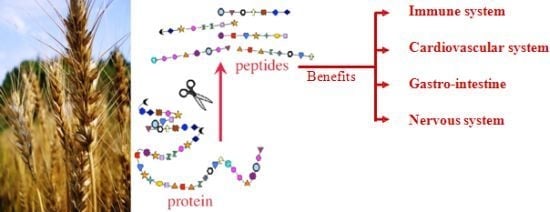
Protein Hydrolysates from Agricultural Crops—Bioactivity and Potential for Functional Food Development
Abstract
:1. Introduction

2. Preparation of Protein Hydrolysates
2.1. Protein Hydrolysis
2.2. Post-Hydrolysis Treatment
| Process | Function |
|---|---|
| Heat inactivation | Inactivation of proteolytic enzymes |
| Ultrafiltration | Removal of high molecular weight proteins and peptides |
| Use of specific enzymes | Reduce content of specific amino acids |
| Hydrolysis by exoproteases | Hydrolysis, reduction of bitterness |
| Activated carbon | Reduction of bitterness |
| Absorption chromatography | Reduce content of aromatic amino acids |
3. Bioactivity of Protein Hydrolysates
3.1. Antioxidant
3.2. Anti-hypertensive
3.3. Cardiovascular Disease
3.4. Exercise and Performance Enhancement
3.5. Other Clinical Applications
3.6. Further Uses
4. Techno-Functional Properties of Protein Hydrolysates
4.1. Solubility
4.2. Emulsifying Properties
4.3. Foaming
4.4. Gelation
5. Safety of Protein Hydrolysates
6. Conclusions
Acknowledgements
References
- Schaafsma, G. Safety of protein hydrolysates, fractions thereof and bioactive peptides in human nutrition. Eur. J. Clin. Nutr. 2009, 63, 1161–1168. [Google Scholar] [CrossRef]
- Grimble, G.; Keohane, P.; Higgins, B.; Kaminski, M., Jr.; Silk, D. Effect of peptide chain length on amino acid and nitrogen absorption from two lactalbumin hydrolysates in the normal human jejunum. Clin. Sci. 1986, 71, 65–69. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotech. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Frokjaer, S. Use of hydrolysates for protein supplementation. Food Technol. 1994, 48, 86–88. [Google Scholar]
- Nagodawithana, T.W.; Nelles, L.; Trivedi, N.B. Protein hydrolysates as hypoallergenic, flavors and pallatants for companion animals. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer Dordrecht Heidelberg: New York, NY, USA, 2010; pp. 191–207. [Google Scholar]
- Clemente, A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Tech. 2000, 11, 254–262. [Google Scholar] [CrossRef]
- Pasupuleti, V.K.; Holmes, C.; Demain, A.L. Applications of protein hydrolysates in biotechnology. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer Dordrecht Heidelberg: New York, NY, USA, 2010; pp. 1–9. [Google Scholar]
- Christians, N.E.; Garbutt, J.T.; Liu, D. Preemergence Weed Control Using Plant Protein Hydrolysate. U.S. patent 5,290,749, 1 March 1994. [Google Scholar]
- Ranganathan, Y.; Patel, S.; Pasupuleti, V.K.; Meganathan, R. Protein hydrolysates from non-bovine and plant sources replaces tryptone in microbiological media. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer Dordrecht Heidelberg: New York, NY, USA, 2010; pp. 115–125. [Google Scholar]
- Mandalari, G.; Faulds, C.B.; Sancho, A.I.; Saija, A.; Bisignano, G.; LoCurto, R.; Waldron, K.W. Fractionation and characterisation of arabinoxylans from brewers’ spent grain and wheat bran. J. Cereal Sci. 2005, 42, 205–212. [Google Scholar] [CrossRef]
- O’Toole, D.K. Characteristics and use of okara, the soybean residue from soy milk production a review. J. Agric. Food Chem. 1999, 47, 363–371. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic hydrolysis of brewers’ spent grain proteins and technofunctional properties of the resulting hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef]
- Lahl, W.J.; Grindstaff, D.A. Spices and seasonings: Hydrolyzed proteins. In Proceedings of the 6th SIFST Symposium on Food Ingredients—Applications, Status and Safety, Singapore Institute of Food Science and Technology, Singapore, 27–29 April 1989; pp. 51–65.
- Pasupuleti, V.K.; Braun, S. State of the art manufacturing of protein hydrolysates. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer Dordrecht Heidelberg: New York, NY, USA, 2010; pp. 11–32. [Google Scholar]
- Pedersen, B. Removing bitterness from protein hydrolysates. Food Technol. 1994, 48, 96–98. [Google Scholar]
- Yee, J.; Shipe, W.; Kinsella, J. Antioxidant effects of soy protein hydrolysates on copper catalyzed methyl linoleate oxidation. J. Food Sci. 1980, 45, 1082–1083. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y. Antioxidant activity of soy protein hydrolysates in a liposomal system. J. Food Sci. 2002, 67, 2952–2956. [Google Scholar] [CrossRef]
- Peña-Ramos, E.A.; Xiong, Y.L. Whey and soy protein hydrolysates inhibit lipid oxidation in cooked pork patties. Meat Sci. 2003, 64, 259–263. [Google Scholar]
- Fan, J.; Saito, M.; Yanyan, Z.; Szesze, T.; Wang, L.; Tatusmi, E.; Li, L. Gel-forming ability and radical-scavenging activity of soy protein hydrolysate treated with transglutaminase. J. Food Sci. 2005, 70, C87–C92. [Google Scholar]
- Moure, A.; Dominguez, H.; Parajo, J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process. Biochem. 2006, 41, 447–456. [Google Scholar]
- Xin, W.; LianZhou, J.; Xia, W.; RuiHong, T. Study on alkaline protease hydrolysis of soy protein isolate and its antioxidant activity. Dongbei Nongye Daxue Xuebao 2011, 42, 24–31. [Google Scholar]
- Roblet, C.; Amiot, J.; Lavigne, C.; Marette, A.; Lessard, M.; Jean, J.; Ramassamy, C.; Moresoli, C.; Bazinet, L. Screening of in vitro bioactivities of a soy protein hydrolysate separated by hollow fiber and spiral-wound ultrafiltration membranes. Food Res. Int. 2012, 46, 237–249. [Google Scholar] [CrossRef]
- Zhang, S.B.; Wang, Z.; Xu, S.Y. Antioxidant and antithrombotic activities of rapeseed peptides. J. Am. Oil Chem. Soc. 2008, 85, 521–527. [Google Scholar] [CrossRef]
- Xue, Z.; Yu, W.; Liu, Z.; Wu, M.; Kou, X.; Wang, J. Preparation and antioxidative properties of a rapeseed (Brassica napus) protein hydrolysate and three peptide fractions. J. Agric. Food Chem. 2009, 57, 5287–5293. [Google Scholar] [CrossRef]
- Pan, M.; Jiang, T.S.; Pan, J.L. Antioxidant activities of rapeseed protein hydrolysates. Food Bioprocess. Tech. 2011, 4, 1144–1152. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; Yust, M.M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Affinity purification of copper-chelating peptides from sunflower protein hydrolysates. J. Agric. Food Chem. 2007, 55, 6509–6514. [Google Scholar]
- Megías, C.; Pedroche, J.; Yust, M.M.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin. LWT Food Sci. Technol. 2008, 41, 1973–1977. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process. Biochem. 2006, 41, 1296–1302. [Google Scholar]
- Tang, C.H.; Peng, J.; Zhen, D.W.; Chen, Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009, 115, 672–678. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Connolly, A.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. In vitro antioxidant and anti-inflammatory effects of brewers’ spent grain protein rich isolate and its associated hydrolysates. Food Res. Int. 2013, 50, 205–212. [Google Scholar] [CrossRef]
- Yokomizo, A.; Takenaka, Y.; Takenaka, T. Antioxidative activity of peptides prepared from okara protein. Food Sci. Technol. Res. 2002, 8, 357–359. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, J.; Cheng, Y.; Li, L. Improvement of the antioxidant activity of Chinese traditional fermented okara (Meitauza) using Bacillus subtilis B2. Food Control. 2008, 19, 654–661. [Google Scholar] [CrossRef]
- World Health Organisation 2009, Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. 2009. Available online: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf (accessed on 2 July 2012).
- Matsui, T.; ChunHui, L.; Tanaka, T.; Maki, T.; Osajima, Y.; Matsumoto, K. Depressor effect of wheat germ hydrolysate and its novel angiotensin I-converting enzyme inhibitory peptide, Ile-Val-Tyr, and the metabolism in rat and human plasma. Biol. Pharmaceut. Bull. 2000, 23, 427–431. [Google Scholar] [CrossRef]
- Li, C.H.; Matsui, T.; Matsumoto, K.; Yamasaki, R.; Kawasaki, T. Latent production of angiotensin I-converting enzyme inhibitors from buckwheat protein. J. Pept. Sci. 2002, 8, 267–274. [Google Scholar] [CrossRef]
- Jia, J.; Ma, H.; Zhao, W.; Wang, Z.; Tian, W.; Luo, L.; He, R. The use of ultrasound for enzymatic preparation of ACE-inhibitory peptides from wheat germ protein. Food Chem. 2010, 119, 336–342. [Google Scholar] [CrossRef]
- Van der Ven, C.; Gruppen, H.; de Bont, D.; Voragen, A.G.J. Optimisation of the angiotensin converting enzyme inhibition by whey protein hydrolysates using response surface methodology. Int. Dairy J. 2002, 12, 813–820. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-Surface Methods in R, using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar]
- Wu, J.; Ding, X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 2002, 35, 367–375. [Google Scholar] [CrossRef]
- Chiang, W.D.; Tsou, M.J.; Tsai, Z.Y.; Tsai, T.C. Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor. Food Chem. 2006, 98, 725–732. [Google Scholar] [CrossRef]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef]
- Suh, H.; Whang, J.; Lee, H. A peptide from corn gluten hydrolysate that is inhibitory toward angiotensin I converting enzyme. Biotechnol. Lett. 1999, 21, 1055–1058. [Google Scholar] [CrossRef]
- Kim, J.; Whang, J.; Kim, K.; Koh, J.; Suh, H. Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process. Biochem. 2004, 39, 989–994. [Google Scholar]
- Yang, Y.; Marczak, E.D.; Yokoo, M.; Usui, H.; Yoshikawa, M. Isolation and antihypertensive effect of angiotensin I-converting enzyme (ACE) inhibitory peptides from spinach Rubisco. J. Agric. Food Chem. 2003, 51, 4897–4902. [Google Scholar] [CrossRef]
- Megías, C.; del Mar Yust, M.; Pedroche, J.; Lquari, H.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Purification of an ACE inhibitory peptide after hydrolysis of sunflower (Helianthus annuus L.) protein isolates. J. Agric. Food Chem. 2004, 52, 1928–1932. [Google Scholar]
- Megías, C.; Pedroche, J.; Yust, M.M.; Alaiz, M.; Girón-Calle, J.; Millán, F.; Vioque, J. Purification of angiotensin converting enzyme inhibitory peptides from sunflower protein hydrolysates by reverse-phase chromatography following affinity purification. LWT Food Sci. Technol. 2009, 42, 228–232. [Google Scholar] [CrossRef]
- Jamdar, S.; Rajalakshmi, V.; Pednekar, M.; Juan, F.; Yardi, V.; Sharma, A. Influence of degree of hydrolysis on functional properties, antioxidant activity and ACE inhibitory activity of peanut protein hydrolysate. Food Chem. 2010, 121, 178–184. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wada, Y.; Schott, M.; Wäsche, A. Functional and bioactive properties of rapeseed protein concentrates and sensory analysis of food application with rapeseed protein concentrates. LWT Food Sci. Technol. 2006, 39, 503–512. [Google Scholar] [CrossRef]
- Hull, J.N.; Kannan, A.; Hettiarachchy, N.S. Antioxidant and antihypertensive activities of rice bran peptides. Discovery Student J. Dale Bumpers College Agric. Food Life Sci. 2011, 11, 52–57. [Google Scholar]
- Chen, Z.Y.; Peng, C.; Jiao, R.; Wong, Y.M.; Yang, N.; Huang, Y. Anti-hypertensive nutraceuticals and functional foods. J. Agric. Food Chem. 2009, 57, 4485–4499. [Google Scholar] [CrossRef]
- Ringseis, R.; Matthes, B.; Lehmann, V.; Becker, K.; Schöps, R.; Ulbrich-Hofmann, R.; Eder, K. Peptides and hydrolysates from casein and soy protein modulate the release of vasoactive substances from human aortic endothelial cells. BBA Gen. Subj. 2005, 1721, 89–97. [Google Scholar] [CrossRef]
- World Health Organisation. 2011. Available online: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed on 2 July 2012).
- Hilleboe, H.E. Some epidemiologic aspects of coronary artery disease. J. Chronic Dis. 1957, 6, 210–228. [Google Scholar] [CrossRef]
- Terpstra, A.H.; Hermus, R.J.; West, C.E. The role of dietary protein in cholesterol metabolism. World Rev. Nutr. Diet. 1983, 42, 1–55. [Google Scholar]
- Clifton, P.M. Protein and coronary heart disease: The role of different protein sources. Curr. Atheroscler. Rep. 2011, 13, 493–498. [Google Scholar] [CrossRef]
- Manson, J.E.; Tosteson, H.; Ridker, P.M.; Satterfield, S.; Hebert, P.; O’Connor, G.T.; Buring, J.E.; Hennekens, C.H. The primary prevention of myocardial infarction. N. Engl. J. Med. 1992, 326, 1406–1416. [Google Scholar]
- Ignatowsky, M.A. Influence de la nourriture animale sur l’organisme des lapins. Arch. Med. Exp. Anat. Pathol. 1908, 20, 1–20. [Google Scholar]
- Velasquez, M.T.; Bhathena, S.J. Role of dietary soy protein in obesity. Int. J. Med. Sci. 2007, 4, 72–82. [Google Scholar] [CrossRef]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-analysis of the effects of soy protein intake on serum lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar]
- Aoyama, T.; Fukui, K.; Takamatsu, K.; Hashimoto, Y.; Yamamoto, T. Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice (yellow KK). Nutrition 2000, 16, 349–354. [Google Scholar] [CrossRef]
- Nagaoka, S.; Miwa, K.; Eto, M.; Kuzuya, Y.; Hori, G.; Yamamoto, K. Soy protein peptic hydrolysate with bound phospholipids decreases micellar solubility and cholesterol absorption in rats and Caco-2 cells. J. Nutr. 1999, 129, 1725–1730. [Google Scholar]
- Tsou, M.J.; Kao, F.J.; Tseng, C.K.; Chiang, W.D. Enhancing the anti-adipogenic activity of soy protein by limited hydrolysis with Flavourzyme and ultrafiltration. Food Chem. 2010, 122, 243–248. [Google Scholar] [CrossRef]
- Park, J.H.; Park, M.N.; Lee, I.S.; Kim, Y.K.; Kim, W.S.; Lee, Y.S. Effects of soy protein, its hydrolysate and peptide fraction on lipid metabolism and appetite-related hormones in rats. Korean J. Nutr. 2010, 43, 342–350. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; del Mar Yust, M.; Alaiz, M.; Girón-Calle, J.; Millán, F.; Vioque, J. Sunflower protein hydrolysates reduce cholesterol micellar solubility. Plant. Food Hum. Nutr. 2009, 64, 86–93. [Google Scholar] [CrossRef]
- Revilla, E.; Maria, C.S.; Miramontes, E.; Bautista, J.; García-Martínez, A.; Cremades, O.; Cert, R.; Parrado, J. Nutraceutical composition, antioxidant activity and hypocholesterolemic effect of a water-soluble enzymatic extract from rice bran. Food Res. Int. 2009, 42, 387–393. [Google Scholar] [CrossRef]
- Bergström, J.; Hultman, E. Muscle glycogen synthesis after exercise: An enhancing factor localized to the muscle cells in man. Nature 1966, 210, 309–310. [Google Scholar] [CrossRef]
- Bergström, J.; Hultman, E. A study of the glycogen metabolism during exercise in man. Scand. J. Clin. Lab. Inv. 1967, 19, 218–228. [Google Scholar] [CrossRef]
- Bergström, J.; Hermansen, L.; Hultman, E.; Saltin, B. Diet, muscle glycogen and physical performance. Acta. Physiol. Scand. 1967, 71, 140–150. [Google Scholar] [CrossRef]
- Van Loon, L.J.C.; Saris, W.H.M.; Kruijshoop, M.; Wagenmakers, A.J.M. Maximizing postexercise muscle glycogen synthesis: Carbohydrate supplementation and the application of amino acid or protein hydrolysate mixtures. Am. J. Clin. Nutr. 2000, 72, 106–111. [Google Scholar]
- Koikawa, N.; Nakamura, A.; Ngaoka, I.; Aoki, K.; Sawaki, K.; Suzuki, Y. Delayed-onset muscle injury and its modification by wheat gluten hydrolysate. Nutrition 2009, 25, 493–498. [Google Scholar] [CrossRef]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; MacLean, D.A. Plasma glucagon and insulin responses depend on the rate of appearance of amino acids after ingestion of different protein solutions in humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar]
- Di Pasquale, M.G. Amino Acids and Proteins for the Athlete: The Anabolic Edge; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Van Loon, L.J.C.; Saris, W.H.M.; Verhagen, H.; Wagenmakers, A.J.M. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef]
- Berry, H.; Sutherland, B.; Hunt, M.; Fogelson, M.; O’Grady, D. Treatment of children with phenylketonuria using a phenylalanine-free protein hydrolysate (Albumaid XP). Am. J. Clin. Nutr. 1976, 29, 351–357. [Google Scholar]
- Acosta, P.B.; Yannicelli, S.; Marriage, B.; Mantia, C.; Gaffield, B.; Porterfield, M.; Hunt, M.; McMaster, N.; Bernstein, L.; Parton, P. Nutrient intake and growth of infants with phenylketonuria undergoing therapy. J. Pediatr. Gastr. Nutr. 1998, 27, 287–291. [Google Scholar] [CrossRef]
- Bickel, H.; Gerrard, J.; Hickmans, E.M. The influence of phenylalanine intake on the chemistry and behaviour of a phenylketonuria child. Acta. Paediatr. 1954, 43, 64–77. [Google Scholar] [CrossRef]
- Delvivo, F.M.; Vieira, C.R.; Biasutti, E.A.R.; Capobiango, M.; Silva, V.D.M.; Afonso, W.O.; Silvestre, M.P.C. Effect of adsorption medium, hydrolytic parameters and ultrafiltration on the phenylalanine removal from pancreatic whey hydrolysates. Am. J. Food Technol. 2006, 1, 94–104. [Google Scholar] [CrossRef]
- Yamashita, M.; Arai, S.; Fujimaki, M. A low-phenylalanine high-tyrosine plastein as an acceptable dietetic food. Method of preparation by use of enzymatic protein hydrolysis and resynthesis. J. Food Sci. 1976, 41, 1029–1032. [Google Scholar] [CrossRef]
- Morgan, M.Y.; Marshall, A.; Milsom, J.P.; Sherlock, S. Plasma amino-acid patterns in liver disease. Gut 1982, 23, 362–370. [Google Scholar] [CrossRef]
- Schenker, S.; Beer, W.H. Nutrients in the pathogenesis and treatment of hepatic encephalopathy. In Nutrition and the Origins of Disease; Halsted, C.H., Rucker, B.B., Eds.; Academic Press: London, UK, 1989; pp. 285–307. [Google Scholar]
- Bautista, J.; Hernandez-Pinzon, I.; Alaiz, M.; Parrado, J.; Millan, F. Low molecular weight sunflower protein hydrolysate with low concentration in aromatic amino acids. J. Agric. Food Chem. 1996, 44, 967–971. [Google Scholar] [CrossRef]
- Bautista, J.; Corpas, R.; Cremades, O.; Hernández-Pinzón, I.; Ramos, R.; Villanueva, A.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Vioque, J. Sunflower protein hydrolysates for dietary treatment of patients with liver failure. J. Am. Oil Chem. Soc. 2000, 77, 121–126. [Google Scholar]
- Siemensma, A.; Babcock, J.; Wilcox, C.; Huttinga, H. Towards an understanding of how protein hydrolysates stimulate more efficient biosynthesis in cultured cells. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer Dordrecht Heidelberg: New York, NY, USA, 2010; pp. 33–54. [Google Scholar]
- Christians, N.; Liu, D.; Unrah, J.B. The use of protein hydrolysates for weed control. In Protein Hydrolysates in Biotechnology; Pasupuleti, V.K., Demain, A.L., Eds.; Springer Dordrecht Heidelberg: New York, NY, USA, 2010; pp. 127–133. [Google Scholar]
- Zayas, J.F. Functionality of Proteins in Foods; Springer-Verlag: Berlin, Germany, 1997; p. 6. [Google Scholar]
- Kinsella, J.E.; Melachouris, N. Functional properties of proteins in foods: A survey. Crit. Rev. Food Sci. 1976, 7, 219–280. [Google Scholar]
- Vojdani, F. Solubility. In Methods of Testing Protein Functionality; Hall, G.M., Ed.; Blackie Academic & Professional: London, UK, 1996; pp. 11–60. [Google Scholar]
- Adler-Nissen, J. Enzymatic Hydrolysis of Food Proteins; Elsevier Applied Science Publishers: London, UK, 1986; pp. 122–124. [Google Scholar]
- Chobert, J.M.; Bertrand-Harb, C.; Nicolas, M.G. Solubility and emulsifying properties of caseins and whey proteins modified enzymically by trypsin. J. Agric. Food Chem. 1988, 36, 883–892. [Google Scholar]
- Yalcin, E.; Celik, S. Solubility properties of barley flour, protein isolates and hydrolysates. Food Chem. 2007, 104, 1641–1647. [Google Scholar] [CrossRef]
- Claver, I.P.; Zhou, H. Enzymatic hydrolysis of defatted wheat germ by proteases and the effect on the functional properties of resulting protein hydrolysates. J. Food Biochem. 2005, 29, 13–26. [Google Scholar]
- Vioque, J.; Sánchez-Vioque, R.; Clemente, A.; Pedroche, J.; Millán, F. Partially hydrolyzed rapeseed protein isolates with improved functional properties. J. Am. Oil Chem. Soc. 2000, 77, 447–450. [Google Scholar] [CrossRef]
- Yoshie-Stark, Y.; Wada, Y.; Wäsche, A. Chemical composition, functional properties, and bioactivities of rapeseed protein isolates. Food Chem. 2008, 107, 32–39. [Google Scholar] [CrossRef]
- Chiang, W.D.; Shih, C.J.; Chu, Y.H. Functional properties of soy protein hydrolysate produced from a continuous membrane reactor system. Food Chem. 1999, 65, 189–194. [Google Scholar] [CrossRef]
- Tsumura, K.; Saito, T.; Tsuge, K.; Ashida, H.; Kugimiya, W.; Inouye, K. Functional properties of soy protein hydrolysates obtained by selective proteolysis. LWT Food Sci. Technol. 2005, 38, 255–261. [Google Scholar]
- Mahmoud, M.I. Physicochemical and functional properties of protein hydrolysates in nutritional products. J. Food Sci. 1994, 59, 89–95. [Google Scholar]
- Chan, W.M.; Ma, C.Y. Acid modification of proteins from soymilk residue (okara). Food Res. Int. 1999, 32, 119–127. [Google Scholar] [CrossRef]
- Barca, A.; Ruiz-Salazar, R.; Jara-Marini, M. Enzymatic hydrolysis and synthesis of soy protein to improve its amino acid composition and functional properties. J. Food Sci. 2000, 65, 246–253. [Google Scholar]
- Achouri, A.; Zhang, W.; Shiying, X. Enzymatic hydrolysis of soy protein isolate and effect of succinylation on the functional properties of resulting protein hydrolysates. Food Res. Int. 1999, 31, 617–623. [Google Scholar] [CrossRef]
- Kong, X.; Zhou, H.; Qian, H. Enzymatic preparation and functional properties of wheat gluten hydrolysates. Food Chem. 2007, 101, 615–620. [Google Scholar] [CrossRef]
- German, J.B.; O’Neill, T.E.; Kinsella, J.E. Film forming and foaming behavior of food proteins. J. Am. Oil Chem. Soc. 1985, 62, 1358–1366. [Google Scholar]
- Zhang, H.J.; Zhang, H.; Wang, L.; Guo, X.N. Preparation and functional properties of rice bran proteins from heat-stabilized defatted rice bran. Food Res. Int. 2010, 47, 359–363. [Google Scholar] [CrossRef]
- Yalcın, E.; Celik, S.; Ibanoglu, E. Foaming properties of barley protein isolates and hydrolysates. Eur. Food Res. Technol. 2008, 226, 967–974. [Google Scholar]
- Harper, W.; Boer, R.; Jelen, P.; Puhan, Z. Functional properties of whey protein concentrates and their relationship to ultrafiltration. In New Applications of Membrane Processes, International Dairy Federation Special Issue 9201; International Dairy Federation: Brussels, Belgium, 1992; pp. 77–108. [Google Scholar]
- Babiker, E.E. Effect of transglutaminase treatment on the functional properties of native and chymotrypsin-digested soy protein. Food Chem. 2000, 70, 139–145. [Google Scholar] [CrossRef]
- Sakamoto, H.; Kumazawa, Y.; Toiguchi, S.; Seguro, K.; Soeda, T.; Motoki, M. Gel strength enhancement by addition of microbial transglutaminase during onshore surimi manufacture. J. Food Sci. 2006, 60, 300–304. [Google Scholar]
- Fan, J.; Saito, M.; Yanyan, Z.; Szesze, T.; Wang, L.; Tatusmi, E.; Li, L. Gel-forming ability and radical-scavenging activity of soy protein hydrolysate treated with transglutaminase. J. Food Sci. 2005, 70, C87–C92. [Google Scholar]
- Sanchez, A.C.; Burgos, J. Factors affecting the gelation properties of hydrolyzed sunflower proteins. J. Food Sci. 1997, 62, 284–288. [Google Scholar] [CrossRef]
- Léger, L.W.; Arntfield, S.D. Thermal gelation of the 12S canola globulin. J. Am. Oil Chem. Soc. 1993, 70, 853–861. [Google Scholar] [CrossRef]
- Uruakpa, F.O.; Arntfield, S. Emulsifying characteristics of commercial canola protein-hydrocolloid systems. Food Res. Int. 2005, 38, 659–672. [Google Scholar] [CrossRef]
- Food and Drug Administration. Available online: http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/default.html (accessed on 17 August 2012).
- Commission of the European Communities. Available online: http://ec.europa.eu/food/food/biotechnology/novelfood/index_en.html (accessed on 17 August 2012).
- Halken, S.; Host, A.; Hansen, L.G.; Østerballe, O. Safety of a new, ultra filtrated whey hydrolysate formula in children with cow milk allergy: A clinical investigation. Pediatr. Allergy Immun. 1993, 4, 53–59. [Google Scholar] [CrossRef]
- Szajewska, H.; Albrecht, P.; Stoinska, B.; Prochowska, A.; Gawecka, A.; Laskowska-Klita, T. Extensive and partial protein hydrolysate preterm formulas: The effect on growth rate, protein metabolism indices, and plasma amino acid concentrations. J. Pediatr. Gastr. Nutr. 2001, 32, 303–309. [Google Scholar] [CrossRef]
- Decsi, T.; Veitl, V.; Szász, M.; Pinter, Z.; Mehes, K. Plasma amino acid concentrations in healthy, full-term infants fed hydrolysate infant formula. J. Pediatr. Gastr. Nutr. 1996, 22, 62–67. [Google Scholar] [CrossRef]
- Lynch, B.; Simon, R.; van Otterdijk, F.; Emmen, H.; Giuseppin, M.; Kemme-Kroonsberg, C. Subchronic toxicity evaluation of potato protein isolates. Food Chem. Toxicol. 2012, 50, 373–384. [Google Scholar]
- Mejia, L.A.; Korgaonkar, C.K.; Schweizer, M.; Chengelis, C.; Marit, G.; Ziemer, E.; Grabiel, R.; Empie, M. A 13-week sub-chronic dietary toxicity study of a cruciferin-rich canola protein isolate in rats. Food Chem. Toxicol. 2009, 47, 2645–2654. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Moure, A.; Domínguez, H.; Parajó, J.C. Fractionation and enzymatic hydrolysis of soluble protein present in waste liquors from soy processing. J. Agric. Food Chem. 2005, 53, 7600–7608. [Google Scholar]
- Hartmann, R.; Wal, J.M.; Bernard, H.; Pentzien, A.K. Cytotoxic and allergenic potential of bioactive proteins and peptides. Curr. Pharm. Design 2007, 13, 897–920. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McCarthy, A.L.; O'Callaghan, Y.C.; O'Brien, N.M. Protein Hydrolysates from Agricultural Crops—Bioactivity and Potential for Functional Food Development. Agriculture 2013, 3, 112-130. https://doi.org/10.3390/agriculture3010112
McCarthy AL, O'Callaghan YC, O'Brien NM. Protein Hydrolysates from Agricultural Crops—Bioactivity and Potential for Functional Food Development. Agriculture. 2013; 3(1):112-130. https://doi.org/10.3390/agriculture3010112
Chicago/Turabian StyleMcCarthy, Aoife L., Yvonne C. O'Callaghan, and Nora M. O'Brien. 2013. "Protein Hydrolysates from Agricultural Crops—Bioactivity and Potential for Functional Food Development" Agriculture 3, no. 1: 112-130. https://doi.org/10.3390/agriculture3010112