Mapping of QTLs for Brown Rice Traits Based on Chromosome Segment Substitution Line in Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Evaluation of Brown Rice Traits
2.3. Identification of QTLs
2.4. Statistical Analysis
3. Results
3.1. Phenotypic Variation of Brown Rice Traits in Parents and CSSLs
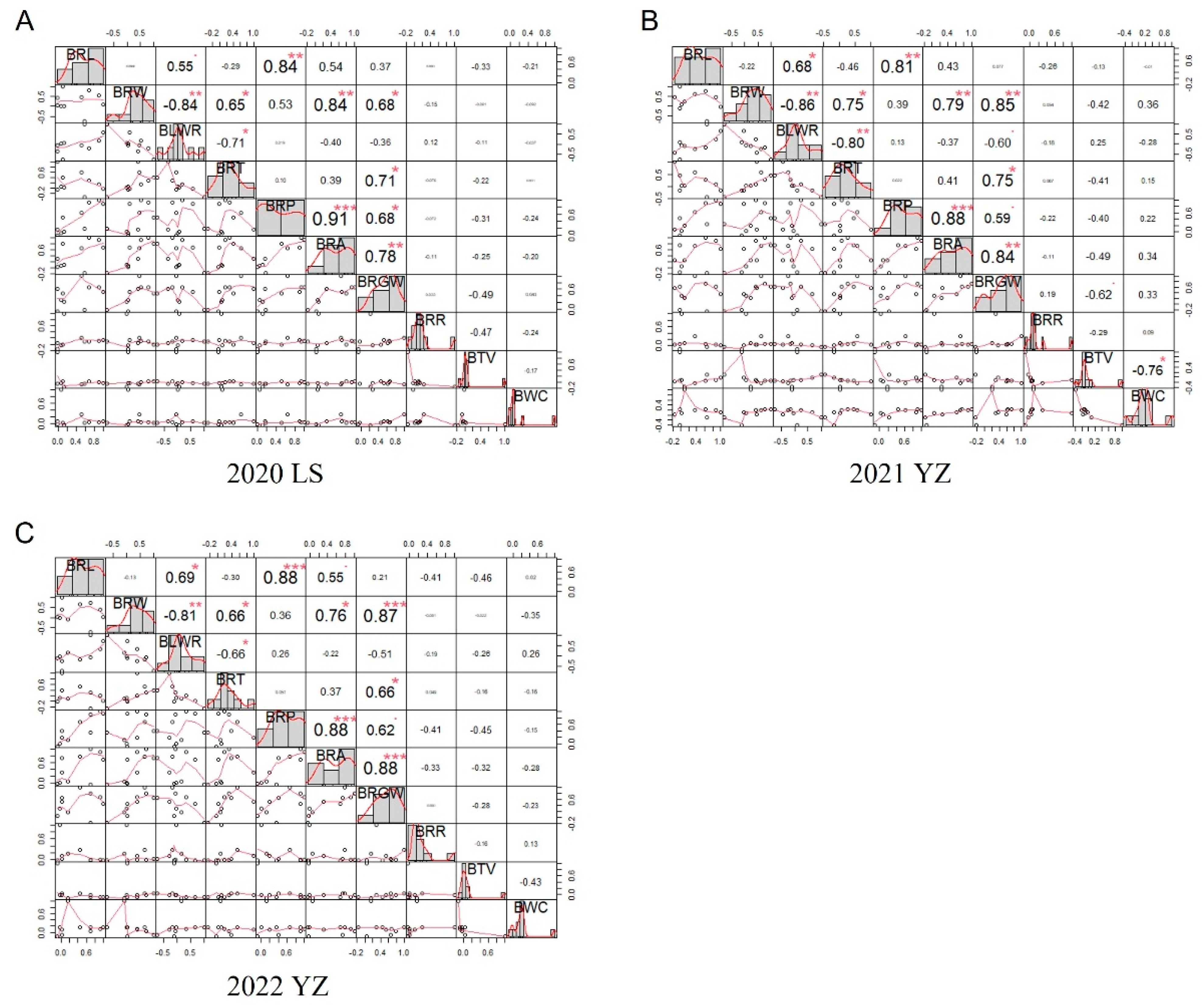
3.2. Correlation Analysis of Brown Rice Traits
3.3. QTL Analysis for Brown Rice Traits
3.4. Digenic Epistasis QTLs for Brown Rice Traits
3.5. Pleiotropic QTLs for Brown Rice Traits
3.6. Comparative Genetic Analysis for QTLs and Grain Size Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.Y. Rice Reproductive Biology; Zhejiang University Press: Hangzhou, China, 2005; pp. 19–25. [Google Scholar]
- Ravichanthiran, K.; Ma, Z.F.; Zhang, H.; Cao, Y.; Wang, C.W.; Muhammad, S.; Aglago, E.K.; Zhang, Y.; Jin, Y.; Pan, B. Phytochemical profile of brown rice and its nutrigenomic implications. Antioxidants 2018, 7, 71. [Google Scholar] [CrossRef]
- Tan, Y.F.; Sum, M.; Xing, Y.Z.; Hua, J.P.; Sun, X.L.; Zhang, Q.F.; Corke, H. Mapping quantitative trait loci for milling quality, protein content and color characteristics of rice using a recombinant inbred line population derived from an elite rice hybrid. Theor. Appl. Genet. 2001, 103, 1037–1045. [Google Scholar] [CrossRef]
- Dong, W.; Cheng, Z.J.; Xu, J.L.; Zheng, T.Q.; Wang, X.L.; Zhang, H.Z.; Wang, J.; Wan, J.-M. Identification of QTLs underlying folate content in milled rice. J. Integr. Agric. 2014, 13, 1827–1834. [Google Scholar] [CrossRef]
- Hu, B.; Huang, D.; Xiao, Y.; He, Q.; Wan, Y.; Fan, Y. QTL analysis for mineral contents in brown rice using a BC2F4:5 population derived from dongxiang wild rice (Oryza rufipogon Griff.). Rice Sci. 2018, 32, 43–50. [Google Scholar]
- Peng, B.; Kong, H.L.; Li, Y.B.; Wang, L.Q.; Zhong, M.; Sun, L.; Gao, G.J.; Zhang, Q.L.; Luo, L.J.; Wang, G.W.; et al. OsAAP6 functions as an important regulator of grain protein content and nutritional quality in rice. Nat. Commun. 2014, 5, 4847. [Google Scholar] [CrossRef]
- Yang, Y.H.; Guo, M.; Sun, S.Y.; Zou, Y.L.; Yin, S.Y.; Liu, Y.N.; Tang, S.Z.; Gu, M.H.; Yang, Z.F.; Yan, C.J. Natural variation of OsGluA2 is involved in grain protein content regulation in rice. Nat. Commun. 2019, 10, 1949. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xia, D.; Li, P.B.; Ao, Y.T.; Xu, X.D.; Wan, S.S.; Li, Y.H.; Wu, B.; Shi, H.; Wang, K.Y.; et al. Genetic architecture and key genes controlling the diversity of oil composition in rice grain. Mol. Plant 2021, 14, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.F.; Wan, J.M.; Xia, J.F.; Zhai, H.Q.; Ikehashi, H. Identification of quantitative trait loci underlying milling quality of rice (Oryza sativa) grains. Plant Breed. 2004, 123, 229–234. [Google Scholar] [CrossRef]
- Aluko, G.; Martinez, C.; Tohme, C.; Castano, C.; Bergman, C.; Oard, J.H. QTL mapping of grain quality traits from the inter specific cross Oryza sativa × O. glaberrima. Theor. Appl. Genet. 2004, 109, 630–639. [Google Scholar] [CrossRef]
- Lou, J.; Chen, L.; Yue, G.H.; Lou, Q.J.; Mei, H.W.; Xiong, L.; Lou, L.J. QTL mapping of grain quality traits in rice. J. Cereal Sci. 2009, 50, 145–151. [Google Scholar] [CrossRef]
- Ren, D.Y.; Rao, Y.C.; Huang, L.C.; Leng, Y.J.; Hu, J.; Lu, M.; Zhang, G.H.; Zhu, L.; Gao, Z.Y.; Dong, G.J.; et al. Fine mapping identifies a new QTL for brown rice rate in rice (Oryza Sativa, L.). Rice 2016, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Pang, Y.; Yuan, Z.; Xing, D.; Xu, J.; Dingkuhn, M.; Li, Z.; Ye, G. Genome-wide association study of grain appearance and milling quality in a worldwide collection of indica rice germplasm. PLoS ONE 2015, 10, e0145577. [Google Scholar] [CrossRef]
- Malik, A.; Kumar, A.; Ellur, R.K.; Krishnan, S.G.; Dixit, D.; Bollinedi, H.; Vinod, K.K.; Nagarajan, M.; Bhowmick, P.K.; Singh, N.K.; et al. Molecular mapping of QTLs for grain dimension traits in Basmati rice. Front. Genet. 2022, 13, 932166. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.X.; Xu, G.Y.; Deng, K.L.; Yu, J.J.; Xiang, S.Q.; Zhou, K.; Zhang, Q.L.; Li, R.X.; Li, M.M.; et al. QTL Analysis of Z414, a chromosome segment substitution line with short, wide grains, and substitution mapping of qGL11 in Rice. Rice 2022, 15, 25. [Google Scholar] [CrossRef]
- Furuta, T.; Uehara, K.; Angeles-Shim, R.B.; Shim, J.; Ashikari, M.; Takashi, T. Development and evaluation of chromosome segment substitution lines (CSSLs) carrying chromosome segments derived from Oryza rufipogon in the genetic background of Oryza sativa L. Breed. Sci. 2014, 63, 468–475. [Google Scholar] [CrossRef]
- Bian, J.M.; Jiang, L.; Liu, L.L.; Wei, X.J.; Xiao, Y.H.; Zhang, L.J.; Zhao, Z.G.; Zhai, H.Q.; Wan, J.M. Construction of a new set of rice chromosome segment substitution lines and identification of grain weight and related traits QTLs. Breed. Sci. 2010, 60, 305–313. [Google Scholar] [CrossRef]
- Hao, W.; Zhu, M.Z.; Gao, J.P.; Sun, S.Y.; Lin, H.X. Identification of quantitative trait loci for rice quality in a population of chromosome segment substitution lines. J. Integr. Plant Biol. 2009, 51, 500–512. [Google Scholar] [CrossRef]
- Wang, D.Y.; Chen, S.; Wang, Z.M.; Ji, C.L.; Xu, C.M.; Zhang, X.F.; Bhagirath, S.C. Optimizing hill seeding density for high-yielding hybrid rice in a single rice cropping system in south China. PLoS ONE 2014, 9, e109417. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.J.; Wang, S.L.; Wang, R.A.; Tao, T.; Jia, S.W.; Song, T.; Xu, L.N.; Cai, X.L.; Jin, S.K.; Gao, J.P. Multi-environmental genetic analysis of grain size traits based on chromosome segment substitution line in rice (Oryza sativa L.). Phyton-Int. J. Exp. Bot. 2022, 91, 943–958. [Google Scholar] [CrossRef]
- Li, H.H.; Ye, G.Y.; Wang, J.K. A modifed algorithm for the improvement of composite interval mapping. Genetics 2007, 175, 361–374. [Google Scholar] [CrossRef]
- Li, M.; Li, H.H.; Zhang, L.Y.; Wang, J.K. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J. 2015, 3, 269–283. [Google Scholar]
- McCouch, S.R.; Cho, Y.G.; Yano, M.; Paul, E.; Blinstrub, M.; Morishima, H.; Kinosita, T. Report on QTL nomenclature. Rice Genet. Newsl. 1997, 14, 11–13. [Google Scholar]
- Voorrips, R. MapChart: Sofware for the graphical presentation of linkage maps and QTLs. J. Hered. 2022, 93, 77–78. [Google Scholar] [CrossRef]
- Dai, L.P.; Wang, L.; Leng, Y.J.; Yang, Y.L.; Huang, L.C.; Chen, L.; Wang, Y.Q.; Ren, D.Y.; Hu, J.; Zhang, G.H.; et al. Quantitative trait loci mapping for appearance quality in short-grain rice. Crop Sci. 2016, 56, 1484–1492. [Google Scholar] [CrossRef]
- Liu, Q.; Han, R.X.; Wu, K.; Zhang, J.Q.; Ye, Y.F.; Wang, S.S.; Chen, J.F.; Pan, Y.J.; Li, Q.; Xu, X.P.; et al. G-Protein βγ subunits determine grain size through interaction with MADS-domain transcription factors in rice. Nat. Commun. 2018, 9, 852. [Google Scholar] [CrossRef]
- Yu, J.P.; Miao, J.L.; Zhang, Z.Y.; Xiong, H.Y.; Zhu, X.Y.; Sun, X.M.; Pan, Y.H.; Liang, Y.T.; Zhang, Q.; Rehman, R.M.A.; et al. Alternative splicing of OsLG3b controls grain length and yield in japonica rice. Plant Biotechnol. J. 2018, 16, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Fan, C.C.; Xing, Y.Z.; Jiang, Y.H.; Luo, L.J.; Sun, L.; Shao, D.; Xu, C.J.; Li, X.H.; Xiao, J.H.; et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Kuroha, T.; Ayano, M.; Furuta, T.; Nagai, K.; Komeda, N.; Segami, S.; Miura, K.; Ogawa, D.; Kamura, T.; et al. Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proc. Natl. Acad. Sci. USA 2015, 112, 76–81. [Google Scholar] [CrossRef]
- Si, L.Z.; Chen, J.Y.; Huang, X.H.; Gong, H.; Luo, J.H.; Hou, Q.Q.; Zhou, T.Y.; Lu, T.T.; Zhu, J.J.; Shangguan, Y.Y.; et al. OsSPL13 controls grain size in cultivated rice. Nat. Genet. 2016, 48, 447–457. [Google Scholar] [CrossRef]
- Wang, S.K.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.Q.; Wang, S.S.; Wang, Y.; Chen, X.B.; Zhang, Y.; Gao, C.X.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xiong, G.S.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.X.; Zeng, L.J.; Xu, E.B.; Xu, J.; et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Miao, J.; Gu, H.Y.; Peng, X.R.; Leburu, M.; Yuan, F.H.; Gu, H.W.; Gao, Y.; Tao, Y.J.; Zhu, J.Y.; et al. Natural variations in SLG7 regulate grain shape in rice. Genetics 2015, 201, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Y.; Wan, J.M.; Weng, J.F.; Jiang, L.; Bi, J.C.; Wang, C.M.; Zhai, H.Q. Stability of QTLs for rice grain dimension and endosperm chalkiness characteristics across eight environments. Theor. Appl. Genet. 2005, 110, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Shanmugavadivel, P.S.; Mithra, S.A.; Dokku, P.; Kumar, K.A.R.; Rao, G.J.N.; Singh, V.P.; Singh, A.K.; Singh, N.K.; Mohapatra, T. Mapping quantitative trait loci (QTL) for grain size in rice using a RIL population from Basmati × indica cross showing high segregation distortion. Euphytica 2013, 194, 401–416. [Google Scholar] [CrossRef]
- Zeng, W.; Cai, Z.Q.; Wang, Y.L.; Jiang, Q.G.; Wang, J.; Li, S.R.; Peng, L.; Qin, B.X.; Chen, B.S.; Li, R.B.; et al. Quantitative trait locus analysis for grain size related traits of rice. Mol. Plant Breed. 2016, 7, 1–19. [Google Scholar]
- Lin, L.H.; Wu, W.R. Mapping of QTLs underlying grain shape and grain weight in rice. Mol. Plant Breed. 2003, 1, 337–342. [Google Scholar]
- Li, Z.F.; Wan, J.M.; Xia, J.F.; Zhai, H.Q. Mapping quantitative trait loci underlying appearance quality of rice grains (Oryza sativa L.). Acta Genet. Sinica 2003, 30, 251–259. [Google Scholar]
- Bai, X.; Luo, L.; Yan, W.; Kovi, M.R.; Zhan, W.; Xing, Y. Genetic dissection of rice grain shape using a recombinant inbred line population derived from two contrasting parents and fine mapping a pleiotropic quantitative trait locus qGL7. BMC Genet. 2010, 11, 16. [Google Scholar] [CrossRef]
- Ge, X.J.; Xing, Y.Z.; Xu, C.G.; He, Y.Q. QTL analysis of cooked rice grain elongation, volume expansion, and water absorption using a recombinant inbred population. Plant Breed. 2005, 124, 121–126. [Google Scholar] [CrossRef]
- Dang, X.J.; Thi, T.G.T.; Edzesi, W.M.; Liang, L.J.; Liu, Q.M.; Liu, E.B.; Wang, Y.; Qiang, S.; Liu, L.H.; Hong, D.L. Population genetic structure of Oryza sativa in East and Southeast Asia and the discovery of elite alleles for grain traits. Sci. Rep. 2015, 5, 11254. [Google Scholar] [CrossRef]
- Jin, S.K.; Xu, L.N.; Yang, Q.Q.; Zhang, M.Q.; Wang, S.L.; Wang, R.A.; Tao, T.; Hong, L.M.; Guo, Q.Q.; Jia, S.W.; et al. High-resolution quantitative trait locus mapping for rice grain quality traits using genotyping by sequencing. Front. Plant Sci. 2023, 13, 1050882. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Hou, L.; Xie, J.; Cao, F.; Wei, R.; Yang, M.; Qi, Z.; Zhu, R.; Zhang, Z.; Xin, D.; et al. Construction of chromosome segment substitution lines and inheritance of seed-pod characteristics in wild soybean. Front. Plant Sci. 2022, 13, 869455. [Google Scholar] [CrossRef] [PubMed]
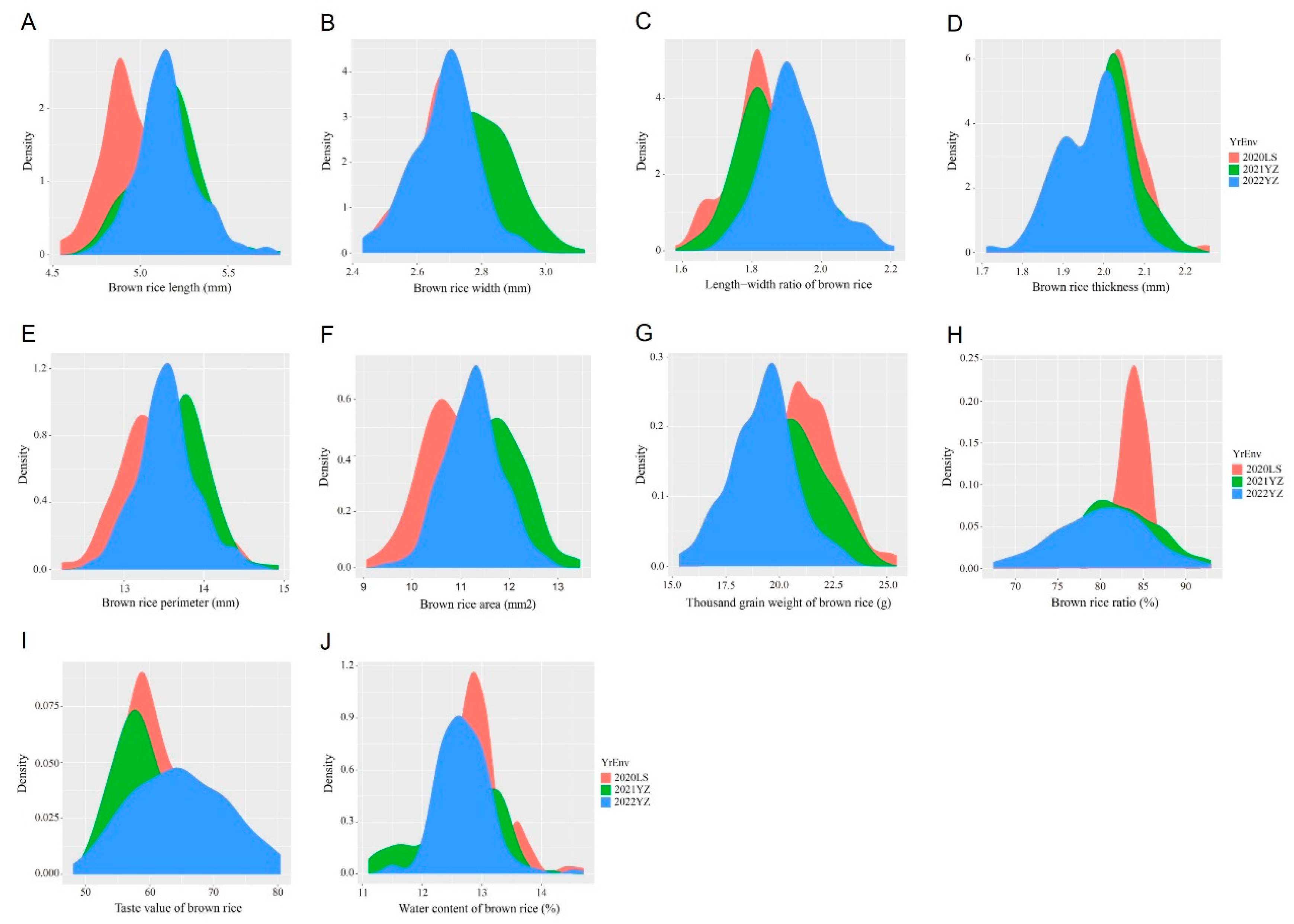

| Year and Location | Traits | Parents (Mean ± SD) | CSSLs Population | ||||
|---|---|---|---|---|---|---|---|
| Koshihikari | Nona Bokra | Mean ± SD | Range | Skewness | Kurtosis | ||
| 2020 Lingshui | BRL (mm) | 4.95 ± 0.31 | 6.14 ± 0.29 ** | 4.95 ± 0.18 | 4.54~5.50 | 0.59 | 0.67 |
| BRW (mm) | 2.76 ± 0.20 | 2.86 ± 0.18 * | 2.72 ± 0.13 | 2.46~3.12 | 0.42 | −0.02 | |
| BLWR | 1.80 ± 0.05 | 2.15 ± 0.07 ** | 1.83 ± 0.10 | 1.58~2.17 | 0.47 | 1.00 | |
| BRT | 2.05 ± 0.02 | 2.09 ± 0.03 ** | 2.02± 0.07 | 1.86~2.26 | 0.14 | 0.51 | |
| BRP (mm) | 13.41 ± 0.71 | 16.11 ± 0.81 ** | 13.37 ± 0.44 | 12.22~14.53 | 0.31 | 0.16 | |
| BRA (mm2) | 10.87 ± 1.03 | 14.36 ± 1.22 ** | 10.80 ± 0.70 | 9.05~13.11 | 0.41 | 0.42 | |
| BRGW(g) | 21.61 ± 0.39 | 25.98 ± 0.08 ** | 21.20 ± 1.55 | 17.46~25.46 | 0.06 | 0.06 | |
| BRR (%) | 83.60 ± 0.50 | 81.99 ± 0.56 * | 83.94 ± 1.81 | 76.42~93.02 | 0.41 | 5.15 | |
| BTV | 57.67 ± 1.37 | 52.67 ± 1.53 ** | 59.75 ± 4.79 | 50.00~77.00 | 0.55 | 0.54 | |
| BWC (%) | 12.34 ± 0.86 | 13.48 ± 0.39 * | 12.91 ± 0.50 | 11.40~14.70 | 0.32 | 2.23 | |
| 2021 Yangzhou | BRL (mm) | 5.16 ± 0.20 | — | 5.15 ± 0.18 | 4.71~5.80 | 0.18 | 0.75 |
| BRW (mm) | 2.80 ± 0.20 | — | 2.77 ± 0.12 | 2.46~3.10 | −0.13 | −0.25 | |
| BLWR | 1.84 ± 0.01 | — | 1.86 ± 0.11 | 1.64~2.17 | 0.50 | −0.13 | |
| BRT | 1.94 ± 0.02 | — | 2.01 ± 0.08 | 1.75~2.22 | −0.30 | 0.49 | |
| BRP (mm) | 13.67 ± 0.61 | — | 13.65 ± 0.41 | 12.60~14.93 | −0.04 | 0.15 | |
| BRA (mm2) | 11.70 ± 1.10 | — | 11.63 ± 0.68 | 9.78~13.45 | −0.04 | −0.02 | |
| BRGW (g) | 19.58 ± 0.75 | — | 20.31 ± 1.78 | 15.97~24.33 | −0.08 | −0.43 | |
| BRR | 76.96 ± 3.45 | — | 81.84 ± 4.82 | 68.40~92.70 | −0.04 | −0.20 | |
| BTV | 51.67 ± 2.08 | — | 61.07 ± 6.37 | 51.00~77.50 | 0.71 | −0.33 | |
| BWC (%) | 13.13 ± 0.12 | — | 12.62 ± 0.59 | 11.10~14.15 | −0.50 | 0.18 | |
| 2022 Yangzhou | BRL (mm) | 5.21 ± 0.29 | — | 5.15 ± 0.17 | 4.71~5.74 | 0.51 | 1.02 |
| BRW (mm) | 2.79 ± 0.23 | — | 2.68 ± 0.10 | 2.43~2.93 | −0.20 | 0.15 | |
| BLWR | 1.87 ± 0.01 | — | 1.92 ± 0.09 | 1.71~2.21 | 0.51 | 0.51 | |
| BRT | 1.92 ± 0.02 | — | 1.96 ± 0.08 | 1.71~2.14 | −0.52 | 0.08 | |
| BRP (mm) | 13.70 ± 0.71 | — | 13.53 ± 0.37 | 12.57~14.68 | 0.25 | 0.45 | |
| BRA (mm2) | 11.36 ± 1.42 | — | 11.24 ± 0.59 | 9.48~12.76 | 0.00 | 0.12 | |
| BRGW (g) | 19.22 ± 0.39 | — | 19.23 ± 1.52 | 15.33~23.08 | −0.03 | 0.08 | |
| BRR | 74.57 ± 0.50 | — | 80.17 ± 5.18 | 67.43~92.48 | −0.12 | −0.26 | |
| BTV | 53.00 ± 2.00 | — | 64.61 ± 7.40 | 48.00~80.50 | 0.09 | −0.66 | |
| BWC (%) | 13.03 ± 0.15 | — | 12.64 ± 0.59 | 11.50~14.55 | 0.63 | 2.75 | |
| Traits | QTL | Chr. | Marker | Position (cm) | LOD | Additive Effect | PVE (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2020LS | 2021YZ | 2022YZ | 2020LS | 2021YZ | 2022YZ | 2020LS | 2021YZ | 2022YZ | |||||
| BRL | qBRL1-1 | 1 | STS1-12 | 132.60 | 4.56 | — | — | 0.16 | — | — | 8.41 | — | — |
| qBRL1-2 | 1 | SSR1-104 | 167.85 | — | — | 4.99 | — | — | 0.10 | — | — | 6.49 | |
| qBRL2-1 | 2 | SSR2-24 | 92.20 | — | — | 3.46 | — | — | −0.07 | — | — | 4.38 | |
| qBRL2-2 | 2 | SSRW2-309 | 96.09 | — | 2.73 | — | — | −0.07 | — | — | 8.06 | — | |
| qBRL3-1 | 3 | SSR3-272 | 1.46 | 3.34 | — | — | 0.13 | — | — | 6.05 | — | — | |
| qBRL3-2 | 3 | STS3-1 | 113.84 | 5.78 | — | 11.58 | 0.10 | — | 0.14 | 10.86 | — | 16.67 | |
| qBRL3-3 | 3 | SSR3-337 | 132.32 | — | — | 4.31 | — | — | −0.09 | — | — | 5.55 | |
| qBRL3-4 | 3 | SSR3-36 | 147.67 | — | 2.57 | — | — | −0.17 | — | — | 7.59 | — | |
| qBRL5-1 | 5 | SSR5-1 | 14.19 | — | — | 3.51 | — | — | 0.10 | — | — | 4.46 | |
| qBRL5-2 | 5 | SSR5-126 | 76.21 | 3.18 | — | — | 0.07 | — | — | 5.74 | — | — | |
| qBRL8 | 8 | SSR8-390 | 111.97 | 3.74 | — | 4.22 | 0.09 | — | 0.09 | 6.81 | — | 5.41 | |
| BRW | qBRW2 | 2 | SSR2-24 | 92.20 | — | — | 3.18 | — | — | −0.04 | — | — | 5.89 |
| qBRW3 | 3 | SSR3-120 | 41.63 | — | 6.93 | 6.90 | — | −0.07 | −0.06 | — | 19.48 | 13.53 | |
| qBRW7 | 7 | SSR7-145 | 20.13 | 9.81 | — | — | 0.09 | — | — | 21.93 | — | — | |
| qBRW8 | 8 | SSR8-417 | 90.25 | 4.43 | 3.51 | — | 0.06 | 0.06 | — | 9.10 | 9.35 | — | |
| qBRW12 | 12 | SSR12-107 | 66.43 | — | — | 6.26 | — | — | −0.07 | — | — | 12.15 | |
| BLWR | qBLWR1-1 | 1 | SSR1-442 | 159.81 | 11.61 | — | — | −0.10 | — | — | 11.90 | — | — |
| qBLWR1-2 | 1 | SSR1-104 | 167.85 | 16.68 | 3.62 | 3.30 | 0.11 | 0.05 | 0.04 | 18.55 | 5.11 | 5.60 | |
| qBLWR3-1 | 3 | SSR3-288 | 17.12 | 6.80 | — | — | 0.07 | — | — | 6.47 | — | — | |
| qBLWR3-2 | 3 | SSR3-120 | 41.63 | 9.40 | 14.96 | 11.52 | 0.06 | 0.08 | 0.07 | 9.31 | 25.38 | 22.30 | |
| qBLWR3-3 | 3 | STS3-1 | 113.84 | — | 4.78 | — | — | 0.05 | — | — | 6.87 | — | |
| qBLWR7-1 | 7 | SSR7-76 | 56.11 | 2.89 | — | — | −0.03 | — | — | 2.60 | — | — | |
| qBLWR7-2 | 7 | SSR7-174 | 99.43 | — | 2.96 | — | — | 0.04 | — | — | 4.14 | — | |
| qBLWR10 | 10 | SSR10-169 | 80.71 | — | 5.64 | 4.63 | — | 0.06 | 0.05 | — | 8.22 | 8.05 | |
| qBLWR12 | 12 | STS12-8 | 57.18 | — | 4.05 | 4.21 | — | 0.04 | 0.04 | — | 5.76 | 7.28 | |
| BRT | qBRT2 | 2 | SSR2-213 | 106.43 | — | 2.68 | 3.56 | — | 0.03 | 0.04 | — | 5.50 | 8.15 |
| qBRT3 | 3 | SSR3-120 | 41.63 | — | 3.51 | — | — | −0.03 | — | — | 7.30 | — | |
| qBRT6 | 6 | SSR6-111 | 6.25 | 3.27 | — | — | 0.03 | — | — | 8.77 | — | — | |
| qBRT7 | 7 | SSR7-174 | 99.43 | 2.82 | 3.93 | — | −0.04 | −0.04 | — | 7.51 | 8.22 | — | |
| qBRT11 | 11 | SSR11-235 | 3.61 | — | 4.23 | 3.52 | — | 0.04 | 0.04 | — | 8.90 | 8.05 | |
| BRP | qBRP1 | 1 | STS1-12 | 132.60 | — | — | 3.34 | — | — | 0.25 | — | — | 4.07 |
| qBRP2 | 2 | SSR2-24 | 92.20 | 4.53 | 4.81 | 5.95 | −0.21 | −0.21 | −0.18 | 8.44 | 11.28 | 7.52 | |
| qBRP3-1 | 3 | STS3-1 | 113.84 | 4.51 | — | 12.00 | 0.22 | — | 0.30 | 8.40 | — | 16.65 | |
| qBRP3-2 | 3 | SSR3-337 | 132.32 | — | — | 5.47 | — | — | −0.22 | — | — | 6.87 | |
| qBRP3-3 | 3 | SSR3-36 | 147.67 | — | 4.08 | — | — | −0.45 | — | — | 9.45 | — | |
| qBRP5 | 5 | SSR5-1 | 14.19 | — | — | 3.20 | — | — | 0.21 | — | — | 3.88 | |
| qBRP7 | 7 | SSR7-145 | 20.13 | 4.34 | — | — | 0.19 | — | — | 8.06 | — | — | |
| qBRP8 | 8 | SSR8-390 | 111.97 | 3.24 | 2.52 | 4.92 | 0.21 | 0.19 | 0.20 | 5.92 | 5.70 | 6.13 | |
| qBRP12 | 12 | SSR12-107 | 66.43 | 4.19 | — | — | −0.25 | — | — | 7.8 | — | — | |
| BRA | qBRA2 | 2 | SSR2-24 | 92.20 | 4.05 | 5.97 | 4.94 | −0.31 | −0.38 | −0.30 | 7.60 | 11.81 | 11.02 |
| qBRA3-1 | 3 | SSR3-105 | 53.44 | — | 2.70 | — | — | −0.31 | — | — | 5.07 | — | |
| qBRA3-2 | 3 | SSR3-36 | 147.67 | — | 3.56 | 3.13 | — | −0.67 | −0.49 | — | 6.79 | 6.78 | |
| qBRA7 | 7 | SSR7-145 | 20.13 | 7.74 | — | — | 0.42 | — | — | 15.36 | — | — | |
| qBRA8 | 8 | SSR8-417 | 90.25 | 3.04 | 4.24 | — | 0.27 | 0.33 | — | 5.61 | 8.16 | — | |
| qBRA12 | 12 | SSR12-107 | 66.43 | 4.63 | — | 4.25 | −0.41 | — | −0.36 | 8.77 | — | 9.36 | |
| BRGW | qBRGW2 | 2 | SSR2-24 | 92.20 | 3.93 | — | — | −0.72 | — | — | 8.06 | — | — |
| qBRGW6 | 6 | SSR6-111 | 6.25 | 4.41 | — | — | 0.66 | — | — | 9.13 | — | — | |
| qBRGW8 | 8 | SSR8-417 | 90.25 | 4.71 | — | — | 0.82 | — | — | 9.80 | — | — | |
| BTV | qBTV5 | 5 | SSR5-83 | 99.01 | 3.35 | 5.69 | 5.67 | 1.80 | 3.19 | 3.56 | 5.94 | 13.32 | 15.87 |
| qBTV6-1 | 6 | STS6-1 | 32.33 | — | — | 2.66 | — | — | 3.93 | — | — | 6.96 | |
| qBTV6-2 | 6 | SSR6-71 | 38.71 | — | 5.96 | — | — | 5.56 | — | — | 14.01 | — | |
| qBTV6-3 | 6 | SSR6-20 | 52.21 | — | — | 3.05 | — | — | 3.20 | — | — | 8.07 | |
| qBTV6-4 | 6 | SSR6-248 | 107.23 | 7.65 | — | — | 3.07 | — | — | 14.61 | — | — | |
| qBTV6-5 | 6 | SSR6-135 | 115.54 | 3.86 | — | — | −2.17 | — | — | 6.91 | — | — | |
| qBTV7 | 7 | SSR7-174 | 99.43 | 4.22 | 2.73 | 4.51 | 2.55 | 2.66 | 3.95 | 7.59 | 6.08 | 12.28 | |
| BWC | qBWC3 | 3 | SSR3-337 | 132.32 | — | 3.42 | — | — | −0.38 | — | — | 9.4 | — |
| qBWC6 | 6 | SSR6-71 | 38.71 | — | 3.07 | — | — | −0.40 | — | — | 8.33 | — | |
| qBWC7-1 | 7 | SSR7-145 | 20.13 | 3.42 | — | — | −0.25 | — | — | 10.9 | — | — | |
| qBWC7-2 | 7 | SSR7-76 | 56.11 | — | — | 2.99 | — | — | −0.18 | — | — | 8.71 | |
| qBWC7-3 | 7 | STS7-5 | 82.36 | — | — | 5.55 | — | — | −0.33 | — | — | 16.13 | |
| qBWC12 | 12 | STS12-4 | 3.37 | — | — | 3.10 | — | — | 0.34 | — | — | 8.52 | |
| Traits | Environment | Chr. | Marker | Chr. | Marker | LOD | Epistasis (AA) | PVE (%) |
|---|---|---|---|---|---|---|---|---|
| BRL | 2022YZ | 1 | SSR1-338 | 3 | STS3-1 | 13.12 | −0.08 | 5.17 |
| 2022YZ | 3 | STS3-1 | 3 | SSR3-35 | 12.64 | 0.08 | 5.01 | |
| 2022YZ | 3 | STS3-1 | 4 | SSR4-274 | 12.92 | −0.08 | 5.11 | |
| 2022YZ | 3 | STS3-1 | 4 | SSR4-302 | 12.98 | −0.08 | 5.12 | |
| BRW | 2020LS | 2 | SSR2-255 | 7 | SSR7-145 | 11.38 | 0.08 | 4.20 |
| 2020LS | 2 | SSR2-53 | 7 | SSR7-145 | 11.32 | 0.08 | 4.18 | |
| 2020LS | 5 | SSR5-241 | 7 | SSR7-145 | 13.42 | −0.09 | 4.81 | |
| 2020LS | 5 | STS5-1 | 7 | SSR7-145 | 13.15 | −0.11 | 4.74 | |
| 2020LS | 6 | STS6-1 | 7 | SSR7-145 | 11.58 | −0.08 | 4.26 | |
| 2020LS | 7 | SSR7-145 | 8 | SSR8-417 | 14.18 | −0.08 | 5.44 | |
| 2020LS | 7 | SSR7-145 | 8 | SSR8-552 | 11.97 | −0.09 | 4.38 | |
| 2020LS | 7 | SSR7-145 | 8 | SSR8-170 | 11.71 | −0.08 | 4.30 | |
| 2020LS | 7 | SSR7-145 | 8 | SSR8-390 | 11.64 | −0.08 | 4.28 | |
| 2020LS | 7 | SSR7-145 | 9 | SSR9-306 | 11.53 | −0.08 | 4.25 | |
| BLWR | 2020LS | 2 | SSR2-19 | 3 | SSR3-120 | 11.27 | −0.04 | 26.50 |
| 2021YZ | 3 | SSR3-120 | 3 | SSR3-105 | 17.13 | −0.06 | 13.14 | |
| 2021YZ | 3 | SSR3-120 | 8 | SSR8-235 | 16.56 | 0.04 | 12.80 | |
| 2021YZ | 3 | SSR3-120 | 12 | STS12-4 | 16.12 | 0.04 | 12.54 | |
| 2022YZ | 3 | SSR3-120 | 3 | SSR3-105 | 14.62 | −0.07 | 15.37 | |
| 2022YZ | 3 | SSR3-120 | 6 | SSR6-71 | 12.90 | 0.04 | 13.90 | |
| BRP | 2022YZ | 1 | SSR1-4 | 3 | STS3-1 | 13.68 | 0.17 | 2.42 |
| 2022YZ | 1 | SSR1-492 | 3 | STS3-1 | 13.77 | 0.17 | 2.44 | |
| 2022YZ | 3 | STS3-1 | 3 | SSR3-35 | 14.13 | 0.24 | 2.49 | |
| 2022YZ | 3 | STS3-1 | 4 | SSR4-3 | 13.53 | 0.19 | 2.40 | |
| 2022YZ | 3 | STS3-1 | 6 | SSR6-20 | 13.98 | 0.21 | 2.46 | |
| 2022YZ | 3 | STS3-1 | 9 | SSR9-3 | 13.69 | 0.17 | 2.43 | |
| 2022YZ | 3 | STS3-1 | 11 | SSR11-235 | 13.74 | 0.17 | 2.43 | |
| BRA | 2020LS | 5 | SSR5-241 | 7 | SSR7-145 | 11.36 | −0.50 | 8.78 |
| 2020LS | 5 | STS5-1 | 7 | SSR7-145 | 11.21 | −0.57 | 8.69 | |
| 2020LS | 7 | SSR7-145 | 8 | SSR8-390 | 10.09 | −0.38 | 7.95 |
| Chr. | Marker | Position (cm) | QTLs |
|---|---|---|---|
| 1 | STS1-12 | 132.60 | qBRL1-1 (2020LS), qBRP1 (2022YZ) |
| 1 | SSR1-104 | 167.85 | qBRL1-2 (2022YZ), qBLWR1-2 (2020LS, 2021YZ, 2022YZ) |
| 2 | SSR2-24 | 92.20 | qBRL2-1 (2022YZ), qBRW2 (2022YZ), qBRP2 (2020LS, 2021YZ, 2022YZ), qBRA2 (2020LS, 2021YZ, 2022YZ), qBRGW2 (2020LS) |
| 3 | SSR3-120 | 41.63 | qBRW3 (2021YZ, 2022YZ), qBLWR3-2 (2020LS, 2021YZ, 2022YZ), qBRT3 (2021YZ) |
| 3 | STS3-1 | 113.84 | qBRL3-2 (2020LS, 2022YZ), qBLWR3-3 (2021YZ), qBRP3-1 (2020LS, 2022YZ) |
| 3 | SSR3-337 | 132.32 | qBRL3-3 (2022YZ), qBRP3-2 (2022YZ), qBWC3 (2021YZ) |
| 3 | SSR3-36 | 147.67 | qBRL3-4 (2021YZ), qBRP3-3 (2021YZ), qBRA3-2 (2021YZ, 2022YZ) |
| 5 | SSR5-1 | 9.60 | qBRL5-1 (2022YZ), qBRP5 (2022YZ) |
| 6 | SSR6-111 | 6.25 | qBRT6 (2020LS), qBRGW6 (2020LS) |
| 6 | SSR6-71 | 38.71 | qBTV6-2 (2021YZ), qBWC6 (2021YZ) |
| 7 | SSR7-145 | 20.13 | qBRW7 (2020LS), qBRP7 (2020LS), qBRA7 (2020LS), qBWC7-1 (2020LS) |
| 7 | SSR7-76 | 56.11 | qBLWR7-1 (2020LS), qBWC7-2 (2022YZ) |
| 7 | SSR7-174 | 99.43 | qBLWR7-2 (2021YZ), qBRT7 (2020LS, 2021YZ), qBTV7 (2020LS, 2021YZ, 2022YZ) |
| 8 | SSR8-417 | 90.25 | qBRW8 (2020LS, 2021YZ), qBRA8 (2020LS, 2021YZ), qBRGW8 (2020LS) |
| 8 | SSR8-390 | 111.97 | qBRL8 (2020LS, 2022YZ), qBRP8 (2020LS, 2021YZ, 2022YZ) |
| 12 | SSR12-107 | 66.43 | qBRW12 (2022YZ), qBRP12 (2020LS), qBRA12 (2020LS, 2022YZ) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Y.; Hong, L.; Tao, T.; Guo, Q.; Yang, Q.; Zhang, M.; Ren, X.; Jin, S.; Cai, X.; Gao, J. Mapping of QTLs for Brown Rice Traits Based on Chromosome Segment Substitution Line in Rice (Oryza sativa L.). Agriculture 2023, 13, 928. https://doi.org/10.3390/agriculture13050928
Leng Y, Hong L, Tao T, Guo Q, Yang Q, Zhang M, Ren X, Jin S, Cai X, Gao J. Mapping of QTLs for Brown Rice Traits Based on Chromosome Segment Substitution Line in Rice (Oryza sativa L.). Agriculture. 2023; 13(5):928. https://doi.org/10.3390/agriculture13050928
Chicago/Turabian StyleLeng, Yujia, Lianmin Hong, Tao Tao, Qianqian Guo, Qingqing Yang, Mingqiu Zhang, Xinzhe Ren, Sukui Jin, Xiuling Cai, and Jiping Gao. 2023. "Mapping of QTLs for Brown Rice Traits Based on Chromosome Segment Substitution Line in Rice (Oryza sativa L.)" Agriculture 13, no. 5: 928. https://doi.org/10.3390/agriculture13050928






