 230–235. [Google Scholar]
- Coppock, G.E. Properties of young and mature ‘Valencia’ oranges related to selective harvest by mechanical means. Am. Soc. Agric. Biol. Eng. 1972, 15, 235–238. [Google Scholar] [CrossRef]
- FDOC. Citrus Mechanical Harvesting Website. Florida Department of Citrus, Lakeland, FL, USA. 2016. Available online: http://citrusmh.ifas.ufl.edu/index.asp?s=2&p=2 (accessed on 22 October 2017).
- Morris, R.A.; Erick, C.; Estes, M. The Incidence of Greening and Canker Infection in Florida Citrus Groves from September 2007 through August 20081FE823.EDIS; FE823 Florida Cooperative Extension Service; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2009. [Google Scholar]
- Singerman, A. 2014/15 Picking, Roadsiding and Hauling Charges for Florida Citrus. University of Florida, IFAS, Citrus Research and Education Center, Lake Alfred, FL, USA, 2015. Available online: http://www.crec.ifas.ufl.edu/extension/economics/pdf/Estimated_Average_Picking_2014-15.pdf (accessed on 25 September 2017).
- Zekri, M.; Obreza, T.A.; Koo, R. Irrigation, Nutrition, and Citrus Fruit Quality; EDIS, SL 207, Florida Cooperative Extension Service; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2009. [Google Scholar]
- Hamido, S.A.; Morgan, K.T.; Kadyampakeni, D.M. The effect of Huanglongbing on young citrus tree water use. HortTechnology 2017, 27, 659–665. [Google Scholar] [CrossRef]
- Enciso, J.; Sauls, J.W.; Wiedenfeld, R.P.; Nelson, S.D. Impacts of Irrigation on Citrus in the Lower Rio Grande Valley; B-6205 05-08; Agrilife Extension-Texas A & M System: College Station, TX, USA, 2008. [Google Scholar]
- Dynamax. Dynagage Installation and Operation Manual; Dynamax: Houston, TX, USA, 1990; p. 80. [Google Scholar]
- Garnier, E.; Berger, A. Testing water potential in peach trees as an indicator of water stress. J. Hortic. Sci. 1985, 60, 47–56. [Google Scholar] [CrossRef]
- SAS Institute. SAS System for Windows Release 9.1.3; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Jones, H.G. Irrigation scheduling: Advantages and pitfalls of plant-based methods. J. Exp. Bot. 2004, 407, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.M.; Allen, S.J. Measurements of sap flow in plant stems. J. Exp. Bot. 1996, 47, 1833–1844. [Google Scholar] [CrossRef]
- Hamido, S.A.; Morgan, K.T.; Ebel, R.C.; Kadyampakeni, D.M. Improved irrigation management of sweet orange with Huanglongbing. HortScience 2017, 52, 916–921. [Google Scholar] [CrossRef]
- Morgan, K.T.; Barkataky, S.; Kadyampakeni, D.; Ebel, R.; Roka, F. Effects of short-term drought stress and mechanical harvesting on sweet orange tree health, water uptake, and yield. HortScience 2014, 49, 835–842. [Google Scholar]
- Xu, X.; Tong, L.; Li, F.; Kang, S.; Qu, Y. Sap flow of irrigated Populus alba var. pyramidalis and its relationship with environmental factors and leaf area index in an arid region of Northwest China. J. For. Res. 2011, 16, 144–152. [Google Scholar] [CrossRef]
- Yin, G.; Zhou, G.; Wang, X.; Chu, G.; Huang, Z. A study on sap flux density of two eucalyptus (Eucalyptus ueophylla) plantation in southeastern China by heat-pulse method. Acta Ecol. Sin. 2003, 23, 1984–1990. [Google Scholar]
- Chone, X.; van Leeuwen, C.; Dubourdieu, D.; Gaudillère, J.P. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. 2001, 87, 477–483. [Google Scholar] [CrossRef]
- Castro-Garcia, S.; Blanco-Roldan, G.L.; Ferguson, L.; Gonzalez-Sanchez, E.J.; Gil-Ribes, J.A. Frequency response of late-season ‘Valencia’ orange to selective harvesting by vibration for juice industry. Biosyst. Eng. 2017, 155, 77–83. [Google Scholar] [CrossRef]


| Experiments | Sap Flow | Stem Water Potential | Harvesting | |
|---|---|---|---|---|
| Start Date | End Date | Date | Date | |
| A (early season) | 26 March 2015 | 7 April 2015 | NA | 30 March 2015 |
| B (late season) | 7 April 2015 | 24 April 2015 | NA | 14 April 2015 |
| C (early season) | 3 March 2016 | 13 April 2016 | 29 March and 5 April | 4 April 2016 |
| D (late season) | 13 April 2016 | 27 April 2016 | 14 and 21 April | 20 April 2016 |
| Experiment (Site)\Mean | Harvesting Method | Hand Harvesting | Mechanical Harvesting | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hand | Mechanical | Difference % | Before | After | Difference % | Before | After | Difference % | |
| A | 56.4 | 45.2 | 19.86 | 68.8 | 44.1 | 35.90 | 48.4 | 41.9 | 13.43 |
| B | 45.3 | 39.4 | 13.02 | 51.9 | 38.7 | 25.43 | 47.7 | 31.0 | 35.01 |
| C | 77.2 | 53.8 | 30.02 | 82.7 | 71.7 | 13.30 | 58.4 | 49.2 | 15.75 |
| D | 89.0a | 50.4 | 43.37 | 93.2 | 84.8 | 9.01 | 53.7 | 47.1 | 12.29 |
| ANOVA | |||||||||
| Harvesting method | Sampling time-hand harvesting | Sampling time-mechanical harvesting | |||||||
| F value | Pr > F | F value | Pr > F | F value | Pr > F | ||||
| A | 14.6 | 0.0068 | 9.2 | 0.0061 | 0.4 | 0.5567 | |||
| B | 4.1 | 0.0118 | 8.5 | 0.0080 | 3.4 | 0.0808 | |||
| C | 5.1 | 0.0047 | 1.2 | 0.2982 | 1.3 | 0.2622 | |||
| D | 9.0 | 0.0001 | 0.4 | 0.5189 | 0.7 | 0.4278 | |||
| Experiment\Mean | Harvesting Method | Hand Harvesting | Mechanical Harvesting | |||
|---|---|---|---|---|---|---|
| Hand | Mechanical | Before | After | Before | After | |
| C | −0.93 | −1.14 | −0.99 | −0.86 | −1.14 | −1.13 |
| D | −0.79 | −0.92 | −0.91 | −0.68 | −0.92 | −0.91 |
| ANOVA | ||||||
| Harvesting method | Sampling time-Hand | Sampling time-Mechanical | ||||
| F value | Pr > F | F value | Pr > F | F value | Pr > F | |
| C | 18.8 | <0.0001 | 11.98 | 0.0022 | 0.07 | 0.7971 |
| D | 31.2 | <0.0001 | 58.21 | <0.0001 | 0.07 | 0.8005 |
| Experiment | Harvesting Dates | Number of Trees | Yield (kg Fruit tree−1) | p Value | ||
|---|---|---|---|---|---|---|
| Hand | Mechanical | Hand | Mechanical | |||
| A (early season) | 30 March 2015 | 18 | 18 | 41.4 | 34.5 | <0.0001 |
| B (late season) | 14 April 2015 | 26 | 23 | 53.7 | 33.7 | <0.0001 |
| C (early season) | 4 April 2016 | 25 | 25 | 32.5 | 15.9 | <0.0001 |
| D (late season) | 20 April 2016 | 25 | 25 | 24.5 | 8.8 | <0.0001 |
| 38.1 | 23.2 | <0.0001 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamido, S.A.; Morgan, K.T. Harvesting Method Affects Water Dynamics and Yield of Sweet Orange with Huanglongbing. Agriculture 2018, 8, 38. https://doi.org/10.3390/agriculture8030038
Hamido SA, Morgan KT. Harvesting Method Affects Water Dynamics and Yield of Sweet Orange with Huanglongbing. Agriculture. 2018; 8(3):38. https://doi.org/10.3390/agriculture8030038
Chicago/Turabian StyleHamido, Said A., and Kelly T. Morgan. 2018. "Harvesting Method Affects Water Dynamics and Yield of Sweet Orange with Huanglongbing" Agriculture 8, no. 3: 38. https://doi.org/10.3390/agriculture8030038





