The Efficacy of 5-Aminolevulinic Acid-Producing Luteovulum sphaeroides Strains on Saline Soil Fertility, Nutrient Uptakes, and Yield of Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Bacteria Source
2.3. Fertilizers
2.4. Soil Preparation
2.5. Experimental Design
2.6. Saline Watering
2.7. Bacterial Inoculation to Rice Seeds
2.8. Plant Biochemical Analysis
2.9. Growth Measurement
2.10. Analysis of Yield Components and Yield
2.11. Soil Analysis
2.12. Analysis of Nutrients Concentration in Straw and Seeds
2.13. Statistical Analysis
3. Results
3.1. The Impact of the Water Salinity and the Supplementation of Luteovulum sphaeroides on the Soil Chemical Characteristics
3.2. The Impact of the Water Salinity and Supplementation of Luteovulum sphaeroides on Proline Concentration
3.3. The Impact of the Water Salinity and the Supplementation of Luteovulum sphaeroides on the Dry Biomass and the Concentration, Uptake, and Total Uptake of N, P, K, and Na in Seeds and Straw
3.3.1. The Dry Biomass and the Concentration of N, P, K, and Na in Seeds and Straws
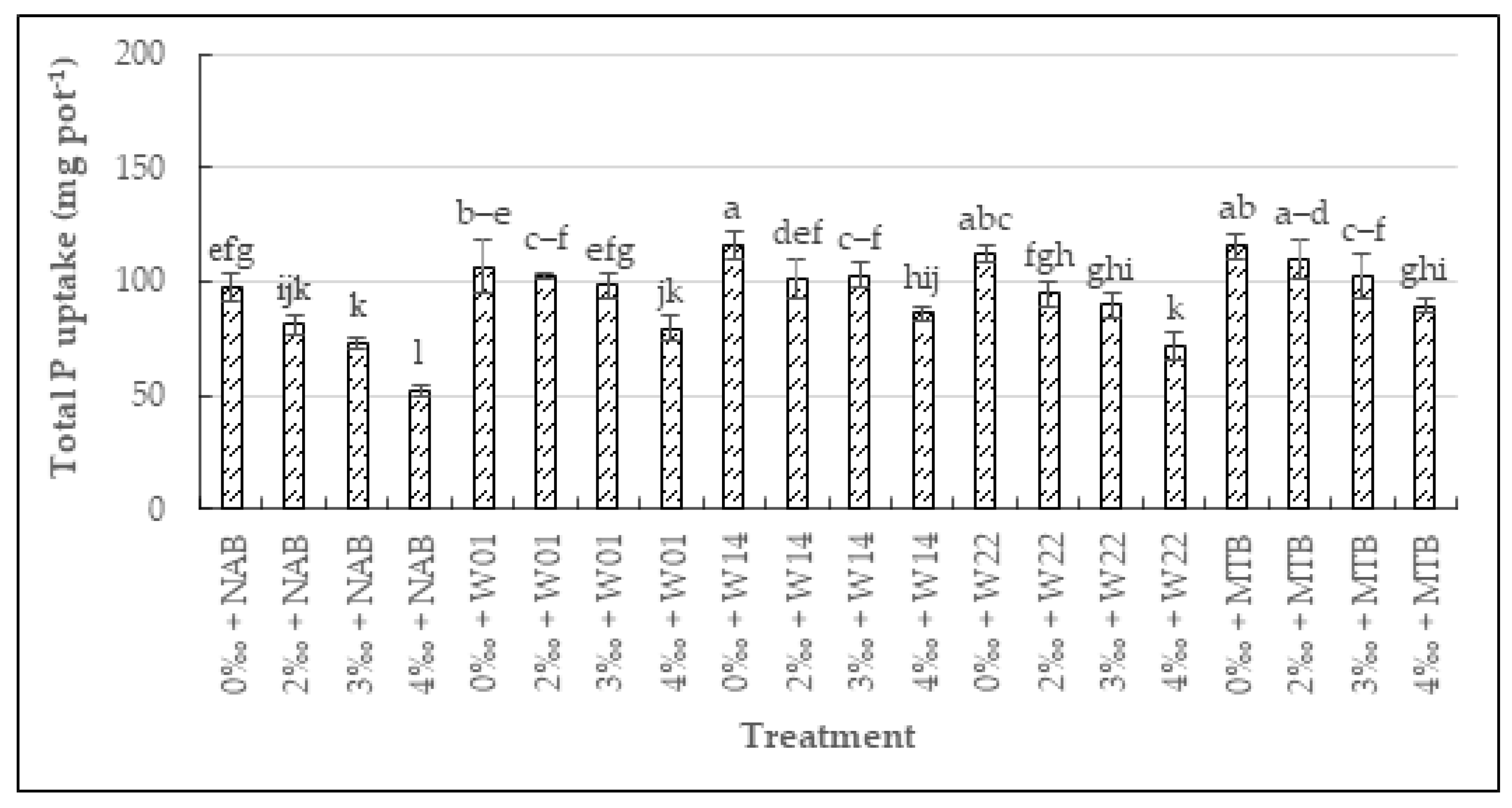
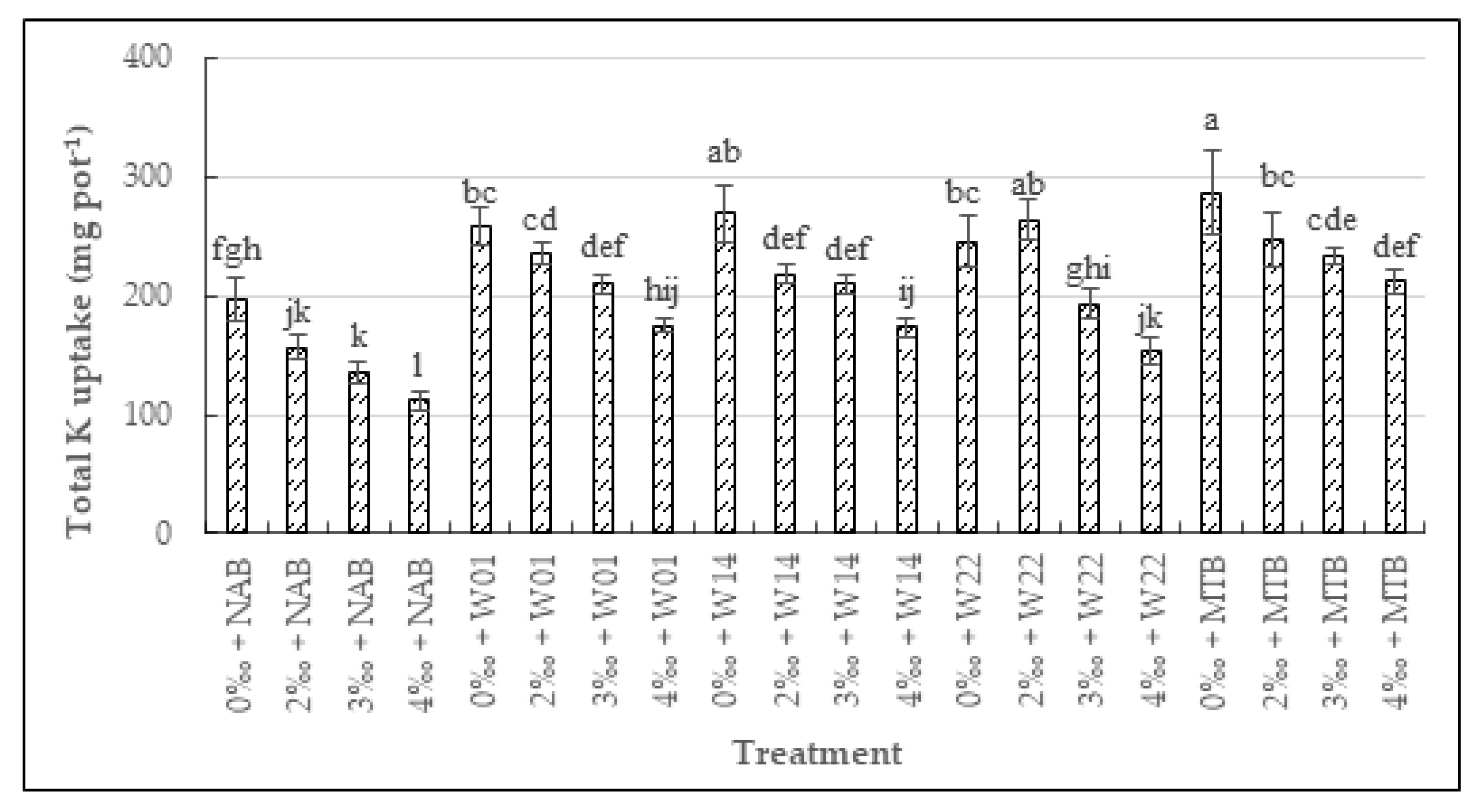
3.3.2. The N, P, K, and Na Uptake in Seeds and Straw
3.3.3. The Total N, P, K, and Na Uptake in Rice
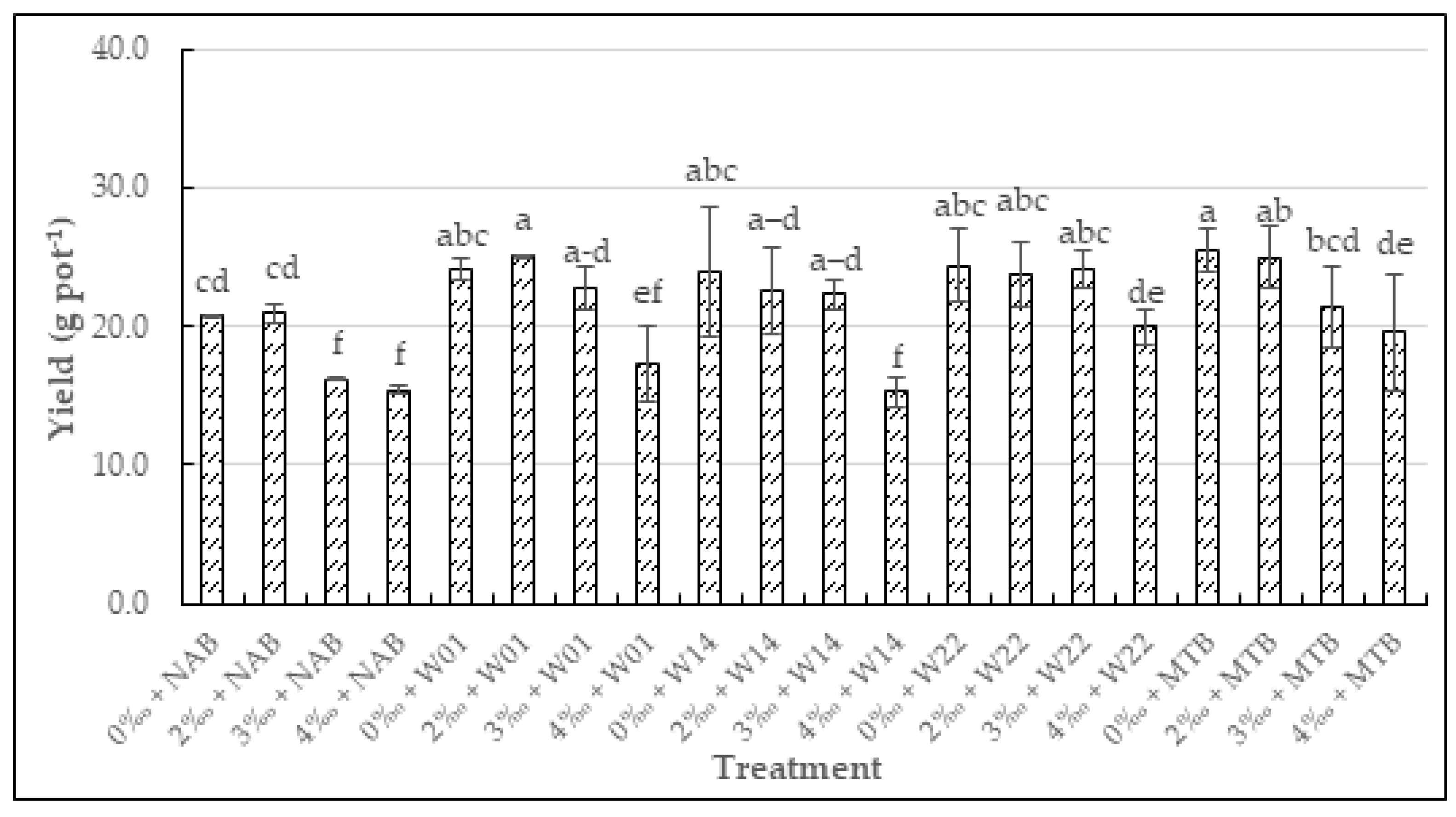
3.4. The Impact of the Water Salinity and the Supplementation of Luteovulum sphaeroides on the Growth, the Yield Components, and the Grain Yield of Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van, P.D.T.; Popescu, I.; Van Griensven, A.; Solomatine, D.P.; Trung, N.H.; Green, A. A study of the climate change impacts on fluvial flood propagation in the Vietnamese Mekong Delta. Hydro. Earth Syst. Sci. 2012, 16, 4637–4649. [Google Scholar] [CrossRef]
- Vu, P.H.; Vu, P.T.; Tri, V.P.D. Classification of risk zones in agriculture under impacts of salt water intrusion in Bac Lieu province. Can Tho Univ. J. Sci. 2016, 42, 70–80. [Google Scholar] [CrossRef]
- Ismail, L.M.; Soliman, M.I.; Abd El-Aziz, M.H.; Abdel-Aziz, H.M.M. Impact of silica ions and nano silica on growth and productivity of pea plants under salinity stress. Plants 2022, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.M.; Altaey, D.K.; Altaee, N.H. The impact of selenium, nano (SiO2) and organic fertilization on growth and yield of potato Solanum tuberosum L. under salt stress conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 735, 012042. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Rady, M.M.; Taha, R.S.; Abd El Azeam, S.; Simpson, C.R.; Semida, W.M. Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci. Hortic. 2020, 261, 108930. [Google Scholar] [CrossRef]
- Shams, M.; Ekinci, M.; Ors, S.; Turan, M.; Agar, G.; Kul, R.; Yildirim, E. Nitric oxide mitigates salt stress effects of pepper seedlings by altering nutrient uptake, enzyme activity and osmolyte accumulation. Physiol. Mol. Biol. Plants 2019, 25, 1149–1161. [Google Scholar] [CrossRef]
- Tolay, I. The impact of different zinc (Zn) levels on growth and nutrient uptake of basil (Ocimum basilicum L.) grown under salinity stress. PLoS ONE 2021, 16, e0246493. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity stress affects photosynthesis, malondialdehyde formation, and proline content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Salinas, R.; Sánchez, E.; Ruíz, J.M.; Lao, M.T.; Romero, L. Proline, betaine, and choline responses to different phosphorus levels in green bean. Commun. Soil Sci. Plant Anal. 2013, 44, 465–472. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Imhoff, J.F. Diversity of anaerobic anoxygenic phototrophic purple bacteria. In Modern Topics in the Phototrophic Prokaryotes; Hallenbeck, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 47–85. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, G.; Li, X.; Zhang, J. Microbial production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2014, 98, 7349–7357. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, Z.; Wu, X.; Chen, X. Light and oxygen facilitating the directly treatment food wastewater and poly-β-hydroxybutyrate, 5-aminolevulinic acid, pigment productions by Rubrivivax gelatinosus. Water Sci. Technol. 2023, 87, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Sakpirom, J.; Kantachote, D.; Nunkaew, T.; Khan, E. Characterizations of purple non-sulfur bacteria isolated from paddy fields, and identification of strains with potential for plant growth-promotion, greenhouse gas mitigation and heavy metal bioremediation. Res. Microbiol. 2017, 168, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Khuong, N.Q.; Kantachote, D.; Dung, N.T.T.; Huu, T.N.; Thuc, L.V.; Thu, L.T.M.; Quang, L.T.; Xuan, D.T.; Nhan, T.C.; Tien, P.D.; et al. Potential of potent purple nonsulfur bacteria isolated from rice-shrimp systems to ameliorate rice (Oryza sativa L.) growth and yield in saline acid sulfate soil. J. Plant Nutr. 2023, 46, 473–494. [Google Scholar] [CrossRef]
- Kang, Z.; Ding, W.; Gong, X.; Liu, Q.; Du, G.; Chen, J. Recent advances in production of 5-aminolevulinic acid using biological strategies. World J. Microbiol. Biotechnol. 2017, 33, 200. [Google Scholar] [CrossRef] [PubMed]
- Kantha, T.; Kantachote, D.; Klongdee, N. Potential of biofertilizers from selected Rhodopseudomonas palustris strains to assist rice (Oryza sativa L. subsp. indica) growth under salt stress and to reduce green-house gas emissions. Ann. Microbiol. 2015, 65, 2109–2118. [Google Scholar] [CrossRef]
- Nookongbut, P.; Kantachote, D.; Meghrai, M.; Naidu, R. Reduction in arsenic toxicity and uptake in rice (Oryza sativa L.) by As-resistant purple nonsulfur bacteria. Environ. Sci. Pollut. Res. 2018, 23, 1681–1690. [Google Scholar] [CrossRef]
- Korkmaz, A.; Şirikçi, R.; Kocaçınar, F.; Değer, Ö.; Demirkırıan, A.R. Alleviation of salt-induced adverse effects in pepper seedlings by seed application of glycinebetaine. Sci. Hortic. 2012, 148, 197–205. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Najeeb, U.; El-Beltagi, H.S.; Huang, Q.; Lu, H.; Xu, L.; Shi, B.; Zhou, W. Promotive role of 5-aminolevulinic acid or salicylic acid combined with citric acid on sunflower growth by regulating manganese absorption. Antioxidants 2023, 12, 580. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alyemeni, M.N.; Moustakas, M.; Ahmad, P. 5-aminolevulinic acid induces chromium [Cr (VI)] tolerance in tomatoes by alleviating oxidative damage and protecting photosystem II: A mechanistic approach. Plants 2023, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Watanabe, M.; Tanaka, T. Biosynthesis, biotechnological production and applications of 5-aminolevulinic acid. Appl. Microbiol. Biotechnol. 2002, 58, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, T.; Summer, M.E. Methods of Soil Analysis. Part 3—Chemical Methods, 1st ed.; SSSA Book Series 5.3; SSSA/ASA: Madison, WI, USA, 1996. [Google Scholar] [CrossRef]
- Walinga, I.; van Vark, W.; Houba, V.J.G.; van der Lee, J.J. Soil and plant analysis, Part 7. In Plant Analysis Procedures: Department of Soil Science and Plant Nutrition; Wageningen Agricultural University: Wageningen, The Netherlands, 1997; p. 263. [Google Scholar]
- Khuong, N.Q.; Kantachote, D.; Onthong, J.; Sukhoom, A. The potential of acid-resistant purple nonsulfur bacteria isolated from acid sulfate soils for reducing toxicity of Al3+ and Fe2+ using biosorption for agricultural application. Biocatal. Agric. Biotechnol. 2017, 68, 217–228. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Kantachote, D.; Onthong, J.; Xuan, L.N.T.; Sukhoom, A. Enhancement of rice growth and yield in actual acid sulfate soils by potent acid-resistant Rodopseudomonas palustris trains for producing safe rice. Plant Soil 2018, 429, 483–501. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Kantachote, D.; Nookongbut, P.; Onthong, J.; Xuan, L.N.T.; Sukhoom, A. Mechanisms of acid-resistant Rhodopseudomonas palustris strains to ameliorate acidic stress and promote plant growth. Biocatal. Agric. Biotechnol. 2020, 24, 101520. [Google Scholar] [CrossRef]
- Carlozzi, P.; Di Lorenzo, T.; Ghanotakis, D.F.; Touloupakis, E. Effects of pH, temperature and salinity on P3HB synthesis culturing the marine Rhodovulum sulfidophilum DSM-1374. Appl. Microbiol. Biotechnol. 2020, 104, 2007–2015. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; He, T.; Cao, M.; Sha, L.; Hu, Y.; Li, Q.; Li, J. Soil properties drive a negative correlation between species diversity and genetic diversity in a tropical seasonal rainforest. Sci. Rep. 2016, 6, 20652. [Google Scholar] [CrossRef]
- Cheah, C.; Cheow, Y.L.; Ting, A.S.Y. Comparative analysis on metal removal potential of exopolymeric substances with live and dead cells of bacteria. Int. J. Environ. Res. 2022, 16, 1–12. [Google Scholar] [CrossRef]
- Panwichian, S.; Kantachote, D.; Wittayaweerasak, B.; Mallavarapu, M. Removal of heavy metals by exopolymeric substances produced by resistant purple nonsulfur bacteria isolated from contaminated shrimp ponds. Electron. J. Biotechnol. 2011, 14, 2. [Google Scholar] [CrossRef]
- Nguyen, K.Q.; Kantachote, D.; Onthong, J.; Sukhoom, A. Al3+ and Fe2+ toxicity reduction potential by acid-resistant strains of Rhodopseudomonas palustris isolated from acid sulfate soils under acidic conditions. Ann. Microbiol. 2018, 68, 217–228. [Google Scholar] [CrossRef]
- Nunkaew, T.; Kantachote, D.; Nitoda, T.; Kanzaki, H.; Ritchie, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Gondal, A.H.; Hussain, I.; Ijaz, A.B.; Zafar, A.; Ch, B.I.; Zafar, H.; Sohail, M.D.; Niazi, H.; Touseef, M.; Kan, A.A.; et al. Influence of soil pH and microbes on mineral solubility and plant nutrition: A review. Int. J. Agric. Biol. Sci. 2021, 5, 71–81. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Kantachote, D.; Thuc, L.V.; Nookongbut, P.; Xuan, L.N.T.; Nhan, T.C.; Xuan, L.N.T.; Tantirungkij, M. Potential of Mn2+-resistant purple nonsulfur bacteria isolated from acid sulfate soils to act as bioremediators and plant growth promoters via mechanisms of resistance. J. Soil Sci. Plant Nutr. 2020, 20, 2364–2378. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Sakpirom, J.; Oanh, T.O.; Thuc, L.V.; Thu, L.T.M.; Xuan, D.T.; Quang, L.T.; Xuan, L.N.T. Isolation and characterization of novel potassium-solubilizing purple nonsulfur bacteria from acidic paddy soils using culture-dependent and culture-independent techniques. Braz. J. Microbiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Zhang, F. Growth-promoting ability of Rhodopseudomonas palustris G5 and its effect on induced resistance in cucumber against salt stress. J. Plant Growth Regul. 2019, 38, 180–188. [Google Scholar] [CrossRef]
- Ábrahám, E.; Hourton-Cabassa, C.; Erdei, L.; Szabados, L. Methods for determination of proline in plants. In Plant Stress Tolerance, Methods in Molecular Biology; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 317–331. [Google Scholar] [CrossRef]
- Sakarika, M.; Spanoghe, J.; Sui, Y.; Wambacq, E.; Grunert, O.; Haesaert, G.; Spiller, M.; Vlaeminck, S.E. Purple non-sulphur bacteria and plant production: Benefits for fertilization, stress resistance and the environment. Micro. Biotechnol. 2020, 13, 1336–1365. [Google Scholar] [CrossRef]
- Lee, K.H.; Koh, R.H.; Song, H.G. Enhancement of growth and yield of tomato by Rodopseudomonas sp. under greenhouse condition. J. Microbiol. Biotechnol. 2008, 46, 641–646. [Google Scholar]
- Kantha, T.; Chaiyasut, C.; Kantachote, D.; Sukrong, S.; Muangprom, A. Selection of photosynthetic bacteria producing 5-aminolevulinic acid from soil of organic saline paddy fields from the Northeast region of Thailand. Afr. J. Microbiol. Res. 2010, 4, 1848–1855. [Google Scholar]
- Nunkaew, T.; Kantachote, D.; Kanzaki, H.; Nitoda, T.; Ritchie, R.J. Effects of 5-aminolevulinic acid (ALA)-containing supernatants from selected Rodopseudomonas palustris trains on rice growth under NaCl stress, with mediating effects on chlorophyll, photosynthetic electron transport and antioxidative enzymes. Electron. J. Biotechnol. 2014, 17, 4. [Google Scholar] [CrossRef]
- Iwai, R.; Uchida, S.; Yamaguchi, S.; Sonoda, F.; Tsunoda, K.; Nagata, H.; Nagata, D.; Koga, A.; Goto, M.; Maki, T.-A.; et al. Effects of seed bio-priming by purple non-sulfur bacteria (PNSB) on the root development of rice. Microorganisms 2022, 10, 2197. [Google Scholar] [CrossRef] [PubMed]
- Huu, T.N.; Giau, T.T.N.; Ngan, P.N.; Van, T.T.B.; Khuong, N.Q. Potential of phosphorus solubilizing purple nonsulfur bacteria isolated from acid sulfate soil in improving soil property, nutrient uptake, and yield of pineapple (Ananas comosus L. Merrill) under acidic stress. Appl. Environ. Soil Sci. 2022, 2022, 8693479. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Thuc, L.V.; Giang, C.T.; Xuan, L.N.T.; Thu, L.T.M.; Isao, A.; Jun-Ichi, S. Improvement of nutrient uptake, yield of black sesame (Sesamum indicum L.), and alluvial soil fertility in dyke by spent rice straw from mushroom cultivation as biofertilizer containing potent strains of Rhodopseudomonas palustris. Sci. World J. 2023, 2023, 1954632. [Google Scholar] [CrossRef] [PubMed]
- Khuong, N.Q.; Kantachote, D.; Huu, T.N.; Nhan, T.C.; Nguyen, P.C.; Thu, L.T.M.; Van, T.T.B.; Xuan, N.T.T.; Xuan, L.N.T.; Xuan, D.T. Use of potent acid resistant strains of Rhodopseudomonas spp. in Mn-contaminated acidic paddies to produce safer rice and improve soil fertility. Soil Tillage Res. 2022, 221, 105393. [Google Scholar] [CrossRef]
- Maeda, I. Potential of phototrophic purple nonsulfur bacteria to fix nitrogen in rice fields. Microorganisms 2021, 10, 28. [Google Scholar] [CrossRef]
- Sundar, L.S.; Chao, Y.Y. Potential of purple non-sulfur bacteria in sustainably enhancing the agronomic and physiological performances of rice. Agronomy 2022, 12, 2347. [Google Scholar] [CrossRef]
- Lu, L.; Chang, M.; Han, X.; Wang, Q.; Wang, J.; Yang, H.; Guan, Q.; Dai, S. Beneficial effects of endophytic Pantoea ananatis with ability to promote rice growth under saline stress. J. Appl. Microbiol. 2021, 131, 1919–1931. [Google Scholar] [CrossRef]
- Puvanitha, S.; Mahendran, S. Effect of salinity on plant height, shoot and root dry weight of selected rice cultivars. Scholars J. Agric. Vet. Sci. 2017, 4, 126–131. [Google Scholar] [CrossRef]
- Shan, Y.K.; Sundar, L.S.; Chao, Y.Y. Foliar application of Rhodopseudomonas palustris enhances the rice crop growth and yield under field conditions. Plants 2022, 11, 2452. [Google Scholar] [CrossRef]
- Wongkantrakorn, N.; Sunohara, Y.; Matsumoto, H. Mechanism of growth amelioration of NaCl—Stressed rice (Oryza sativa L.) by δ-aminolevulinic acid. J. Pestic. Sci. 2009, 34, 89–95. [Google Scholar] [CrossRef]
- Khuong, N.Q.; Minh, D.P.T.; Thu, L.T.M.; Thuc, L.V. The potential of bacterial strains of Luteovulum sphaeroides W22 and W47 for producing δ-aminolevulinic acid to improve soil quality, growth and yield of saline-irrigated rice cultivated in salt-contaminated soil. Agronomy 2023, 13, 1409. [Google Scholar] [CrossRef]


| Parameters | Unit | Values |
|---|---|---|
| pHwater | - | 3.15 ± 0.13 |
| pHKCl | - | 2.77 ± 0.09 |
| EC | mS cm−1 | 5.10 ± 0.36 |
| Ntotal | %N | 0.84 ± 0.03 |
| NH4+ | mg kg−1 | 120 ± 2.89 |
| Ptotal | %P2O5 | 0.017 ± 0.003 |
| Psoluble | mg P kg−1 | 9.39 ± 1.27 |
| Al-P | mg P kg−1 | 13.9 ± 1.65 |
| Fe-P | mg P kg−1 | 53.9 ± 5.37 |
| Ca-P | mg P kg−1 | 22.4 ± 2.22 |
| CEC | meq CEC 100 g−1 | 6.86 ± 0.85 |
| Na+ | meq Na+ 100 g−1 | 1.78 ± 0.08 |
| K+ | meq K+ 100 g−1 | 0.56 ± 0.11 |
| Mg2+ | meq Mg2+ 100 g−1 | 9.00 ± 1.29 |
| Factor | pHwater | pHKCl | EC | Ntotal | NH4+ | Ptotal | Psoluble | ||
|---|---|---|---|---|---|---|---|---|---|
| mS cm−1 | % | mg kg−1 | % | mg kg−1 | |||||
| The water salinity (A) (‰) | 0 | 3.65 ± 0.17 | 3.19 ± 0.10 | 0.48 ± 0.05 b | 0.128 ± 0.014 | 18.8 ± 2.8 b | 0.048 ± 0.002 | 51.5 ± 2.1 d | |
| 2 | 3.53 ± 0.17 | 3.18 ± 0.08 | 0.53 ± 0.03 a | 0.133 ± 0.011 | 18.9 ± 2.9 b | 0.048 ± 0.003 | 55.3 ± 2.5 c | ||
| 3 | 3.58 ± 0.18 | 3.16 ± 0.08 | 0.57 ± 0.04 a | 0.128 ± 0.013 | 68.7 ± 5.6 a | 0.046 ± 0.002 | 59.9 ± 2.5 b | ||
| 4 | 3.64 ± 0.21 | 3.18 ± 0.11 | 0.56 ± 0.06 a | 0.133 ± 0.013 | 71.2 ± 2.9 a | 0.047 ± 0.002 | 64.7 ± 2.3 a | ||
| The bacteria (B) (1.812 × 105 CFU g−1 dry soil) | NAB | 3.51 ± 0.12 | 3.19 ± 0.06 | 0.58 ± 0.04 a | 0.131 ± 0.009 | 39.3 ± 3.3 c | 0.047 ± 0.003 | 53.5 ± 1.9 d | |
| W01 | 3.63 ± 0.27 | 3.15 ± 0.07 | 0.54 ± 0.04 b | 0.131 ± 0.013 | 45.4 ± 2.5 ab | 0.048 ± 0.003 | 56.2 ± 2.9 c | ||
| W14 | 3.57 ± 0.20 | 3.18 ± 0.11 | 0.53 ± 0.05 b | 0.132 ± 0.014 | 47.9 ± 6.2 a | 0.049 ± 0.003 | 60.9 ± 2.3 a | ||
| W22 | 3.56 ± 0.12 | 3.17 ± 0.13 | 0.53 ± 0.03 b | 0.133 ± 0.016 | 44.2 ± 2.3 b | 0.047 ± 0.002 | 58.5 ± 2.4 b | ||
| MTB | 3.72 ± 0.21 | 3.18 ± 0.08 | 0.51 ± 0.06 b | 0.125 ± 0.011 | 45.2 ± 3.4 ab | 0.047 ± 0.001 | 60.2 ± 2.3 ab | ||
| F (A) | ns | ns | * | ns | * | ns | * | ||
| F (B) | ns | ns | * | ns | * | ns | * | ||
| F (A × B) | ns | ns | * | ns | * | * | * | ||
| CV (%) | 5.76 | 3.30 | 10.2 | 10.1 | 9.74 | 6.73 | 4.55 | ||
| Factor | Ca-P | Al-P | Fe-P | CEC | Ca2+ | Mg2+ | Na+ | K+ | |
| mg kg−1 | meq 100 g−1 | ||||||||
| The water salinity (A) (‰) | 0 | 13.5 ± 1.3 | 23.5 ± 2.7 c | 106.0 ± 5.4 | 13.5 ± 0.9 | 2.57 ± 0.33 | 1.32 ± 0.13 | 0.481 ± 0.033 c | 0.187 ± 0.017 |
| 2 | 12.5 ± 1.0 | 24.6 ± 3.6 c | 107.4 ± 6.2 | 14.1 ± 0.9 | 2.40 ± 0.35 | 1.32 ± 0.12 | 0.555 ± 0.044 b | 0.187 ± 0.017 | |
| 3 | 12.8 ± 2.4 | 26.9 ± 3.2 b | 104.0 ± 10.2 | 14.1 ± 1.1 | 2.40 ± 0.30 | 1.30 ± 0.39 | 0.608 ± 0.053 a | 0.175 ± 0.012 | |
| 4 | 12.8 ± 1.1 | 29.2 ± 2.3 a | 103.8 ± 4.8 | 14.0 ± 1.3 | 2.37 ± 0.29 | 1.25 ± 0.17 | 0.634 ± 0.036 a | 0.183 ± 0.012 | |
| The bacteria (B) (1.812 × 105 CFU g−1 dry soil) | NAB | 16.2 ± 1.6 a | 33.5 ± 2.4 a | 121.9 ± 13.4 a | 13.5 ± 1.3 | 2.50 ± 0.29 | 1.31 ± 0.19 | 0.614 ± 0.065 a | 0.169 ± 0.010 b |
| W01 | 12.4 ± 2.1 b | 22.8 ± 2.4 c | 97.7 ± 3.4 c | 13.9 ± 0.7 | 2.40 ± 0.31 | 1.38 ± 0.32 | 0.564 ± 0.027 b | 0.185 ± 0.011 a | |
| W14 | 11.7 ± 1.0 b | 23.5 ± 4.1 bc | 106.1 ± 7.5 b | 14.3 ± 1.1 | 2.52 ± 0.30 | 1.28 ± 0.18 | 0.563 ± 0.040 b | 0.188 ± 0.020 a | |
| W22 | 11.8 ± 1.8 b | 25.7 ± 3.6 b | 95.6 ± 3.3 c | 13.9 ± 1.1 | 2.30 ± 0.29 | 1.18 ± 0.17 | 0.547 ± 0.042 b | 0.184 ± 0.017 a | |
| MTB | 12.4 ± 0.9 b | 24.9 ± 2.3 bc | 105.1 ± 5.6 b | 14.1 ± 1.0 | 2.45 ± 0.39 | 1.33 ± 0.15 | 0.561 ± 0.033 b | 0.190 ± 0.013 a | |
| F (A) | ns | * | ns | ns | ns | ns | * | ns | |
| F (B) | * | * | * | ns | ns | ns | * | * | |
| F (A × B) | ns | * | ns | ns | ns | ns | * | * | |
| CV (%) | 13.9 | 12.4 | 8.26 | 8.44 | 13.8 | 22.6 | 7.84 | 9.05 | |
| Factor | Proline (µmol g−1 Dry Weight) | ||
|---|---|---|---|
| 35 DAS | 50 DAS | ||
| The water salinity (A) (‰) | 0 | 2.57 ± 0.26 d | 3.03 ± 0.29 d |
| 2 | 3.31 ± 0.44 c | 3.97 ± 0.44 c | |
| 3 | 7.06 ± 0.41 b | 5.15 ± 0.39 b | |
| 4 | 7.74 ± 0.40 a | 5.99 ± 0.50 a | |
| The bacteria (B) (1.812 × 105 CFU g−1 dry soil) | NAB | 5.54 ± 0.53 a | 5.32 ± 0.25 a |
| W01 | 5.08 ± 0.22 ab | 5.05 ± 0.57 ab | |
| W14 | 5.24 ± 0.36 ab | 3.08 ± 0.35 d | |
| W22 | 5.04 ± 0.56 b | 4.39 ± 0.42 c | |
| MTB | 4.98 ± 0.22 b | 4.83 ± 0.43 b | |
| F (A) | * | * | |
| F (B) | * | * | |
| F (A × B) | * | * | |
| CV (%) | 9.55 | 9.96 | |
| Factor | Biomass | Concentration (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g pot−1) | N | P | K | Na | |||||||
| Seeds | Straw | Seeds | Straw | Seeds | Straw | Seeds | Straw | Seeds | Straw | ||
| The water salinity (A) (‰) | 0 | 19.9 ± 1.1 a | 20.8 ± 1.0 a | 1.846 ± 0.085 c | 1.142 ± 0.082 | 0.349 ± 0.025 a | 0.192 ± 0.014 | 0.467 ± 0.038 ab | 0.758 ± 0.066 a | 0.045 ± 0.008 d | 0.186 ± 0.008 d |
| 2 | 18.2 ± 0.9 b | 19.2 ± 0.9 b | 2.126 ± 0.113 b | 1.121 ± 0.044 | 0.337 ± 0.012 ab | 0.189 ± 0.013 | 0.489 ± 0.034 a | 0.699 ± 0.034 b | 0.055 ± 0.006 c | 0.216 ± 0.022 c | |
| 3 | 17.3 ± 1.0 c | 18.7 ± 0.7 b | 2.116 ± 0.095 b | 1.112 ± 0.085 | 0.333 ± 0.014 b | 0.189 ± 0.013 | 0.446 ± 0.037 b | 0.630 ± 0.040 c | 0.067 ± 0.011 b | 0.228 ± 0.017 b | |
| 4 | 14.3 ± 0.8 d | 16.9 ± 0.7 c | 2.468 ± 0.089 a | 1.118 ± 0.082 | 0.313 ± 0.013 c | 0.184 ± 0.013 | 0.408 ± 0.022 c | 0.624 ± 0.031 c | 0.101 ± 0.008 a | 0.252 ± 0.016 a | |
| The bacteria (B) (1.812 × 105 CFU g−1 dry soil) | NAB | 15.4 ± 0.9 c | 16.8 ± 0.6 c | 1.965 ± 0.101 c | 1.049 ± 0.079 c | 0.312 ± 0.014 b | 0.163 ± 0.011 c | 0.372 ± 0.016 c | 0.546 ± 0.036 c | 0.082 ± 0.010 a | 0.259 ± 0.017 a |
| W01 | 17.4 ± 0.7 b | 20.3 ± 0.5 a | 2.291 ± 0.118 a | 1.136 ± 0.088 ab | 0.337 ± 0.019 a | 0.188 ± 0.012 b | 0.456 ± 0.044 b | 0.688 ± 0.054 b | 0.053 ± 0.005 c | 0.214 ± 0.018 c | |
| W14 | 18.7 ± 1.0 a | 18.9 ± 0.7 b | 2.193 ± 0.066 b | 1.177 ± 0.020 a | 0.339 ± 0.016 a | 0.201 ± 0.016 a | 0.443 ± 0.028 b | 0.703 ± 0.025 ab | 0.072 ± 0.009 b | 0.212 ± 0.015 c | |
| W22 | 16.8 ± 0.9 b | 18.5 ± 1.4 b | 2.017 ± 0.092 c | 1.106 ± 0.083 bc | 0.344 ± 0.023 a | 0.182 ± 0.013 b | 0.469 ± 0.037 b | 0.721 ± 0.035 ab | 0.079 ± 0.009 a | 0.235 ± 0.015 b | |
| MTB | 18.9 ± 1.1 a | 19.9 ± 1.0 a | 2.228 ± 0.100 ab | 1.148 ± 0.096 ab | 0.331 ± 0.009 a | 0.210 ± 0.014 a | 0.523 ± 0.038 a | 0.731 ± 0.064 a | 0.050 ± 0.009 c | 0.183 ± 0.015 d | |
| F (A) | * | * | * | ns | * | ns | * | * | * | * | |
| F (B) | * | * | * | * | * | * | * | * | * | * | |
| F (A × B) | * | * | * | ns | * | * | * | * | * | * | |
| CV (%) | 1.65 | 5.42 | 4.90 | 7.96 | 5.88 | 7.76 | 6.98 | 8.08 | 13.9 | 8.09 | |
| Factor | Uptake (mg pot−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Na | ||||||
| Seeds | Straw | Seeds | Straw | Seeds | Straw | Seeds | Straw | ||
| The water salinity (A) (‰) | 0 | 369.0 ± 28.3 b | 237.3 ± 17.0 a | 69.5 ± 6.7 a | 40.1 ± 3.8 a | 93.4 ± 10.1 a | 157.9 ± 17.6 a | 9.03 ± 1.67 c | 38.7 ± 2.5 b |
| 2 | 388.2 ± 27.6 a | 215.5 ± 10.9 b | 61.3 ± 4.0 b | 36.6 ± 2.8 b | 89.1 ± 9.0 a | 135.1 ± 9.3 b | 9.98 ± 1.14 c | 41.1 ± 4.8 ab | |
| 3 | 368.6 ± 26.6 b | 208.3 ± 20.3 b | 57.8 ± 3.6 c | 35.5 ± 3.2 b | 77.9 ± 7.3 b | 118.7 ± 10.1 c | 11.5 ± 1.94 b | 42.4 ± 3.3 a | |
| 4 | 358.4 ± 27.3 b | 189.3 ± 16.4 c | 44.7 ± 2.9 d | 31.2 ± 2.7 c | 59.4 ± 4.9 c | 105.8 ± 8.3 d | 14.0 ± 1.48 a | 41.9 ± 3.9 a | |
| The bacteria (B) (1.812 × 105 CFUg−1 dry soil) | NAB | 293.9 ± 22.7 d | 177.1 ± 16.6 c | 48.3 ± 2.5 c | 27.6 ± 2.7 d | 57.9 ± 5.0 c | 92.7 ± 8.2 c | 11.8 ± 1.76 b | 43.1 ± 3.6 a |
| W01 | 393.4 ± 23.7 b | 229.6 ± 15.5 a | 58.6 ± 4.8 b | 38.1 ± 2.4 b | 79.6 ± 9.0 b | 139.9 ± 9.9 a | 8.81 ± 1.01 c | 43.2 ± 3.6 a | |
| W14 | 408.4 ± 29.0 b | 223.5 ± 7.7 a | 63.7 ± 4.4 a | 37.9 ± 3.1 b | 83.9 ± 7.4 b | 133.9 ± 7.5 b | 13.1 ± 1.83 a | 39.9 ± 3.4 b | |
| W22 | 334.3 ± 25.4 c | 205.1 ± 20.5 b | 58.3 ± 5.2 b | 33.9 ± 4.1 c | 78.9 ± 7.1 b | 135.1 ± 15.3 b | 12.7 ± 1.64 b | 42.9 ± 4.3 a | |
| MTB | 418.8 ± 36.3 a | 227.6 ± 20.5 a | 62.7 ± 4.7 a | 41.7 ± 3.3 a | 99.3 ± 10.7 a | 145.6 ± 15.7 a | 9.24 ± 1.52 c | 36.2 ± 3.1 c | |
| F(A) | * | * | * | * | * | * | * | * | |
| F (B) | * | * | * | * | * | * | * | * | |
| F (A × B) | * | ns | * | * | * | * | * | * | |
| CV (%) | 7.59 | 8.88 | 8.50 | 9.76 | 11.1 | 10.5 | 15.1 | 9.66 | |
| Factor | Plant Height | Panicle Length | Panicle Number pot−1 | Seed Number panicle−1 | Filled Seeds Ratio | 1000-Seed Weight | |
|---|---|---|---|---|---|---|---|
| (cm) | (cm) | (panicles) | (seeds) | (%) | (g) | ||
| The water salinity (A) (‰) | 0 | 72.6 ± 1.8 a | 19.3 ± 0.3 a | 20.7 ± 2.3 a | 65.9 ± 3.3 | 86.2 ± 8.8 a | 25.3 ± 1.2 |
| 2 | 72.2 ± 1.7 ab | 18.8 ± 0.9 ab | 20.6 ± 2.5 a | 69.0 ± 3.9 | 81.0 ± 5.8 b | 25.4 ± 1.3 | |
| 3 | 71.2 ± 1.6 bc | 18.7 ± 0.5 ab | 18.7 ± 2.4 b | 68.2 ± 4.3 | 77.9 ± 4.9 bc | 25.2 ± 1.1 | |
| 4 | 70.8 ± 1.5 c | 18.4 ± 0.6 b | 15.7 ± 1.9 c | 65.8 ± 3.6 | 75.7 ± 8.5 c | 25.5 ± 1.5 | |
| The bacteria (B) (1.812 × 105 CFU g−1 dry soil) | NAB | 69.3 ± 1.7 b | 18.2 ± 0.6 c | 16.3 ± 2.7 b | 67.8 ± 1.8 | 76.6 ± 5.3 b | 25.3 ± 1.4 |
| W01 | 71.8 ± 1.4 a | 19.2 ± 0.5 ab | 19.7 ± 2.7 a | 68.2 ± 3.5 | 82.9 ± 7.7 a | 25.6 ± 1.6 | |
| W14 | 72.0 ± 1.9 a | 18.6 ± 1.0 bc | 19.1 ± 1.9 a | 65.1 ± 3.1 | 76.7 ± 9.4 b | 25.2 ± 1.3 | |
| W22 | 72.2 ± 1.8 a | 18.8 ± 0.5 ab | 20.0 ± 2.8 a | 68.5 ± 5.7 | 81.2 ± 6.1 ab | 25.4 ± 1.3 | |
| MTB | 73.1 ± 1.4 a | 19.3 ± 0.3 a | 19.4 ± 1.3 a | 66.4 ± 4.9 | 83.6 ± 6.6 a | 25.3 ± 0.8 | |
| F (A) | * | * | * | ns | * | ns | |
| F (B) | * | * | * | ns | * | ns | |
| F (A × B) | ns | ns | ns | ns | * | ns | |
| CV (%) | 2.59 | 3.87 | 13.7 | 6.76 | 9.87 | 5.50 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuong, N.Q.; Dung, N.T.T.; Thu, L.T.M.; Quang, L.T.; Xuan, L.N.T.; Phong, N.T. The Efficacy of 5-Aminolevulinic Acid-Producing Luteovulum sphaeroides Strains on Saline Soil Fertility, Nutrient Uptakes, and Yield of Rice. Agriculture 2023, 13, 1761. https://doi.org/10.3390/agriculture13091761
Khuong NQ, Dung NTT, Thu LTM, Quang LT, Xuan LNT, Phong NT. The Efficacy of 5-Aminolevulinic Acid-Producing Luteovulum sphaeroides Strains on Saline Soil Fertility, Nutrient Uptakes, and Yield of Rice. Agriculture. 2023; 13(9):1761. https://doi.org/10.3390/agriculture13091761
Chicago/Turabian StyleKhuong, Nguyen Quoc, Nguyen Thi Thuy Dung, Le Thi My Thu, Le Thanh Quang, Ly Ngoc Thanh Xuan, and Ngo Thanh Phong. 2023. "The Efficacy of 5-Aminolevulinic Acid-Producing Luteovulum sphaeroides Strains on Saline Soil Fertility, Nutrient Uptakes, and Yield of Rice" Agriculture 13, no. 9: 1761. https://doi.org/10.3390/agriculture13091761







