Using Colony Monitoring Devices to Evaluate the Impacts of Land Use and Nutritional Value of Forage on Honey Bee Health
Abstract
:1. Introduction
2. Methods
2.1. Apiary Locations
2.2. Pollen Collection and Protein Content Analyses
2.3. Floral Pollen Source Identification
2.4. Pollen Pesticide Residue Analysis
2.5. Hive Weight Data Collection and Analysis
2.6. Statistical Analyses
3. Results
3.1. Pollen-Derived Metrics between Apiary Locales
3.1.1. Pollen Quantity and Quality
3.1.2. Taxonomic Identification of Honey Bee-Collected Pollen
3.1.3. Pesticide Residues in Honey Bee-Collected Pollen
3.1.4. Colony Weight Change Measured by Hive Scales
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sponsler, D.B.; Johnson, R.M. Honey bee success predicted by landscape composition in Ohio, USA. PeerJ 2015, 3, e838. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.; Pettis, J.; Rice, N.; Browning, Z.; Spivak, M. Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS ONE 2016, 11, e0152685. [Google Scholar] [CrossRef] [PubMed]
- Alburaki, M.; Steckel, S.J.; Williams, M.T.; Skinner, J.A.; Tarpy, D.R.; Meikle, W.G.; Adamczyk, J.; Stewart, S.D. Agricultural landscape and pesticide effects on honey bee (Hymenoptera: Apidae) biological traits. J. Econ. Entomol. 2017, 110, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; vanEngelsdorp, D. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS ONE 2013, 8, e70182. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.R.V.; Roth, C.; Carlson, B.; Smart, M.D. Land use change reduces habitat suitability for supporting managed honey bee colonies in the Northern Great Plains. Proc. Natl. Acad. Sci. USA 2016, 113, 10430–10435. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Ducloz, F.; Crauser, D.; LeConte, Y. Diet effects on honeybee immunocompetence. Biol. Lett. 2010. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, G.; Salignon, M.; LeConte, Y.; Belzunces, L.P.; Decourtye, A.; Kretzschmar, A.; Suchail, S.; Brunet, J.L.; Alaux, C. Influence of pollen nutrition on honey bee health: Do pollen quality and diversity matter? PLoS ONE 2013, 8, e72016. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Dantec, C.; Parrinello, H.; LeConte, Y. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genom. 2011, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.D.; Pettis, J.S.; Euliss, N.; Spivak, M.S. Land use in the Northern Great Plains region of the US influences the survival and productivity of honey bees. Agric. Ecosyst. Environ. 2016, 230, 139–149. [Google Scholar] [CrossRef]
- Dolezal, A.G.; Carrillo-Tripp, J.; Miller, W.A.; Bonning, B.C.; Toth, A.L. Intensively cultivated landscape and varroa mite infestation are associated with reduced honey bee nutritional state. PLoS ONE 2016, 11, e0153531. [Google Scholar] [CrossRef] [PubMed]
- Alaux, C.; Allier, F.; Decourtye, A.; Odoux, J.F.; Tamic, T.; Chabirand, M.; Delestra, E.; Decugis, F.; LeConte, Y.; Henry, M. A ‘landscape physiology’ approach for assessing bee health highlights the benefits of floral landscape enrichment and semi-natural habitats. Sci. Rep. 2017, 7, 40568. [Google Scholar] [CrossRef] [PubMed]
- Colwell, M.J.; Williams, G.R.; Evans, R.C.; Shutler, D. Honey bee-collected pollen in agro-ecosystems reveals diet diversity, diet quality, and pesticide exposure. Ecol. Evol. 2017, 7, 7243–7253. [Google Scholar] [CrossRef] [PubMed]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Predictive markers of honey bee colony collapse. PLoS ONE 2012, 7, e32151. [Google Scholar] [CrossRef] [PubMed]
- Simone-Finstrom, M.; Li-Byarlay, H.; Huang, M.H.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016, 6, 32023. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, S.L.; Thoenes, S.C. The electronic scale honey bee colony as a management and research tool. Bee Sci. 1990, 1, 40–47. [Google Scholar]
- Meikle, W.G.; Rector, B.G.; Mercadier, G.; Holst, N. Within-day variation in continuous hive weight data as a measure of honey bee colony activity. Apidologie 2008, 39, 694–707. [Google Scholar] [CrossRef]
- Meikle, W.G.; Holst, N. Application of continuous monitoring of honeybee colonies. Apidologie 2015, 46, 10–22. [Google Scholar] [CrossRef]
- Gil-Lebrero, S.; Quiles-Latorre, F.J.; Ortiz-Lopez, M.; Sanchez-Ruiz, V.; Gamiz-Lopez, V.; Luna-Rodriguez, J.J. Honey bee colonies remote monitoring system. Sensors 2017, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- McLellan, A.R. Honey bee colony weight as an index of honey production and nectar flow: A critical evaluation. J. Appl. Ecol. 1977, 14, 401–408. [Google Scholar] [CrossRef]
- Todd, F.E.; Bishop, R.K. Trapping honeybee-gathered pollen and factors affecting yield. J. Econ. Entomol. 1940, 33, 866–870. [Google Scholar] [CrossRef]
- Richardson, R.T.; Lin, C.H.; Sponsler, D.B.; Quijia, J.O.; Goodell, K.; Johnson, R.M. Application of ITS2 metabarcoding to determine the provenance of pollen collected by honey bees in an agroecosystem. Appl. Plant Sci. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Smart, M.D.; Cornman, R.S.; Iwanowicz, D.D.; McDermott-Kubeczko, M.; Pettis, J.S.; Spivak, M.S.; Otto, C.R.V. A comparison of honey bee-collected pollen from working agricultural lands using light microscopy and ITS metabarcoding. Environ. Entomol. 2017, 46, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Meikle, W.G.; Weiss, M.; Maes, P.W.; Fitz, W.; Snyder, L.A.; Sheehan, T.; Mott, B.M.; Anderson, K.E. Internal hive temperature as a means of monitoring honey bee colony health in a migratory beekeeping operation before and during winter. Apidologie 2017, 48, 666–680. [Google Scholar] [CrossRef]
- Dubois, E.; Reis, C.; Schurr, F.; Cougoule, N.; Ribiere-Chabert, M. Effect of pollen traps on the relapse of chronic bee paralysis virus in honeybee (Apis mellifera) colonies. Apidologie 2017. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture National Agricultural Statistics Service Cropland Data Layer. Published Crop-Specific Data Layer. 2014. Available online: https://nassgeodata.gmu.edu/CropScape/ (accessed on 1 May 2014).
- Cornman, R.S.; Otto, C.R.V.; Iwanowicz, D.; Pettis, J.S. Taxonomic characterization of honey bee (Apis mellifera) pollen foraging based on non-overlapping paired-end sequencing of nuclear ribosomal loci. PLoS ONE 2015, 10, e0145365. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J. DNA protocols for plants. In Molecular Techniques in Taxonomy; Hewitt, G.M., Johnston, A.W.B., Wong, J.P.W., Eds.; Springer: Berlin, Germany, 1991; pp. 283–293. [Google Scholar]
- Kamel, A. Refined methodology for the determination of neonicotinoid pesticides and their metabolites in honey bee and bee products by liquid chromatography-tandem mass spectrometry (LC-MS/MS). J. Agric. Food Chem. 2010, 58, 5926–5931. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 6 November 2017).
- DeGroot, A.P. Protein and amino acid requirements of the honeybee (Apis mellifica L.). Physiol. Comp. Oecol. 1953, 3, 197–285. [Google Scholar]
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies, 1st ed.; Harvard University Press: Cambridge, UK, 1995. [Google Scholar]
- Ziska, L.H.; Pettis, J.S.; Edwards, J.; Hancock, J.E.; Tomecek, M.B.; Clark, A.; Dukes, J.S.; Loladze, I.; Polley, H.W. Rising atmospheric CO2 is reducing the protein concentration of a floral pollen source essential for North American bees. Proc. R. Soc. B 2016, 283, 20160414. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture National Agricultural Statistics Service. Honey Bee Colonies. 2016; ISSN 1949-1492. Available online: http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1943 (accessed on 18 October 2017).
- Pollinator Health Task Force. National Strategy to Promote the Health of Honey Bees and Other Pollinators. 2015. Available online: https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/Pollinator%20Health%20Strategy%202015.pdf (accessed on 26 January 2017).
- Smart, M.D.; Otto, C.R.V. Using colony monitoring devices to evaluate the impacts of land use and forage quality on honey bee health datasets. U.S. Geol. Surv. Data Release 2017. [Google Scholar] [CrossRef]
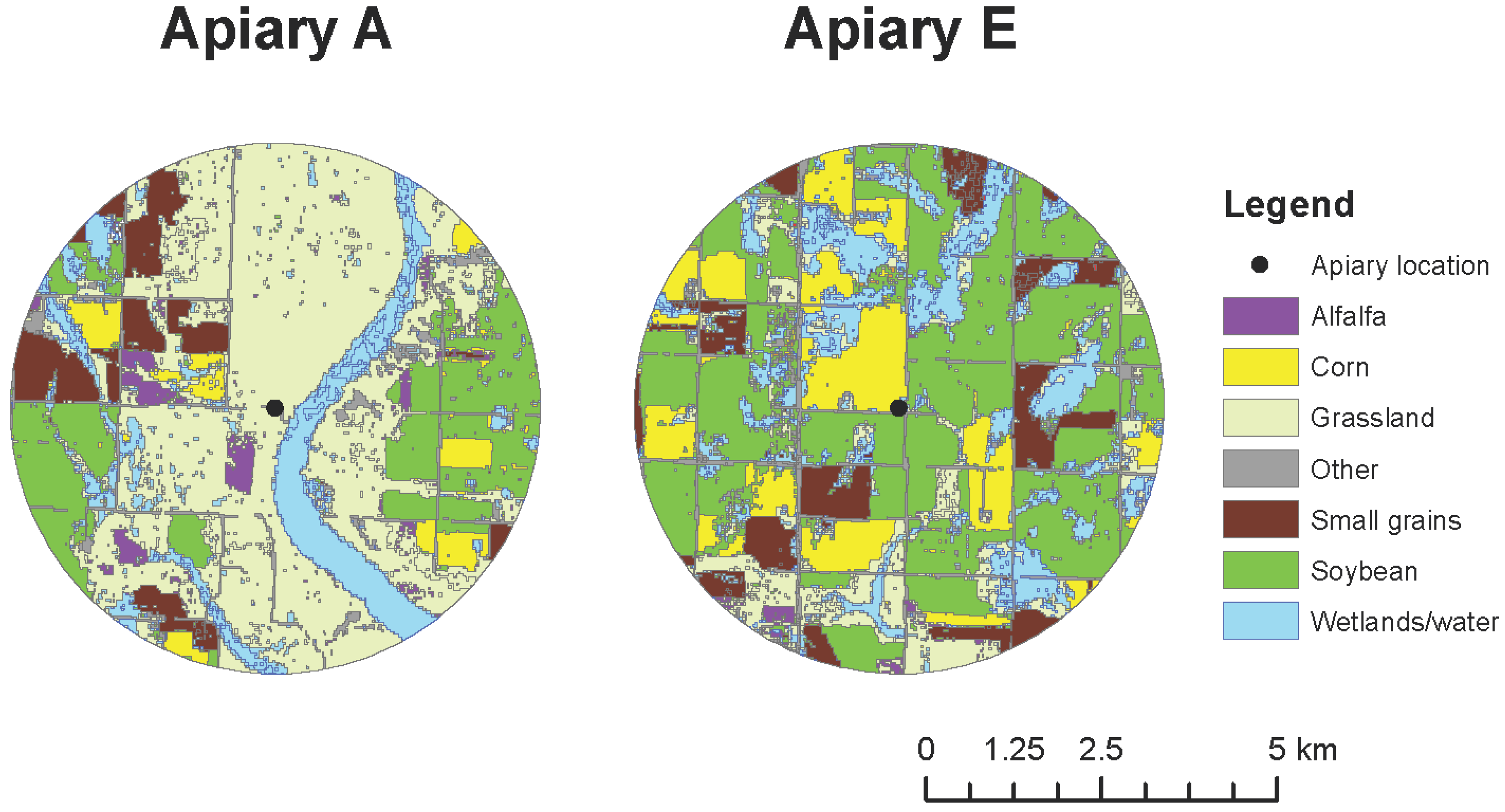

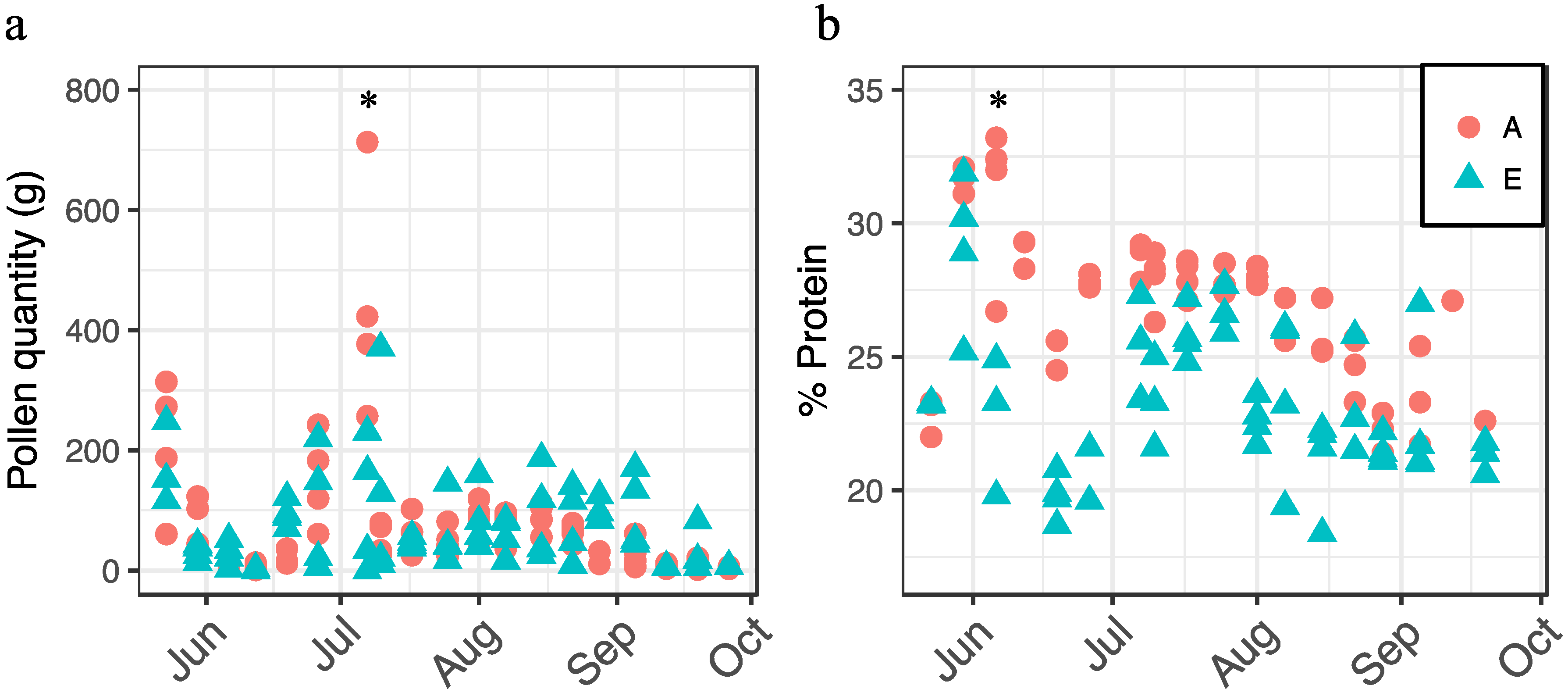

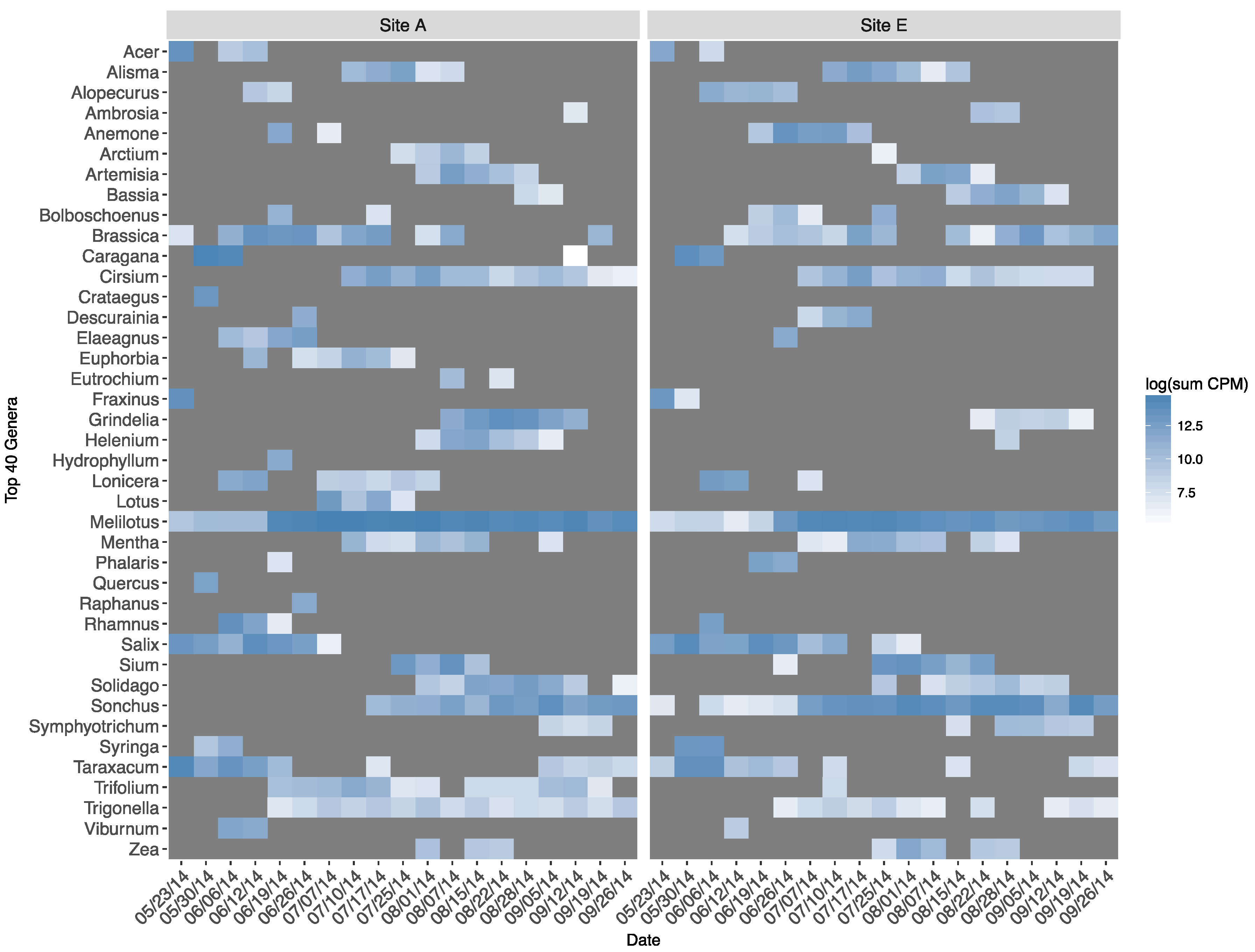

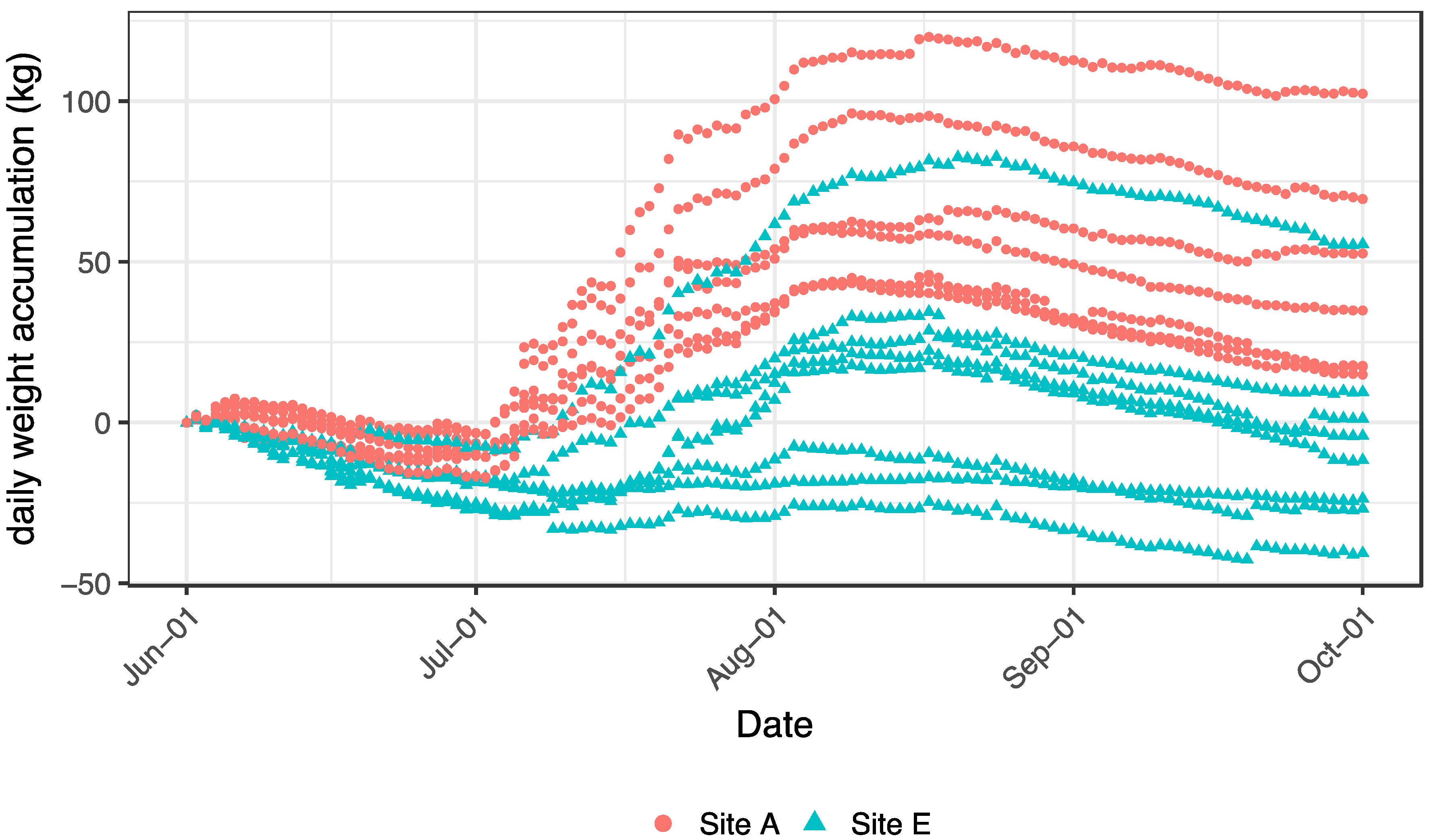
| Apiary A | Apiary E | |||
|---|---|---|---|---|
| Family | Genera | Species | Genera | Species |
| Alismataceae | 1 | - | 1 | - |
| Amaranthaceae | 3 | 2 | 2 | 1 |
| Apiaceae | 1 | 1 | 1 | 1 |
| Apocynaceae | 1 | - | 1 | - |
| Asteraceae | 17 | 14 | 17 | 11 |
| Brassicaceae | 5 | 10 | 2 | 4 |
| Caprifoliaceae | 3 | 3 | 2 | 1 |
| Celastraceae | 1 | 1 | - | - |
| Cyperaceae | 1 | 1 | 1 | 1 |
| Eleagnaceae | 1 | 1 | 1 | 1 |
| Euphorbiaceae | 1 | 1 | - | - |
| Fabaceae | 9 | 5 | 5 | 1 |
| Fagaceae | 1 | - | - | - |
| Hydrophyllaceae | 1 | 1 | - | - |
| Lamiaceae | 1 | - | 1 | - |
| Oleaceae | 2 | 2 | 2 | 1 |
| Onagraceae | 1 | 1 | - | - |
| Poaceae | 6 | 2 | 4 | 2 |
| Polygonaceae | 1 | 1 | 1 | 1 |
| Ranunculaceae | 1 | 1 | 1 | 1 |
| Rhamnaceae | 1 | - | 1 | - |
| Rosaceae | 2 | 1 | - | - |
| Salicaceae | 1 | 1 | 1 | 1 |
| Sapindaceae | 1 | 2 | 1 | 1 |
| Typhaceae * | - | - | - | - |
| Total | 63 | 51 | 45 | 28 |
| Sample Date | ρ | p-Value |
|---|---|---|
| 23 May 2014 | 0.61 | 0.17 |
| 30 May 2014 | 0.68 | 0.11 |
| 6 June 2014 | 0.58 | 0.04 |
| 12 June 2014 | 0.39 | 0.21 |
| 19 June 2014 | 0.28 | 0.32 |
| 26 June 2014 | 0.55 | 0.09 |
| 7 July 2014 | 0.09 | 0.80 |
| 10 July 2014 | 0.29 | 0.33 |
| 17 July 2014 | 0.35 | 0.21 |
| 25 July 2014 | 0.63 | 0.01 |
| 1 August 2014 | 0.44 | 0.08 |
| 7 August 2014 | 0.89 | <0.0001 |
| 15 August 2014 | 0.61 | 0.03 |
| 22 August 2014 | 0.34 | 0.17 |
| 28 August 2014 | 0.48 | 0.08 |
| 5 September 2014 | 0.60 | 0.01 |
| 12 September 2014 | 0.69 | 0.006 |
| 19 September 2014 | 0.90 | <0.0001 |
| 26 September 2014 | 0.91 | 0.0001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smart, M.; Otto, C.; Cornman, R.; Iwanowicz, D. Using Colony Monitoring Devices to Evaluate the Impacts of Land Use and Nutritional Value of Forage on Honey Bee Health. Agriculture 2018, 8, 2. https://doi.org/10.3390/agriculture8010002
Smart M, Otto C, Cornman R, Iwanowicz D. Using Colony Monitoring Devices to Evaluate the Impacts of Land Use and Nutritional Value of Forage on Honey Bee Health. Agriculture. 2018; 8(1):2. https://doi.org/10.3390/agriculture8010002
Chicago/Turabian StyleSmart, Matthew, Clint Otto, Robert Cornman, and Deborah Iwanowicz. 2018. "Using Colony Monitoring Devices to Evaluate the Impacts of Land Use and Nutritional Value of Forage on Honey Bee Health" Agriculture 8, no. 1: 2. https://doi.org/10.3390/agriculture8010002





