1. Introduction
A leafy vegetable, chicory is among the most well-known and popular horticultural plants in the world. Although there are large differences with regard to cultural practices and utilizations, chicory is found in almost every country and is included in the diet of most Western as well as Eastern populations.
Chicory is a traditional European horticultural crop, and although it cannot be considered autochthonous, its domestication as a vegetable crop occurred on continental Europe, where it gradually differentiated into a variety of cultivated types. According to Street et al. [
1],
Cichorium intybus can be divided in four varieties according to the purpose and use for which it was cultivated: (i) “industrial” or “root” chicory, which is predominantly cultivated in northwestern Europe, India, South Africa, and Chile. At present, the taproot utilization of this type appears to be mainly for inulin extraction or, on a more limited scale, for the production of a coffee substitute; (ii) “forage” chicory, a variety that was initially derived from wild chicory, has been domesticated since the mid-1970s to intensify herbage obtainability in perennial pastures for livestock; (iii) “Brussels” or “Witloof” chicory, which is commonly grown in Europe as an industrial crop for etiolated buds obtained by forcing (i.e., the particular whitening process adopted to obtain the commercial product usually named “chicon”); (iv) “leaf” chicory, also called “Radicchio”, which is mainly known as an important component of fresh salads and is often cooked and differently prepared according to traditions and alimentary habits [
2].
Currently,
C. intybus is mainly grown throughout continental Europe, in southwestern Asia, and in limited areas of North America, South Africa, and Australia. Most likely known by the Egyptians as a medicinal plant [
3] and used as a vegetable crop by ancient Greeks and the Romans [
4], chicory gradually underwent a process of naturalization in Europe. Although it cannot be considered as an autochthonous species, it became part of the natural and agricultural European flora. Wild
C. intybus covers a great portion of the entire European continent; it has traditionally become a part of the diet of local populations as an important ingredient of typical local dishes. This might be both the consequence and the cause of the great differentiation among a number of types, which have originated an ever-increasing number of cultivar groups, types, and populations that altogether comprise the horticultural landscape of the genus
Cichorium. This genus is particularly rich and interesting from historical, cultural, agronomical, commercial, and scientific points of view.
The commonly named “root chicory” appears to derive from so-called “Magdebourg chicory”, the ancient root chicory known and traditionally used in some European countries as a coffee substitute since the end of the 16th century and that surged to outstanding importance with the continental block at the time of Napoleon. Also, a very important leafy vegetable, so-called “Witloof Chicory” or “Belgian Endive”, perhaps the best known among leafy chicories, is considered to be a derivative of Magdebourg chicory. It is commonly accepted that the first well-known pale-yellowish sprouts of Witloof chicory were casually obtained by a Belgian farmer around the 1870s, who had observed and harvested the sprouts from a stock of roots piled up in autumn and left apart during the cold season, waiting to be dried, grounded, and toasted [
2].
Lacking comprehensive, homogeneous, sufficiently detailed, and univocal data regarding horticultural production and trade, it is impossible to provide reliable figures on the diffusion and economic importance of the crop in Europe, where chicory is mostly grown [
2]. The most recent statistics concerning the European market [
5] often include chicory under the general declaration “salads”, or it is considered together with lettuce, which is by far the most important leafy vegetable at both European and world-wide scales. The situation is not very different if one considers, as a source of reliable information, the statistics of each single country. Nonetheless, on the basis of accessible data, it is possible to determine that Italy is the almost exclusive producer of leaf chicory. Although this crop does not contribute greatly to the total agricultural income of each country, it is very important at the local level, as it characterizes the agriculture of limited areas, where from 80% to 90% of the country’s production is concentrated. This is indeed the case for Italy, where the Veneto region accounts for 66% of the national acreage and 59% of the national production of the particular type of red or variegated chicory known as “Radicchio”. The cultivation of other chicory types such as chicory of Catalogne in both France and Italy is much less concentrated and may extend far south. Furthermore, it is worth noting that chicory is not only important for the local economy, but it may also have significance at an international trade scale. Altogether, the US imports of chicory in 2011 [
5] equaled 175 Mt, with a value of $185,131,000. Approximately half of the amounts, both quantity and value, are represented by Witloof chicory, for which import from Belgium and the Netherlands reached more than 90% of the total. Thus, although still grown at a regional scale, chicory has a place among more known and used vegetables and may represent a non-negligible source of income for farmers in areas where it has traditionally been grown.
Within this framework, two observations can be made. The first concerns the marked decrease in US not-qualified chicory imports from Europe, in particular from Belgium, the Netherlands and Italy (2622 Mt in 1996, 536 Mt in 2002, 1.856 Mt in 2011), which corresponds with the increase in imports from Central and Southern America (1046 Mt in 1996, 2522 Mt in 2002, 149 Mt in 2011). Compared to the stable or slightly increasing figures recorded during the same period for Witloof chicory (between 2000 and 2400 Mt), this trend appears to indicate that Witloof has benefited from the quality and standardization of the marketable product. The second observation regards Radicchio, which is now considered with increasing attention both in Europe and in the US, as well as abroad, where its cultivation started some years ago and appears to have an improving evaluation, whereby the red or variegated leaves are particularly appreciated as a component of ready-to-eat salads.
In Italy, Radicchio of Chioggia is cultivated on a total area of approximately 16–18,000 ha, half of which is in the Veneto region, with a total production of approximately 270,000 tons (more than 60% obtained using professional seeds), reaching an overall turnover of approximately €10,000,000 per year [
6].
2. Taxonomy and Biology of Chicory
Chicory (
C. intybus L.) belongs to the family Asteraceae, a very large family with approximately 23,000 species subdivided into 1,535 genera grouped into three subfamilies: Barnadesioideae, Cichorioideae, and Asteroideae [
7].
The tribe Lactuceae, in the subfamily Cichorioideae, includes the genus
Cichorium, within which different species are recognized according to the origin. Referring to European flora, Tutin et al. [
8] describe the three species
C. spinosum,
C. intybus, and
C. endivia and subdivide the last into subsp.
endivia (cultivated) and subsp.
divaricatum (wild). Pignatti [
9], taking into account Italian flora, refers to the three wild species as
C. spinosum,
C. intybus, with the var.
glabratum (Presl) Fiori, and
C. pumilum, considering
C. endivia only as a cultivated species.
Integrating morphological characters with molecular observations, Kiers [
10] describes the two cultivated and most known species,
C. intybus and
C. endivia, and the two wild species,
C. spinosum and
C. pumilum. Moreover, two more species, never observed in Europe, are added,
C. calvum and
C. bottae: the first is endemic to the dry and hot environments of the Middle East and southwestern Asia and the second to Yemen and Saudi Arabia. More recently, Conti et al. [
11] recognized three species in the genus with regard to Italian flora:
C. endivia, with the two subspecies
endivia Hegi and
pumilum (Jacq) Cout.;
C. intybus, with the two subspecies
glabratum (C. Presl) Arcang. and
intybus; and C.
spinosum.
Since the early 1990s, when the analysis of DNA polymorphisms became more familiar to taxonomists, several studies attempted to explore, and possibly clarify, the relationships among the cultivated species,
C. intybus, and its wild relative. Using mitochondrial Restriction Fragment Length Polymorphism (RFLP) markers, Vermeulen et al. [
12] suggested that
C. spinosum could be considered an ecotype of
C. intybus rather than a separate species. Applying other and more informative molecular methods, such as nuclear Internal Transcribed Spacer (ITS) and Simple Sequence Repeat (SSR) markers, Gemeinholzer and Bachmann [
13] were unable to discriminate between these two species, which, nonetheless, could be clearly delimited with two diagnostic and one overlapping morphological character. On the basis of chloroplast DNA and nuclear rDNA sequence analysis [
14] or Amplified Fragment Length Polymorphism (AFLP) fingerprints [
15], it was confirmed that
C. intybus is closely related to
C. spinosum, whereas
C. endivia,
C. pumilum, and
C. calvum revealed a high genetic similarity with each other and are fairly well separated from
C. intybus and
C. spinosum. The sixth species,
C. bottae, must be considered a sister species.
In addition to morphological descriptors and molecular similarity or diversity estimates, a distinction among the above six Cichorium species can be made on the basis of their life cycle and reproductive system. Thus, two groups may be established: on one side, C. intybus, C. spinosum, and C. bottae, which are perennials and characterized by a strong self-incompatibility system, on the other, C. endivia, C. pumilum, and C. calvum, which are annual species and show self-compatibility. Although the names of the recognized botanical varieties do not appear within this framework, the various cultivated types have originated from these species.
The origin and differentiation of the genus is concordantly located in southeastern Europe, the eastern Mediterranean basin and southwest Asia. Within this large region, the area of origin of C. intybus tends to be mapped to the southern Balkan Peninsula and the northern Middle East. From there, it firstly migrated throughout the entire Mediterranean basin and toward southern and eastern Asia, where it appears to have found different areas of diffusion and adoption as horticultural crops.
Variously extensive lists of
Cichorium species, subspecies, botanical varieties, and cultivar groups have been published; such lists are present on accessible internet sites, where scientific and technical information is often confused with commercial promotion, forming a mass of information that is not always easily interpreted. The most complete list appears to be the Germplasm Resources Information Network (GRIN) database released by the United States Department of Agriculture (USDA) [
16], in which 212 entries of the genus
Cichorium and 127 of the species
C. intybus are cited. A brief description is given; in most cases, pictures of a not-well grown plant are present plus an actual short, though not always correct, botanical description (i.e., genus, species, and family). Many of the names are synonyms and often refer to differences among commercial types rather than to taxonomic distinctiveness. A smaller list is provided in Mansfeld’s World Database of Agricultural and Horticultural Crops [
17], which includes 49 entries but only 15 if we consider just the species
C. intybus.
On the basis of Kiers’ [
10] findings, Raulier et al. [
18] presented results of genetic diversity within
C. endivia and
C. intybus using SSR markers. The study showed that the two species are strongly differentiated, even though some
C. intybus individuals were genetically closer to
C. endivia, revealing complex genetic relationships between the two species. Regarding
C. intybus, the results confirmed its differentiation into three cultivar groups (i.e., Witloof, root chicory, and leaf chicory). The sub-classification of leaf chicory into Radicchio, Sugarloaf, and Catalogne previously based on morphological factors was also confirmed. Altogether, at least six cultivar groups, mainly differentiated on the basis of their use, are recognizable [
14,
15,
18,
19]. A summary is proposed in
Table 1, where correspondence among taxonomy, cultivar group, and most frequent and known utilization is attempted.
Although chicory is cultivated throughout Europe and tends to expand toward new horticultural areas, most of the cultivar groups are well known. These groups have been extensively adopted as horticultural crops at a local scale only, and as such, their description in the scientific and technical literature is partially complete or not entirely accurate. This is particularly true when the crop is largely differentiated, with subgroups among which it may be very difficult to identify differences and affinities. We are aware that diversity, particularly for this type of horticultural crop, is often the most efficient tool for commercial success, both for the seed producers and the farmers. Thus, in a continuously changing breeding world, it may be unwise to establish a rigid framework within which everything must be ordered. Nevertheless, it seems advisable to have an account, as complete as possible, of the plant materials of chicory.
Five main groups can be recognized within
C. intybus subspecies
intybus, to which all the cultivated types of chicory belong, as stated by Kiers [
10] and re-marked by Raulier et al. [
18]. Excepting wild accessions, the first referring to the var.
foliosum, traditionally includes Witloof chicory. It might be argued that if Witloof chicory was obtained from the roots of Magdebourg, as commonly accepted, then it should be grouped under var.
sativum, together with all the other root types. Nevertheless, most scientific literature refers to Witloof chicory as a type belonging to var.
foliosum [
2,
10,
18,
19,
20,
21,
22,
23]. The cultivar groups Pain de Sucre, Radicchio, and Catalogne are assigned to var.
porphyreum, var.
latifolium, and var.
sylvestre, respectively.
C. intybus (cultivated types) is a biennial species, while wild chicories are perennial. Although there are differences according to the cultivar group, early sowing or transplanting in spring under long days results in almost generalized flowering. Conversely, if sowing or transplanting are delayed until July and August, the plant forms a rather loose rosette or a fairly compact head that remains in the field until the following spring; at this time, between May and June, the central bud develops into a stem bearing inflorescences with clusters of blue flowers (rarely petals white or mauve).
Many clusters of two to four, rarely eight, sessile “flowers” are developed on the flowering stalk in axillary position or single inflorescences at the end of peduncles 4–7 cm long, rarely up to 13. The inflorescence (capitulum) is typical of the entire family and consists of a cluster of 15–25 single hermaphrodite flowers on a receptacle that is protected by an involucre. Each single flower has a gamopetalous and ligulate corolla and bears five filamentous stamens fused by their anthers to form a column surrounding a pistil with a bifid stigma.
At flowering, the style elongates, and the stigma is pushed up through the small channel composed of the anthers; the two halves of the stigma separate and assume a rather pronounced spiral form that may bring the inner receptive surface, completely free from pollen, in contact with the outer surface of the pistil, which, extruding from the staminal column, has remained densely covered with pollen grains. Thus, independently of the intervention of external agents, self-pollination is possible in addition to open-pollination. In general,
C. intybus is characterized by a strong sporophytic incompatibility system that usually inhibits self-fertilization and hence favorites cross-fertilization [
2,
10,
24,
25,
26,
27,
28]. Flowering occurs in the early part of the morning, mainly between 7:00 and 10:00 a.m., according to the air temperature and humidity. In the afternoon, after 1:00 p.m. the flowers wilt. The flowers can remain turgid and the pollen viable until the first hours of the afternoon only under exceptionally cold and humid conditions or during cloudy days.
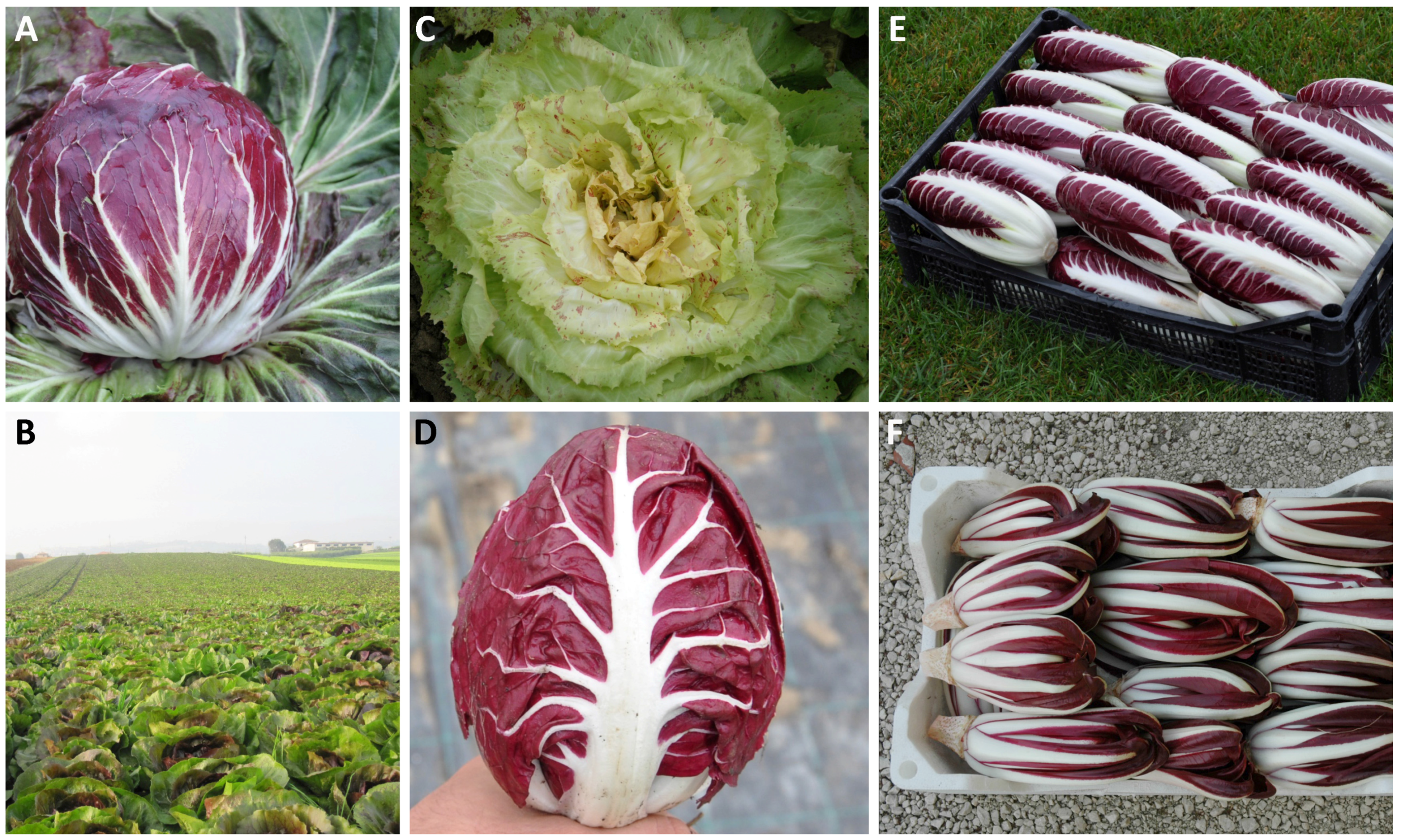
3. Italian Radicchio Biotypes
Here we only discuss Radicchio (i.e., var. latifolium). The Italian common name “Radicchio”, which has been adopted by all common languages, indicates a very differentiated group of chicories with red or variegated leaves that are traditionally cultivated in northeastern Italy.
Radicchio is considered a promising crop, as it is colorful; indeed, Radicchio is added to the color variety of fresh-cut mixed salads, for which there is an increasing demand by consumers [
29]. There is no documented history about the origin of colored leaf chicory in Italy. All red types of Radicchio currently cultivated appear to derive from red-leaved individuals first introduced in the 15th century. According to Bianchedi [
30], the cultivation of red chicory dates to the first half of the 16th century. It is clear that the original type should be identified with “Rosso di Treviso Tardivo (Late Red of Treviso)” which was long the only cultivated Radicchio in the Venetian territories. After spreading to nearby territories, the original type underwent accentuated selection according to very different criteria suggested by personal preference, but at least partially due to or dependent on the various environmental situations. Thus, after years of repeated selection and intercrossing by farmers between individuals inside the population tending to produce a heavy head with imbricated leaves, a new type called “Rosso di Treviso Precoce (Early Red of Treviso)” became popular. It is worth noting that the adjectives “late” and “early” do not refer to the cycle but instead indicate two different varietal types. As a matter of fact, there are early and late varieties (this characteristic is usually indicated in days from transplanting) of both types. Similarly, in the area of Verona, a small winter hardy type forming a rosette of deep-red colored leaves with a nice egg shape was initially selected from “Rosso di Treviso”; from this, the most recent populations of “Rosso di Verona” (Red of Verona) were obtained around 1960. Currently, both winter varieties and also very early ones belong to this type, which are also suitable for September harvesting and spring production. Later, possibly due to spontaneous or controlled crosses between red-leaved individuals and plants of
C. endivia, types with red spotted or variegated leaves were originated; these are currently known as “Variegato di Castelfranco” (Variegated of Castelfranco, a small medieval country in the province of Treviso). In the area of Chioggia, a traditional horticultural area established on the sandy soils extending southward from this small sea-side town just south of Venice, a variegated type able to form rather conical, firm, and tightly closed heads was generated most likely from the Variegated of Castelfranco; in the field, the new biotype was originally selected circa 1930. From this type, a large-leaved red type with an accentuated and white midrib and characteristic ball-shaped heads, known as “Rosso di Chioggia” (Red of Chioggia), was initially selected approximately twenty years later, and an almost completely light-yellowish type of very limited cultivation named “Bianco di Chioggia” (White of Chioggia) was obtained toward the end of the last century.
As a result, at least five main cultivated types are grown, named according to their province or town of origin, and may at present be distinguished within this cultivar group (
Figure 1).
The biotype “Red of Chioggia” is by far the most widely grown among the various types of Radicchio and it presents the highest within-type differentiation among cultivars able to guarantee production almost year-round. Indeed, Rosso di Chioggia has exhibited great adaptability to very different environmental situations worldwide, becoming the most grown type of Radicchio outside Italy and thus the most known at the international level [
2]. Independent of the sowing time, it grows in the open field. The great majority of farmers in the typical area of production still use seed from their own populations, which they maintain through yearly conservative selection and on-farm seed production. This seed is often sold through private transactions, outside the official seed market, both inside and outside the typical area. Commercial seed is mainly utilized outside the typical area of cultivation. The majority of commercial varieties are open-pollinated populations derived through selection from the original genetic pool. In recent years, both synthetic and hybrid varieties have been put on the market and have been favorably adopted largely for out-of-season production.
The biotype “Early Red of Treviso” is characterized as having upright long leaves with a large and thick midrib sustaining a rather expanded deep-red-colored lamina. During the vegetative period, the newly developed leaves do not expand in an open rosette as the plant grows but tighten to form closed and firm heads. In the Veneto region, it is sown or transplanted in the field from July to mid-August and harvested in September through December-January. At harvest, the outer green leaves and the major part of the taproot are removed to leave the inside red heart ready for market. Although it is one of the most recent selections, it is becoming increasingly known outside its initially limited area of production. Due to a cultivation technique very similar to the one used for “Red of Chioggia”, this type of Radicchio is beginning to show the same trend of expansion. As a consequence, the seed industry has been looking with increasing attention at the “Early Red of Treviso”. In addition to some open-pollinated commercial populations, some hybrid varieties have been put on the market. Because out-of-season cultivation has started to be adopted, an increasing demand for genetically improved material is foreseeable. At present, most farmers utilize their own populations derived from the original genetic pool and maintained through yearly mass selection. In recent years, synthetic varieties released by private firms and pre-commercial hybrid varieties have been released on the seed market, as occurred ten years prior for Rosso di Chioggia.
Among the cultivated biotypes, the “Late Red of Treviso” is the most ancient type of Radicchio grown in Italy and can be considered the legitimate ancestor of all others. It is a typical winter product, as it is sown or transplanted in the field from July to mid-August and may be harvested in October through February. The plant develops long, deep-green, essentially upright leaves that form a loose rosette; the midrib and lamina produce an always more accentuated reddish color as temperature decreases. At harvest, the entire plants are dug up, stored with all their leaves and roots intact, and maintained at low temperature, approximately 0 °C, as long as possible. According to the market’s request, plants are forced by placing them under a black cover, with their roots in running water, at 10–12 °C. After 10–18 days, the forcing period is concluded according to the air temperature; the plants are cleaned, whereby the outer leaves and a great portion of the tap root is eliminated, leaving a bunch of bright-red colored leaves with a white large midrib and a rather reduced lamina. This crop has at least some features in common with Witloof chicory, with which it shares leaf shape, growth habit, a large taproot, and a forcing process to obtain the commercial product. It is grown in a very restricted area and, together with “Variegated of Castelfranco”, “Early Red of Treviso Precoce”, “Red of Chioggia”, and “Red of Verona”, is one of the five Radicchio types recognized since the late 1990s with the Protected Geographical Indication (PGI) mark. Its peculiar aspect and rather superior culinary quality make this Italian Radicchio the most appreciated type. Its market price, particularly at Christmas, can reach as high as twice or three times the price of any other Radicchio. In spite of this, no named commercial variety is on the seed market except for selected open-pollinated populations. In fact, its cultivation is very limited, and the entire production procedure is rather complicated and much less standardized than that adopted for Witloof chicory. Therefore, unless it reaches a comparable degree of popularity, it seems unlikely that the seed industry would invest in this very unique crop. Almost all of the actual production thus relies on farmer populations, the history of which may extend for generations and which are maintained via yearly mass selection.
Concerning the “Red of Verona”, the first populations of this type of Radicchio were obtained approximately sixty years ago. With respect to previous populations, the actual populations comprise plants with much larger heads that, although on average smaller, may resemble those of “Red of Chioggia”. In comparison, the “Red of Verona” heads are more egg-shaped, formed by less expansive leaves with a brighter red lamina, and a large and thick midrib from which less evident and intersecting veins extend. The cultivation period of this type is reduced compared to that of “Red of Chioggia” and “Early Red of Treviso”. It is a typical winter crop, the popularity and cultivation area of which are both increasing in Italy, where it is extending to more southern regions, and outside the country. The reason for this expansion is much the same as for early “Red of Treviso”. Indeed, its cultivation can be standardized quite easily and there is no need for forcing. Moreover, due to its attractiveness, the product is well accepted by the market, and the consumer recognizes a better culinary quality in comparison to other Radicchio types. In spite of this increasing popularity, the seed market is rather poor, and available commercial varieties are selected open-pollinated populations. The first hybrid variety appears to be forthcoming. The most frequently used seed is, thus, from farmer populations selected during the last decades and maintained through mass selection. It is worth noting that in developing these populations, a procedure suggesting crosses of the initial small-leaved “Red of Verona” with the larger headed type “Red of Chioggia” may have been adopted. Regardless, growers appreciate the type that produces large heads called “Cologna” because it allows higher yields, whereas the market prefers the original small “Verona”.
Together with “Late Red of Treviso”, the “Variegated of Castelfranco” is the second most traditional type of Radicchio grown in northeastern Italy. Its morphological traits make it easily distinguishable from any other type. Directly sown or transplanted between July and the end of August, the plants develop a large rosette of more or less indented brilliant green leaves with a very extended red-spotted or variegated lamina sustained by a not-too-evident white midrib. At harvest, the external green leaves are removed, and the internal leaves open to form a bunch of creamy-yellowish red-spotted leaves that look very much like a flower. Indeed, this Radicchio is also known as “Rose of Castelfranco” and is one of the most appreciated components of fresh salads during the cold season. The selected populations grown at present are all self-blanching; thus, cultivation of “Variegated of Castelfranco” is comparable to that described for other types of Radicchio, with the exception of “Late Red of Treviso”, for which a final forcing process has long been used. The availability of commercial seed is much the same as for the majority of other Radicchios. Selected populations or synthetic varieties are available on the market, but the great proportion of the crop is planted using seed of farmer populations long selected and maintained through mass selection by each farmer.
4. Genetics and Breeding of Radicchio
Productive as well as qualitative traits are the main objectives in Radicchio breeding programs. The general and common goals of breeding new varieties mainly concern the following: (i) single plant size, weight, and yield; (ii) resistance to biotic (fungal diseases and insects) and abiotic stresses; (iii) adaptation to specific climatic or agronomic environments; (iv) uniformity of crop maturity, size, and growth; (v) good market acceptance as far as extrinsic (color, shape, uniformity) and intrinsic (taste and texture) traits.
There is 90 years of breeding history for C. intybus, dating to when the first varieties were bred and sold on the seed market. With regard to Radicchio, the main traits evaluated during selection programs are related to morpho-phenological, agronomic, and organoleptic characteristics. Important features are the time of cultivation, class of earliness, thickness and length of the main root, leaf shape and color, adaptation to local environments, disease resistance, and taste and bitterness of the edible parts.
Regardless, the performance of a cultivar is strictly dependent upon its genetic value, which in turn may be tightly linked to its genetic structure and thus to the strategy adopted for its constitution. Traditionally, varieties of Radicchio were developed by mass selection to obtain uniform populations characterized by valuable production and acceptable commercial head size and shape. Newly released varieties are mainly synthetics produced by intercrossing of a number of phenotypically superior plants selected on the basis of morpho-phenological and commercial traits. More rarely, plants are also evaluated genotypically by means of progeny tests. Synthetics have a large genetic base and are represented by a heterogeneous mixture of highly heterozygous genotypes sharing a common gene pool.
In recent years, methods for breeding F
1 hybrid varieties have been developed by private breeders and seed companies. However, details are not available in the current literature, and it may be presumed that each company has developed its own protocol, mainly in accordance with the genetic material it has at its disposal and the possibility of applying more or less efficient control at the F
1 hybrid seed production phase. In fact, the strong self-incompatibility system, which makes it difficult to obtain highly homozygous parents, has always been considered to be a great barrier for the production of inbred lines and parental lines for the development of hybrid varieties. Increased interest in the production of F
1 hybrid varieties has resulted from the discovery of spontaneous male-sterile mutants [
31,
32]. Male-sterility, or the inability of a plant to produce functional pollen, is important for the commercial production of hybrid seed via the crossing of parental inbred lines appropriately selected through progeny tests; such new commercial varieties can be considered to be true F
1 hybrids.
Chicory breeding materials of the Radicchio group are usually represented by local populations known to possess high variation and adaptation to the natural and anthropological environment where they originated and are still cultivated [
33]. These populations are maintained by farmers through phenotypic selection according to their own criteria. Controlled hybridization among different types is occasionally exploited to obtain recombinant genotypes showing superior agronomic and commercial traits or to avoid inbreeding depression caused by yearly intercrossing among selected individuals of the same population to improve uniformity. The ongoing breeding programs by local breeders and regional seed institutions aim at the following: (i) to isolate, within the best local selections, individuals amenable for use as parents for developing synthetic varieties, and although not easily feasible; (ii) to select inbred lines suitable for the production of commercial F
1 hybrids. These breeding procedures could be greatly assisted by the use of molecular markers that allow the discarding of molecular off-types to better exploit the parental genetic polymorphisms for synthetics and to identify the most genetically distant inbreds as parental lines for hybrids.
A scheme widely used for the constitution of synthetics in all Radicchio types includes the field selection of approximately 50–100 mother plants conforming to a prefixed breeder’s ideotype. These plants should share similar morpho-phenological traits, and productive and qualitative properties. Local varieties and landraces, and especially populations from repeated cycles of mass selection, represent excellent breeding material for the selection of the mother plants. In addition to morpho-phenological, agronomic, and commercial evaluation, characterization based on molecular markers could be undertaken to choose and retain plants of the original population that associate a high genetic uniformity with a superior phenotypic value. The roots of the selected mother plants should be preserved during the winter until the next spring, when they will be transplanted in the field under an isolation cage. The seed from plant intercrossing is the basic stock from which commercial seed lots can be obtained and sold to farmers. It is generally accepted that seed multiplication should no longer be performed in the cultivation areas of Radicchio. Traditional dedicated areas for seed production are the Apennine valleys of central Italy, which allow the production of high-quality seed (i.e., germinability and purity) due to the favorable climate.
The application of molecular markers to Radicchio breeding, as has been achieved with other crops for which they have been extensively and routinely utilized, may be of great help in overcoming theoretical as well as practical problems. Solutions to these problems can improve the efficiency of possible breeding methods and schemes.
Phylogeny, population distinctiveness, and their interrelationships as well as the genetic traceability of the commercial product are only a few examples of the most frequent uses. For instance, the possibility of discriminating the five different types of Radicchio grown in Veneto (Italy) was established on this basis [
33]. More refined applications concern the construction of genetic linkage maps, the location of specific loci for genetic factors affecting important qualitative traits and the possibility of quantifying their contribution to the total explained variation of quantitative characters. The mapping of useful genes should facilitate the identification of superior genotypes and minimize the manifestation of linkage drag effects in backcrossing strategies. Most importantly in Radicchio, the implementation of marker-assisted breeding programs based on mapped molecular markers could be very useful for the selection of parental genotypes of synthetic and F
1 hybrid varieties.
Molecular markers could also be useful to test other important varietal attributes in synthetics, such as mother plant relationships, heterozygosity evaluation and prediction, population uniformity and distinctiveness, and to assess the homozygosity and stability of inbred lines and diversity among inbred lines, in order to maximize heterosis in hybrid varieties. A cost- and time-efficient multilocus genotyping method based on a panel of mapped SSR markers is now available for Radicchio [
34]. Its application has utility for assessing the degree of homozygosity of parental inbred lines, as a measure of their genetic stability, and also for predicting the degree of heterozygosity of their F
1 hybrids, as an estimate of the specific combining ability on the basis of the genetic diversity between maternal and paternal inbred lines.
5. The Reproductive System of Chicory
In
C. intybus, the mating system is usually characterized by mechanisms that promote cross-fertilization and reduce or impede self-fertilization. In most cases, this is directly related to the manifestation of inbreeding depression and heterotic vigor as result of selfing and outcrossing, respectively [
28,
35,
36].
C. intybus is a self-incompatible species. Both auto-pollen and allo-pollen grains deriving, respectively, from the same genotype or different but related genotypes are recognized by cells of the pistil and rejected after pollination, either immediately on the stigma surface or later during pollen tube growth in the transmitting tissue of the style. Genes located at the S-locus encode the male (pollen) and female (pistil) recognition determinants of self-incompatibility (SI).
Over 60% of Asteraceae are estimated to be self-incompatible with sporophytic genetic determination [
37,
38]. The existence of an effective system of sporophytic self-incompatibility (SSI) in chicory was demonstrated in two different studies analyzing the progeny of two crosses between inbred lines of Witloof chicory [
24] and the progeny of the crosses between a wild-type chicory plant with a cultivated Radicchio plant of “Red of Chioggia” [
25]. In both studies, di-allelic crosses among the F
1 plants and F
1 backcrosses with both parents were applied to analyze the progeny for incompatibility reactions. The mean number of achenes per inflorescence was chosen as the criterion for evaluating the compatibility or incompatibility of a cross. The results indicated that a one-locus sporophytic self-incompatibility system is present in chicory, and the existence of dominance and co-dominance relationships between
S-alleles in the pollen and styles was noted. In
Brassica, it has been shown that dominant interactions between
S-haplotypes can act independently in pollen and stigma, thereby resulting in extremely complex patterns of incompatibility among individuals [
39]. This also appears to be the case in
Cichorium because only a difference in pollen and stigma allelic activity can explain the seed set observed in certain cross-combinations [
25]. Further studies [
2,
10,
27] confirmed the presence of a sporophytic self-incompatibility system in the chicory.
Indeed, different levels of self- and cross-incompatibility are found when wild and cultivated genotypes are crossed [
25,
40]. It has been suggested that the observed self-compatibility could be due to the presence of heterozygous genotypes bearing
S-alleles with stigma and pollen dominance relationships that are not linear and the presence of homozygous genotypes with weak
S-alleles [
41]. Moreover, recessive
S-haplotypes can reach high frequencies in chicory populations because their effects are masked by dominant
S-haplotypes. It should be underlined that in spite of the SSI mechanism, self-pollinated Radicchio plants very often yield one or a few fertile seeds per flower head, which means that considering the large number of flower heads produced by a single plant during its flowering period (8–10 weeks), the seed set can be significant and this makes possible the production of inbred lines.
Rejection of incompatible pollen on the chicory stigma is very rapid in the case of self-pollination because auto-pollen grains do not adhere to papilla cells. In incompatible crosses, papilla cells frequently permit the development of the pollen tube, which bursts and does not reach the transmitting tissue of the style. The rejection process in
Cichorium differs from that of
Brassica because the incompatibility response manifests within a few minutes due to the inhibition of pollen hydration, pollen germination, or pollen invasion of the stigma epidermis [
42].
Few data are available on the molecular mechanisms operating in chicory or in other species belonging to Asteraceae. In Radicchio, molecular investigations have sought to identify orthologs of
SRK and
SLG, the female components of the
Brassica S-locus. The
SRK-like genes in chicory were found to not be exclusively expressed in stigmas, thus, indicating that they are unlikely candidates for stigma
S-genes [
43]. Analyses of stigma proteins in
Senecio squalidus have revealed a family of polymorphic basic proteins associated with specific
S genotypes, though these proteins bear no resemblance to the
S-locus product of
Brassica spp. [
39,
44]. It will be useful to ascertain whether Asteraceae species possess their own system of self-incompatibility at the molecular level, as further information is needed for manipulating the SI system in chicory breeding.
High-density genetic maps including the loci responsible for sporophytic self-incompatibility (
S-locus) and involved in nuclear male sterility (
NMS-1 locus) have been published [
28]. This study was performed using a mapping population of 389 F
1 individuals obtained by crossing two plants, one male-sterile and one male-fertile, both heterozygous at the
S-locus, according to a method described by Eenink [
24] and Varotto et al. [
25]. Phenotyping of progeny plants for the male-sterility or -fertility trait allowed mapping of the
NMS1 locus to linkage group 5, and controlled diallelic and factorial crosses were utilized to identify self-compatible or -incompatible phenotypes for mapping the
S-locus to a 0.8 cm region on linkage group 2. A bulked segregant analysis was performed using the AFLP technology and a total of 2350 out of 31,000 markers were found to be polymorphic between parents and segregating in the progenies. Thirteen and six AFLP markers were found genetically linked to the
NMS1 locus and the
S-locus, respectively. Eight of these markers were converted into locus-specific Sequence Characterized Amplified Region (SCAR) diagnostic assays, among which five exhibited co-dominant polymorphisms [
28].
It is worth mentioning that the chromosomal blocks containing the genomic loci for male-sterility and self-incompatibility were both encompassing a region of less than 1 cm in length. In addition, Gonthier et al. [
28] mapped genes encoding proteins similar to
S-receptor kinase, the female determinant of sporophytic SI in Brassicaceae, and markers related to the putative
S-locus of sunflower, family of Compositae, but none of these genes or markers mapped close to the chicory
S-locus. Concerning male-sterility, neither candidate genes nor diagnostic markers are available for
Cichorium spp.
Male-sterility may have an important function for breeding F
1 hybrids of leaf chicory, particularly because the use of SI for the female line is inadequate for reliable F
1 hybrid seed production on a commercial scale. Male-sterility is another efficient mechanism for outcrossing in angiosperms and is represented by mutants that cannot produce pollen, fertile pollen grains, or functional anthers or gametes, though female fertility is normally preserved [
45,
46]. Two types of male-sterility can be observed in plants, which can be divided into nuclear (NMS) and cytoplasmic male-sterility (CMS) on the basis of inheritance patterns. Nuclear male-sterility is based solely on recessive mutations that affect different functions of nuclear genes, whereas the cytoplasmic type is maternally inherited and mainly due to mutations in mitochondrial genes [
47]. Moreover, male-fertility can be restored in CMS genotypes by nuclear-encoded fertility restorer (
Rf) genes. In fact, CMS has been used in several species to produce female-parental lines and has also been used for the production of hybrid seeds [
47]. In the absence of this system, male floral organs must be removed mechanically.
CMS is preferred by breeders over NMS owing to its modes of transmission to progeny and restoration of fertility [
47,
48]. Regardless, the presence of a naturally occurring CMS system has not been reported in chicory [
2], whereas two spontaneous NMS mutations have been discovered and exploited by breeders: one in root chicory [
49,
50] and the other in leaf chicory belonging to the biotype “Red of Chioggia” [
31,
32].
With regard to the patents by Barcaccia and Tiozzo [
31,
32], the inventors described the mutants at the cytological and genetic levels, affirming that the mutation itself affects a single nuclear gene, providing a recessive trait that causes male-sterility when homozygous. Moreover, the inventors documented that the mutation caused the microspores of each tetrad to arrest development at the uninucleate stage, degenerating before their release from the tetrads. At full flowering in the male-sterile genotypes, all the microspores of dehiscent anthers were found to be shapeless, shrunken, and much smaller than those of wild-type. The inventors demonstrated that pollen grains are never produced in mature anthers, supporting full expressivity of the trait, with mutants being 100% male-sterile. The gene
ms1 responsible for male-sterility was mapped at 5.8 cM upstream and 12.1 cM downstream of two SSR markers [
31,
32]. This information enabled the development of a first diagnostic assay useful to discriminate male-sterile and male-fertile plants of Radicchio with a low genotyping error. The gene
ms1 has been recently mapped on linkage group 4 [
51] into a chromosomal region spanning 8.5 cM (
Figure 2).
As reported by Cadalen et al. [
51], a MADS-box gene is present a few centiMorgans from the SSR marker loci mapped by Barcaccia and Tiozzo [
31,
32] on linkage group 4. Functional analyses by molecular genetic studies in model eudicots, such as
A. thaliana, have shown that transcription factors encoded by MADS-box class L2R2 genes are essential for regulating various aspects of flower development [
52,
53]. Further investigations on this gene were performed in chicory by Collani [
54] on the basis of the findings in rice [
55]. Both gene expression and segregation data have shown that this MADS-box candidate cannot be the genetic factor responsible for male-sterility in Radicchio.
Approaches to genetic engineering of male-sterility have been applied to Magdebourg, Witloof and Chioggia genotypes [
56]. Nevertheless, this technique is currently not in use, as the public opinion disapproves of the application of genetically modified organisms in food production. Moreover, the absence of joint European Union legislation makes the development of such projects not profitable for seed companies.
Another method pursued by different groups [
57,
58] is protoplast symmetric fusion between a breeding line of chicory and a CMS line of sunflower. This technique, which is based on somatic hybridizations, enabled the regeneration of interspecific hybrid plants characterized by the sum of the chromosomes of the two parental protoplast donor species. To transfer the male-sterile cytoplasm from an industrial chicory nuclear environment into a Witloof background, three different CMS cytoplasmic hybrids, or cybrids (i.e., cell lines produced by the fusion of a whole cell with an enucleated cell which contributes only with the cytoplast genome), originating from three different fusion events were characterized at the molecular level and then backcrossed to Witloof [
59]. Furthermore, protoplast asymmetric fusion (i.e., a technique used to obtain hybrids which are usually formatted with the full somatic complements of one parental species while nearly all of the chromosomes of the other parental species are lost), was used to produce male-sterile somatic hybrids between a Radicchio accession and a PET-1 sunflower CMS line [
60].
Other works in sunflower have focused on male-sterility and have led to the identification of several NMS genes as well as CMS and associated restored genes, some of which have been finely mapped [
61,
62,
63]. On the basis of these findings, Habarugira et al. [
64] reported the effects of the nuclear genome on anther development using a chicory CMS mutant. These researchers studied histo-morphological changes during anther development in fertile chicory plants from flower initiation to anthesis. Moreover, using normal development as a reference in fertile plants, alterations in the male-sterile mutant named “524” (i.e., a male-sterile cybrid obtained by protoplast fusion) and in different phenotypes obtained from crosses between this male-sterile plant and two different pollen donors were investigated [
64].
In conclusion, the information provided in this study on flower development will be useful for analyzing the expression of genes involved in the flower development, in general, and in the male sterility occurring in
C. intybus, in particular. This study also represents a starting point for the analysis of fertility restoration in the 524-CMS, which may contribute to the understanding of CMS in chicory [
64].
6. Seed Production in Chicory
Seed production represents the conclusive phase of breeding programs and, as with any other cultivated species, is of paramount importance for the success of the crop. In chicory, the seed is the only plant material used for the commercial propagation of varieties and planting, and it often determines the quantity of yield and the qualitative commercial standard of the crop. Despite this, little research has been conducted on chicory seed production. The fruit (seed) is an achene, obovoid to cylindrical, weakly ribbed, and light brown to completely brown when ripe. Almost all of the chicory commercial seed in Europe is produced in the Netherlands, northern France and Italy, usually in areas with climatic conditions that are sufficiently fair to permit abundant differentiation of flowers, a long flowering period, a good presence of pollinating insects, available water during the filling period, and dry conditions during the last part of the reproductive cycle when the seeds need to mature and dry to allow a high germination rate (i.e., >90%). In Italy, for instance, important seed production activity, which is also performed by Italian and European seed companies, is present in the eastern portion of the Po Valley (Veneto region), south of the Po River (Romagna region) and in the Marche region.
Allogamy is the preferred reproductive strategy in C. intybus. Different varieties, landraces or, more generally, populations have to be considered as interfertile, in other words, cross-pollination due to visitor insects and cross-fertilization are possible. To avoid this situation and any possible contamination, spatial isolation needs to be strictly respected in different fields (1500 to 2000 m) of the seed production areas. Seed producers must also take into account the presence of wild plants, which need to be removed from fallow fields and roadsides. The cultural practices applied to chicory seed crops during the vegetative phase are much the same as those used in growing these plants for the vegetable market, though a lower plant density (approximately five plants per square meter, rather than 7–9) is used. During the vegetative period, fields are repeatedly inspected to remove off-type or diseased plants.
As a biennial plant, Radicchio and, in general, all types of leaf chicories require vernalization to differentiate and produce a seed stalk. Thus, a seed crop needs to be sown in autumn to produce seed the next spring. If seed in the same year of sowing is desired, seeding operations need to occur in winter (end of January to beginning of February), ideally under protection, to let the small seed or transplanted plants be naturally vernalized in the field by the cold temperatures at this time of the year. Another option is to seed in autumn in greenhouses using Styrofoam cell trays and to store the seedlings in cold greenhouses until March. Vernalization will also be assured and flowering will hopefully occur at the same time. However, the genetic control of flower induction and differentiation in chicory has to be better understood. The transition to flowering was demonstrated to be mediated by the
MFL gene [
65], homologue of
AtFLC, the main repressor of flowering in
A. thaliana that is regulated by vernalization. A period of seven days of cold treatment at 4 °C induced
CiMFL full down-regulation under a long-day photoperiod, but not under a short-day photoperiod. This down-regulation was maintained after the cold treatment ended. Furthermore, together with a decrease in
CiMFL transcripts, cold conditions induced changes in the morphology of the shoot apical meristem. Long-day photoperiod itself was not able to induce flowering.
Flowering begins in May to June: the azure-blue flowers open early in the morning and, under optimal light and temperature conditions, anthesis is complete before 10 a.m. The flowers usually do not stay open after the morning, unless under conditions of moderate temperature, shadow, and high humidity. Seeds mature at 50 to 60 days after flowering and are collected in July to August. As the seed heads on the plant do not mature uniformly, particularly under conditions of high temperature and aridity, some shattering can occur when the seeds are collected. This phenomenon could be promoted by the birds that tend to feed on the mature flower heads. Nonetheless, the seeds need to be harvested at a physiological maturation stage because germination could be poor if they are not completely mature. In addition to its genetic value, the intrinsic and extrinsic properties of the seed depend upon the crop’s growing conditions as well as on harvest techniques; overall, these facets determine the commercial quality and field performance of the seed. Harvesting is preferably carried out early in the morning by cutting the seed stalks at their base just before the seeds have dried out completely; in fact, the seeds may fall off the stalk and be lost if they are allowed to fully dry on the plant while still in the field. When the seed stalks are dry, the seed is threshed using adequate equipment and care to allow for optimal genetic purity and physical properties. The seed yield depends on the plant density and architecture, averaging 10 to 15 g per plant, hence a total production of 0.5–0.7 t/ha can be expected. Depending on the cultivar, the 1.000 seed weight is 1.4 to 1.7 g (600 to 700 seeds per gram). Due to their angular shape, commercial chicory seeds are often coated and pelletized with various materials (e.g., cementite) to afford them with a more spherical shape. This facilitates the use of planting machines, pesticide application against seed and soil-borne pathogens, and priming processing to improve sowing performance.
Because leaf chicory F1 hybrids have entered the seed market, special attention should be given to the multiplication of parental lines and to F1 seed production. Well established in vitro techniques or less sophisticated in vivo procedures based on the ability of sliced roots to produce numerous clones may allow for the maintenance and adequate multiplication of chicory parental lines without any risk of genetic contamination.
Since
C. intybus is a biennial species, plants do not enter the reproductive stage without vernalization [
65,
66,
67]. This is why both Witloof and Radicchio, the commercial product of which is a head of tight leaves, are commonly grown as annual plants but must be sown directly in the field in late spring or in summer to produce a head between October and February; alternatively, as a response of the forcing procedure, planting can occur sometime during the winter months according to the producer’s choice. Regardless, anticipated or early flowering (bolting) caused by early spring sowing or late sowing and the sudden drop in temperature has to be avoided. This is because of the net loss in commercial production in addition to the shedding of unwanted seeds, resulting in weed chicory in the field of the following year. This situation poses two problems: (a) the need for non-bolting varieties able to render the crop at least partially independent of the temperature during the first stage of development, and (b) the need for technical procedures able to permit selection and seed production of the selected plants in the same growing season, early enough to proceed according to an annual cycle.
With regard to the first issue, breeding programs could help in the development of new varieties resistant to early flowering. One study on root chicory suggested an absolute vernalization requirement because bolting never occurred in control plants not subjected to low temperatures but maintained in long days in the field. However, because the effects of environmental parameters other than low temperature were not investigated, the same study could not definitively conclude that root chicory is an absolute cold-requiring plant [
68]. Thus, the exact requirements for floral initiation in terms of the duration and intensity of an effective vernalization treatment and the developmental stage during which seeds and/or plants are sensitive to cold treatments remain to be established. Some research suggests that an essential factor for chicory flowering is the post-vernalization requirement for long days [
69], but it is not known whether temperature is as determining as long days during this phase. It is quite evident that better knowledge of the genetics governing flower induction and vernalization requirements in chicory is fundamental for developing varieties with predictable flowering behavior.
In recent years, useful information on the genetic regulation of flowering time has been derived from the model plant
A. thaliana [
70]. Indeed, different classes of commercial earliness have been selected within each of the various types of Radicchio currently grown and, to a certain extent, they show correspondence with as many classes of earliness in flowering time. Furthermore, it is noteworthy that the timing and rhythm of selection cycles might be quite different if one refers to the field head-producing types of Radicchio or to the forced chicories such as Witloof and Radicchio Late Red of Treviso. For these two chicories, selection mainly occurs after forcing on the basis of observations made on the commercial product. The selected plants can be transplanted in pots and later, after a period of acclimation, directly in the field or under an isolation cage.
The second issue, thus, mainly relates to the other four types of Radicchio and what follows herein refers to the procedure generally applied in northeastern Italy, their typical area of production. Three aspects need to be considered: (a) the moment of selection; (b) the conservation of the taproot; and (c) the timing of seed stalk development and seed production. The moment of selection strictly depends on the earliness of the material. In any case, the selection procedure, mainly based on field observations carried out during autumn or winter months and that usually suggest the destruction of the head, must take into account the necessity of preserving the roots of the selected plants until the following spring, the time when they will be transplanted in the field to obtain seed. Once selection has been completed, the selected plants are dug up, and the leaves are removed with a sharp cut at the base of the head, paying close attention to preserve the upper portion of the root where axillary buds are present in great number. Once washed and treated with fungicide, the taproots are transplanted into pots and maintained under plastic tunnels where temperature should not reach below 0–2 °C. During storage, the roots can start to bud, and it may be necessary to thin out the young sprouts. The roots of early material, selected in September or October, are often cold stored, both before and/or after transplanting, to inhibit sprouting. At the beginning of January, the preserved roots are transplanted in a protected environment where, with the aim of accelerating the flowering process, the temperature should be raised to 18 °C and artificial light (150–250 lux at the vegetation level) should be added from 1–2 h before sunset until midnight. The plant density adopted is two plants per square meter, which should result in production of approximately 40 g of viable seed per plant. During this phase, periodic defoliation of the basal portion of the seed stalk might be necessary to facilitate air circulation, and careful disease and insect control should be carried out. As the seed stalk grows, the plants should be clipped to 100–120 cm to promote earliness and simultaneous flowering. At the opening of the first flowers, pollinating agents (bees, flies or, more recently, bumblebees, according to the available volume) must be introduced under the isolation cage while drastically reducing and attentively controlling the use of any chemical spraying. The flowering period may last two to three months, with a peak between the third and the sixth weeks. This means that, independently of any attempt to anticipate flowering, harvesting cannot occur later than the end of mid-June to enable the threshing of the plants and preparation of the seed in time for the mid-July to mid-August sowing.
7. Molecular Markers and Genomics of Chicory
The genetic relationships among the species and cultivar groups of
C. intybus were established by Kiers et al. [
10] using molecular markers and multivariate statistics. At the species level, the results correspond with previously obtained phylogenetic relationships, with
C. bottae as the most divergent species and
C. intybus and
C. spinosum as well as
C. endivia,
C. pumilum, and
C. calvum clustered into distinct subgroups. Based on the congruence between phylogenetic and genetic analyses, unique markers were expected for all species, except for
C. bottae. The analysis of
C. intybus materials resembled the species analysis in terms of sorting cultivars according to cultivar groups.
Chicory (
C. intybus, 2
n = 2
x = 18) is a strictly allogamous species being strongly hampered by an efficient incompatibility system, which prevents inbreeding and promotes outbreeding [
24,
26,
28].
Regarding their genetic structure, the original populations of C.
intybus could be considered as natural because independently of their historic background, the production of both Witloof and Radicchio has long relied on populations maintained by farmers for their own use. In these cases, very little selection, if any, might have been applied according to personal criteria. All these populations obtained by mass selection and maintained through the intercrossing of selected phenotypically superior mother parents can be considered as highly heterozygous and genetically heterogeneous, with behavior and level of adaptation to different environments and/or cultural conditions dependent on the frequency of favorable genes or gene combinations [
2]. As interest in edible products grew, the selection criteria of farmers became increasingly attentive to the consumer’s and market’s demands, and most of them elaborated their own ideotypes. This situation brought about a great deal of genetic and morphological differentiation that was entirely preserved until organized breeding programs were implemented, first by public institutions and in more recent times by private firms. As occurs for most open-pollinated species, detectable heterosis (i.e., hybrid vigor) effects are present in
C. intybus. Hybridization between selected genotypes provides uniform and heterotic progeny due to increased heterozygosity. Thus, the development of F
1 hybrid varieties is feasible. Except for the “Red of Chioggia” and “Early Red of Treviso” biotypes, for which both synthetic and hybrid varieties have been released onto the market by private firms, the vast majority of the Radicchio crop is still based on farmer’s populations. These populations are selected and maintained annually, and the seed is usually reutilized on the farm, but it may also be sold through private and non-officially registered transactions. Usually single populations are very well distinguishable among types, being very often also recognizable within a given type on the basis of morphological and physiological characters and agronomic performance. At the same time, they present an acceptable phenotypic uniformity among individuals. With respect to genetic variation estimated by applying molecular markers, a common observation true for both Radicchio and Witloof chicories is that the vast majority of the genetic variation can be explained at the within-population level, whereas only a minor portion is attributable to among-population differences [
15,
19,
33].
In 2015, two different groups published two independent works on the genetic characterization of leaf chicory breeding stocks based on data collected by exploring mapped SSR markers.
The data presented by Raulier et al. [
18] agree with previous studies. Utilizing 15 SSR markers, the researchers analyzed the genetic diversity of the current industrial chicory germplasm and obtained data concerning the relationships between and within
C. intybus L. and
C. endivia L. A total of 19 cultivated
C. endivia lines, along with 27 wild and 155 cultivated
C. intybus populations were analyzed, corresponding to 42 Witloof, 83 root chicory and 30 leaf chicory accessions (the latter included Radicchio, Sugarloaf and Catalogne materials). Moreover, 1297 samples derived from 15 modern root chicory cultivars were also processed. The authors reported that the
C. endivia and
C. intybus accessions could be clearly separated from each other. However, they also confirmed that seven wild
C. intybus individuals were genetically closer to the
C. endivia macro-cluster rather than the sub-group formed by the total of
C. intybus samples, highlighting the complex genetic interrelationships between these
Cichorium species. Furthermore, based on this analysis, the authors confirmed the existence of three cultivar groups of
C. intybus (i.e., Witloof, root, and leaf chicory) and the three subgroups of leaf chicory (i.e., Radicchio, Sugarloaf, and Catalogne). For industrial root chicory cultivars, evident differentiation was observed among Belgian, Polish and Austrian varieties, whereas no differentiation was detected among the French modern varieties. Overall, the researchers reported that most (i.e., 87%) of the genetic variation occurs within single cultivars, with 7% among cultivars of the same biotype and 6% among cultivars of different biotypes.
In the same year, Ghedina et al. [
34] published a method for genotyping elite breeding stocks of leaf chicory by assaying 27 mapped SSR markers, three per linkage group. As the plant accessions analyzed were inbred lines, the data were not comparable with the data of previous studies, in which open-pollinated varieties or wild accessions were taken into account. The authors demonstrated that most of the genetic differentiation occurs among populations, as expected, and that each population deriving from repeated selfing cycles can be considered as being genetically uniform and distinguishable from the others of the core collection of Radicchio breeding materials. Nevertheless, they also observed that inbreeding coefficients of single lines were low or negative, indicating that the observed heterozygosity is higher than expected. An interesting hypothesis is that maintenance of such levels of heterozygosity despite inbreeding reproductive strategies (i.e., selfing, full-sibling, and back-crossing) could be a consequence of the reproductive system of
C. intybus, which is naturally characterized by a high frequency of allogamy as a result of self-incompatibility [
34]. In addition, the authors also speculated that a fraction of the observed heterozygosity could be a consequence of phenotypic selection of morphologically superior individuals performed by breeders during inbreeding programs.
Because the local farmer’s seed still represents the starting materials for the development of new commercial varieties, it seems reasonable to state that, if preserved from extinction, these local varieties are an invaluable germplasm source on which leaf chicory breeding programs can rely for a long time. Modern varieties of “Red of Chioggia” and “Early Red of Treviso” are mainly synthetics and F
1 hybrids, the latter being bred and adopted with increasing frequency, as predicted by Lucchin et al. [
2] This is particularly true for those biotypes that can take an advantage of the uniformity of the marketed product, as this is often the key to customer’s appreciation.
Although chicory cannot be considered as a model species, some genetic studies using molecular markers have been conducted in order to characterize commercial varieties and experimental materials [
20,
33,
71,
72]; to evaluate the genetic homogeneity and purity of inbreds and hybrids, respectively [
34,
73]; to investigate phylogenetic relationships between cultivars and cultivar groups of
C. intybus and other species, both cultivated and wild, belonging to the same genus [
12,
15,
18,
19,
74]; and to assess the degree of spontaneous gene flow between landraces, cultivars, and wild populations of chicory. Moreover, molecular markers were also applied to understand whether co-existence between transgenic varieties and conventional varieties is possible [
75].
Molecular marker-based investigations have also been aimed at evaluating the genetic relationships among the five types of Radicchio and establishing a molecular reference system that would allow the precise identification of the different cultivated types. The five major cultivated types of Radicchio were investigated by PCR-derived markers [
33]. The experimental material was represented by two outbred populations (one of Variegated of Castelfranco and one of Red of Verona) and by eight partial inbred lines (three of Early Red of Treviso, three of Late Red of Treviso, and two of Red of Chioggia). The different types were well distinguished from one another when analyzed in bulk using AFLP markers at the population level. However, they were not well distinguished when analyzed at the individual level using RAPD (Random Amplified Polymorphic DNA), Inter-SSR (Inter-Simple Sequence Repeat), and AP-PCR (Arbitrarily Primed Polymerase Chain Reaction) markers. The genetic variation was shown to be much higher within types than between types. This result suggested that in each Radicchio type, populations produced by breeders through controlled intercrossing or repeated selfing have maintained good separation of gene pools over the years.
On the basis of the reproductive system of Radicchio, such a finding may be explained by taking the following into account: (i) the SSI system of the species, which -limits both selfing and intercrossing between plants with an identical phenotype at the multi-allelic
S-locus, thus allowing a certain amount of heterozygosity to be maintained even in inbred populations; (ii) the selection criteria of mother plants applied each year by each farmer to maintain his/her own population most likely results in limited contamination between types, thereby preserving the phenotypic identity of each type. At the same time, seed multiplication that has been carried out over the years has produced a clear genetic differentiation between types within the species [
33].
The establishment of a molecular reference system in Radicchio appears to be feasible and suitable both for the precise identification of single biotypes and for evaluating of the extent of the natural hybridization that can occur between different biotypes. Diagnostic molecular markers, along with morphological and phenological descriptors, will be useful for the certification of typical local products of Radicchio and for the recognition of a protected geographical indication (PGI) mark [
2].
In the last two decades, molecular markers have been exploited for the construction of linkage maps and for the identification of Mendelian (qualitative) genes and polygenes (or quantitative trait loci, QTLs). In particular, AFLP and RAPD markers were used to construct the two oldest genetic linkage maps of
C. intybus var.
latifolium reported in the literature [
21,
76]. More recently, Cadalen et al. [
51] used SSR and Sequence-Tagged Site (STS) markers to develop a consensus genetic map for this species containing 472 marker loci and covering 878 cM. This genetic map was integrated by Gonthier et al. [
28] for marker loci involved in nuclear male-sterility and sporophytic self-incompatibility using five SCAR markers showing co-dominant inheritance. An independent work conducted by Muys et al. [
77] enabled the implementation of a genetic map of industrial chicory (
C. intybus var.
sativum) that includes 237 marker loci attributable not only to AFLP and SSR markers, but also to Single Nucleotide Polymorphisms (SNPs), and covering 1208 cM.
Recently, Delporte et al. [
78] reported data on the selection and validation of reference genes for elucidating the phenylpropanoid pathway and for investigating gene expression patterns and models in chicory. This species accumulates four major polyphenols: chlorogenic, isochlorogenic, caftaric, and chicoric acids [
79]. These acids, caffeic esters, are well known for their antioxidant and antidiabetic properties [
80,
81,
82]. Although various biochemical pathways are involved in the synthesis of these secondary metabolites, each of them arises from the phenylpropanoid pathway. Eighteen candidate genes were chosen according to known
A. thaliana stable genes and reference genes commonly used in qRT-PCR studies. Orthologous chicory sequences were retrieved after performing BLASTX searches in a root chicory Expressed Sequence Tag (EST) database and specific primers were also designed and tested [
78].
An all-encompassing work on the transcriptome assemblies of Compositae crops and wild relatives was conducted by Hodgins et al. [
74], the main purpose of which was the development of genomic resources for 12 Compositae species, including some of the genus
Cichorium for which the whole seedlings were used as tissue source (
Table 2). In this work, both wild and cultivated accessions were represented for the two species of
Cichorium:
C. endivia and
C. intybus [
74]. The authors demonstrated a recent domestication for most species of the Compositae family and suggested that all
Cichorium taxonomic entities belong to the same biological species because reproductive barriers between crops and wild progenitors are weak or absent. Nevertheless, compared with self-compatible progenitors, self-incompatible progenitors exhibited greater genetic divergence from their corresponding crops. The exception was chicory, which showed the lowest genetic divergence among self-compatible species [
74]. The authors also observed evidence of introgression when comparing endive and chicory, a result that was replicated in all four comparisons of the wild and cultivated accessions. Putatively introgressed genes were found and the overrepresented biological processes for these genes were carbon fixation, ATP synthesis-coupled electron transport, and mitotic cell cycle spindle assembly checkpoint.
The first genome draft of leaf chicory was recently unveiled by Galla et al. [
6]. The plant material used for sequencing of the leaf chicory genome belongs to the Radicchio biotype Red of Chioggia, specifically the inbred line named “SEG111”. This accession was chosen as the most suitable genotype based on five common criteria [
6]. Briefly, among the Italian biotypes of leaf chicory, the Red of Chioggia is the most relevant one from a commercial point of view. Plus, the accession used is a male-fertile inbred line that is both phenotypically and genotypically well characterized. It is permanently conserved by in vitro culture to allow high availability of clonal materials, and it is distinguished by a high degree of homozygosity, more than 80% [
34]. Last but not least, it shows an excellent general combining ability, as demonstrated in trial experiments where this inbred line was used as seed parent in pairwise crosses with genetically distinct pollen donors for the development of F
1 hybrids.
Regarding methods, sequencing of the genomic DNA library was performed using Illumina HiSeq 2000 and MiSeq platforms to combine the high number of reads originating from the former with the longer sequences produced by the latter; the average coverage in a HiSeq run is approximately 21×. The estimated size of the assembled genome draft is 760 Mb. The authors reported to have obtained 58,392,530 and 389,385,400 raw reads using the MiSeq and HiSeq platforms, respectively. The two datasets were combined in a unique reference genome draft by assembling 724,009,424 nucleotides into 522,301 contigs. The contig length ranges from 200 bp to a maximum of 379,698 bp, with an average contig length of 1386 bp. As much as 68.9% of the recovered sequences are contained within a length spanning 200 nt to 999 nt. The interval length, ranging between 1000 nt and 2,999 nt, is represented by 19.7% of the assembled contigs, whereas the proportion of contigs with a length longer than or equal to 3,000 nt is 11.5%. The functional annotation of more than 18,000 unigenes was performed according to established computational biology protocols by taking advantage of publically available reference transcriptome data for
C. intybus [
6].
The authors presented the main preliminary findings on the genome organization and gene composition of Radicchio, and the potential of the newly annotated expressed sequences and diagnostic microsatellite markers in breeding programs were critically discussed. The main purpose of the sequencing and annotation of the first genome draft of Radicchio was to obtain a powerful tool for breeding programs, and overall statistics on the presence of SSR loci were presented. A total of 66,785 SSR-containing regions were identified. As many as 52,186 and 11,501 sequences were found to contain one or more microsatellites, respectively. The most common SSR elements were those showing a dinucleotide motif (89.0%), followed by trinucleotide (7.1%), and tetranucleotide (3.0%) ones; microsatellites revealing a pentanucleotide and hexanucleotide motif were less than 1.0% of the total.
The functional annotation of the assembled contigs was performed using a BLASTX approach according to which contig sequences were used to query different public protein databases. Data for functional annotations based on the most interesting Gene Ontology (GO) terms in breeding were presented, with biotic and abiotic stresses, hormonal responses, and flower and seed development taken into account. In addition, for each selected metabolic pathway, many microsatellite sequences putatively linked to important traits were presented according to their potential effect on plant characteristics. Based on the Kyoto Encyclopedia of Genes and Genomes database (KEGG) [
83], a total of 22,273 contigs enabled the mapping of 795 enzymes to 157 metabolic pathways that are interesting from a breeding point of view [
6].
In conclusion, the availability of the first genome sequence for this plant species can provide a powerful tool to be exploited for the identification of markers associated with or genes responsible for relevant agronomic traits and that influence crop productivity and product quality. As an example, the data and techniques from this research project will be capitalized on in subsequent years for the planning and development of basic studies as well as applied research on male-sterility and self-incompatibility in this species.
In the era of New Generation Sequencing (NGS) technologies, the availability of the first genome draft of C. intybus and the four leaf transcriptomes of species of the genus Cichorium will have an extraordinary impact from both scientific and economic points of view.
A Genotyping by Sequencing (GBS) approach could be adopted for the cloning of some key genetic factors useful for breeding programs, including Mendelian genes responsible of male-sterility, self-incompatibility, resistance to abiotic and biotic stresses, and also for the mapping of major QTLs related to plant cycle length, flowering time, shelf life, etc. Thus the reconstruction of the nine linkage groups of chicory with a very high number of EST, SNP, and other molecular markers will pave the way for the application of modern marker-assisted breeding strategies in this crop. Moreover, a comparison between leaf transcriptomes obtained from distinct accessions of the different biotypes of chicory could be useful to connect agronomic and morpho-phenological traits to specific differentially expressed genes, biosynthetic pathways, and metabolic networks. It is worth mentioning that of the guaianolides, a large group of the sesquiterpene lactones of chemotaxonomic, are of biological importance, being the compounds mainly responsible for the bitter taste in chicory.
8. Conclusions
In conclusion, it is worth adding some observations related to the significance that chicory has to horticulture within a European framework. The Witloof and Radicchio cultivar groups overall by far represent the largest quantity of chicory produced in Europe and elsewhere. The Radicchio group presents an almost astonishing phenotypic and genetic variability, from which selection is still able to generate new varieties and new commercially valuable forms. From a breeding point of view, Radicchio may represent a variable and rich germplasm resource with regard to useful morphological and/or physiological variants. Nevertheless, useful genes controlling some main quality traits or resistance to the most important pathogens are at very low frequency. In fact, farmer selection has traditionally paid attention to morphological characters, on which the market value mainly relies, whereas little attention has been given to other important characters, such as biotic or abiotic stress resistance, bitterness, and post-harvest rotting. In both cases, useful genes may be identified, isolated, and utilized through traditional breeding schemes or marker-assisted breeding methods through the adequate use of now-available molecular tools. From this point of view, the available numberless farmer populations of Radicchio represent an invaluable reserve of genetic resources that must be adequately studied, analyzed, classified, and compared to exploit them in breeding programs.
Indeed, C. endivia and C. intybus have the same number of chromosomes and are completely interfertile, and the same degree of sexual compatibility exists among different cultivars groups within each species and between these and the wild populations of both species. The complex endivia-intybus might thus be considered, from the breeder’s point of view, as a unique genetic pool, the access to which is not limited by sexual incompatibility. As opposed to the underlying possibility of transferring useful genes across species, cultivars groups, or cultivated and wild types through traditional methods (i.e., selection of superior genotypes from segregating populations), this observation, although trivial, may suggest the feasibility of breeding programs aimed at creating horticultural novelties that might open new commercial perspectives or enlarge the existing ones. This possibility needs to be entirely explored by possibly integrating traditional procedures based on test crosses with more sophisticated molecular approaches.