Toxicity of Insecticides in the Adult and Larva Olive Fruit Fly, after Estimation of the Dislodgeable Foliar and Fruit Residues in Olive Trees by LC-MS/MS and GC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Application
2.1.1. Experimental Area-Olive Orchard
2.1.2. Application of the Insecticides in Olive Trees
2.2. Sampling
2.2.1. Sampling of Olive Leaves and Fruit to Assess Insecticide Residues
2.2.2. Dislodgeable Foliar Residue (DFR) Sampling
2.3. Analytical Procedure
2.3.1. Chemicals, Reagents, and Standard Solutions
2.3.2. Preparation of Standard Solutions
2.3.3. Washing Residue DFR Technique and Sample Preparation
2.3.4. Instrumentation
2.3.5. Validation Design
2.3.6. Confirmation Criteria
2.4. Toxicity Assessment
2.4.1. Toxicity Assessment in Olive Fruit Fly Adults-Leaf Trial
2.4.2. Toxicity Assessment in Olive Fruit Fly Larval Stages-Olive Fruit Trial
2.5. Statistical Analysis
3. Results
3.1. Analytical Method Performance
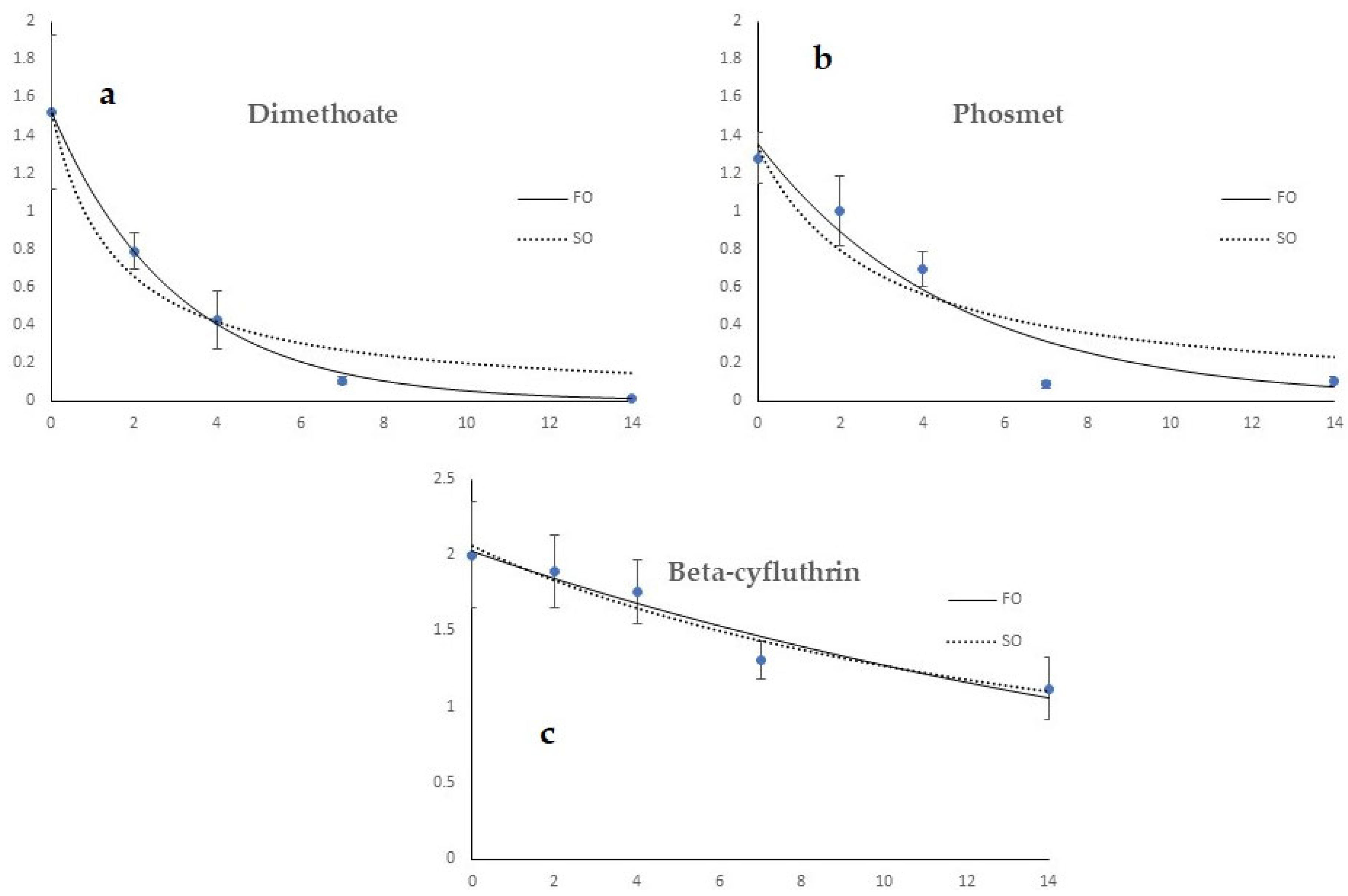
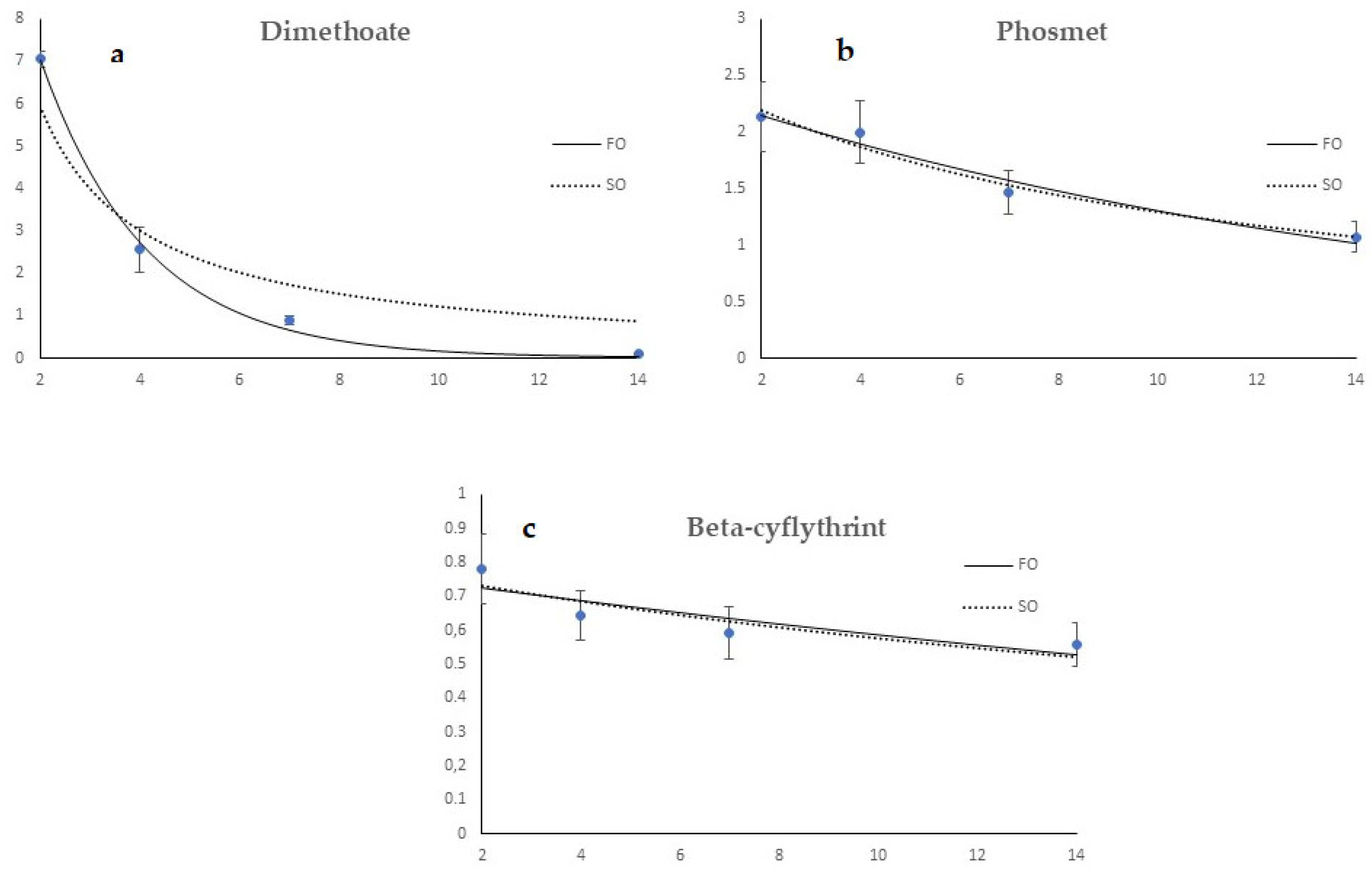
3.2. Dislodgeable Foliar Residue Results
3.3. Assessment of Toxicity of Olive Fly Adults and Immature Stages
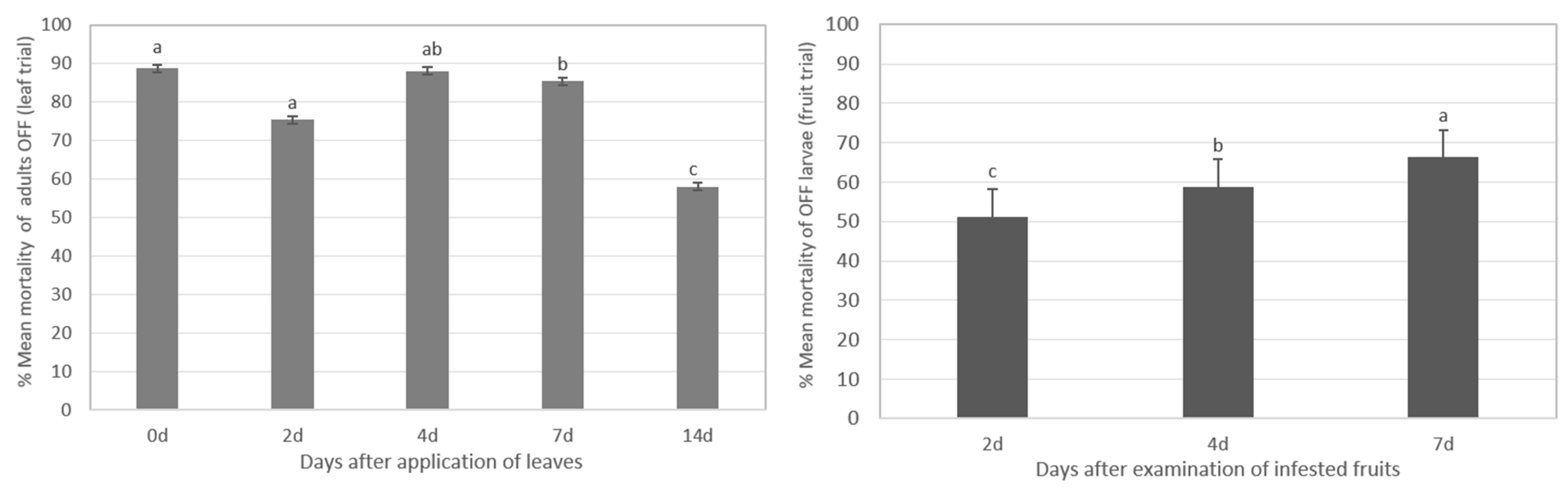
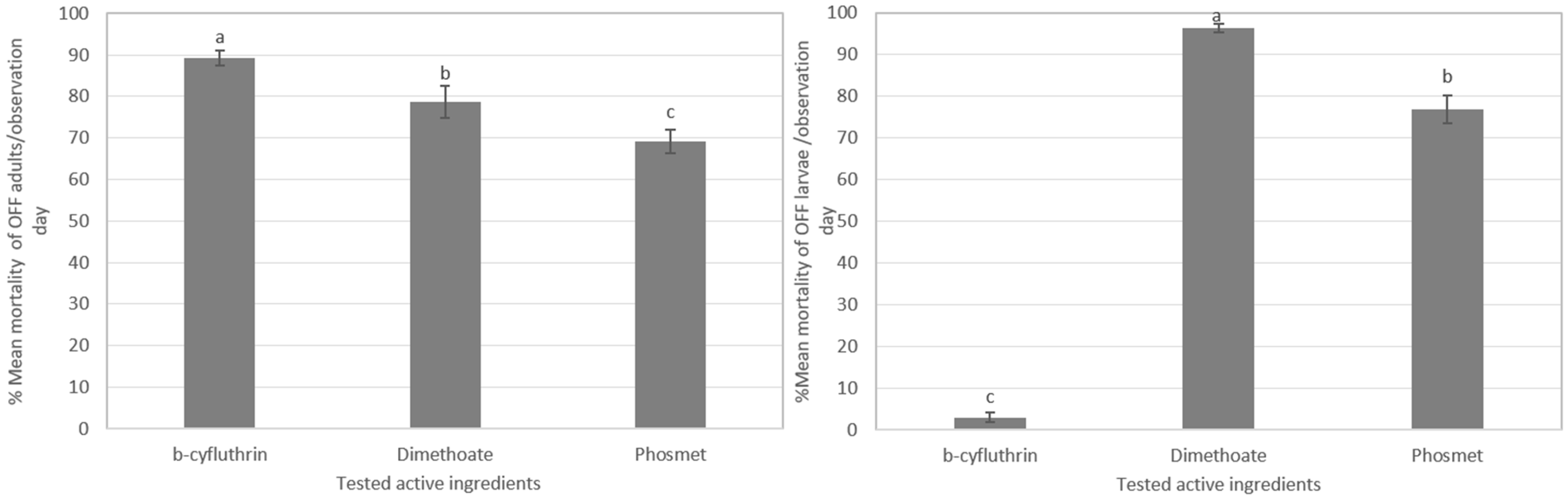
3.3.1. Assessment of Toxicity of Olive Fly Adults
3.3.2. Toxicity Assessment in Olive Fruit Fly Larval Stages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazomenos, B.; Pantazi-Mazomenou, A.; Stefanou, D. Attract and kill of the olive fruit fly Bactrocera oleae in Greece as a part of an integrated control system. IOBC/WPRS 2002, 25, 137–146. [Google Scholar]
- Haniotakis, G.E. Olive pest control: Present status and prospects. In Proceedings of the 1st European Meeting of IOBC/WPRS, Chania, Greece, 29–31 May 2003; pp. 1–9. [Google Scholar]
- Varikou, K.; Garantonakis, N.; Birouraki, A.; Gkilpathi, D.; Kapogia, E. Refreshing bait spots in an olive orchard for the control of Bactrocera oleae (Diptera: Tephritidae). Crop Prot. 2017, 92, 153–159. [Google Scholar] [CrossRef]
- Mangan, R.L. Effects of bait age and prior protein feeding on cumulative tie-dependent mortality of Anastrepha ludens (Diptera: Tephritidae) exposed to GF-120 spinosad baits. J. Econ. Entomol. 2009, 102, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Allwood, A.J.; Vueti, E.T.; Leblanc, L.; Bull, R. Chapter: Eradication of introduced Bactrocera species (Diptera: Tephritidae) in Nauru using male annihilation and protein bait application techniques. In Turning the Tide: The Eradication of Invasive Species; Veight, C.R., Clout, M.N., Eds.; Occasional Papers of the IUCN Species Survival Commission. No. 27; IUCN: Gland, Switzerland, 2002; pp. 19–25. [Google Scholar]
- Dauterman, W.C.; Viado, G.B.; Casida, J.E.; O’Brien, R.D. Insecticide residues, persistence of dimethoate and metabolites following foliar application to plants. J. Agric. Food Chem. 1960, 8, 115–119. [Google Scholar] [CrossRef]
- Tomlin, C. The Pesticide Manual, 13th ed.; British Crop Protection Council: Hampshire, UK, 2003; p. 1457. [Google Scholar]
- EURL-Datapool, European Reference Laboratories for the Pesticide Residues. 2019. Available online: https://www.eurl-pesticides-datapool.eu/Member/Compound (accessed on 16 August 2019).
- Smith, G.J. Pesticide Use and Toxicology in Relation to Wildlife: Organophosphorus and Carbamate Compounds; Smolley, C.K., Ed.; United States Department of the Interior, Fish and Wildlife Service: Boca Raton, FL, USA, 1993; p. 510.
- FAO—Food and Agriculture Organization of the United Nations. Pesticide Residues in Food; Paper 77; FAO Plant Production and Protection: Rome, Italy, 1986. [Google Scholar]
- FAO—Food and Agriculture Organization of the United Nations. FAO Specifications and Evaluations for Plant Protection Products. Beta-cyfluthrin. 2017. Available online: https://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/Specs/beta-cyfluthrin_2017_07_07.pdf (accessed on 20 May 2021).
- Kakani, E.G.; Mathiopoulos, K.D. Organophosphate resistance-related mutations in the acetylcholinesterase gene of Tephritidae. J. Appl. Entomol. 2008, 132, 762–771. [Google Scholar] [CrossRef]
- Amvrazi, E.G.; Albanis, T.A. Pesticide residue assessment in different types of olive oil and preliminary exposure assessment of Greek consumers to the pesticide residues detected. Food Chem. 2009, 113, 253–261. [Google Scholar] [CrossRef]
- Iwata, Y.; Knaak, J.B.; Spear, R.C.; Foster, R.J. Worker reentry into pesticide treated crops. I. Procedures for the determination of dislodgeable pesticide residues on foliage. Bull. Environ. Contam. Toxicol. 1977, 18, 649. [Google Scholar] [CrossRef]
- Holland, P.T. Glossary of terms relating to pesticides. Pure Appl. Chem. 1996, 68, 1167–1193. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Tsakirakis, A.N.; Richard Glass, C.; Charistou, A.N.; Anastassiadou, P.; Gerritsen-Ebben, R.; Machera, K. Assessment of field re-entry exposure to pesticides: A dislodgeable foliar residue study. Sci. Total Environ. 2017, 596–597, 178–186. [Google Scholar] [CrossRef]
- Vojislava Bursić, V.; Vuković, G.; Cara, M.; Kostić, M.; Stojanović, T.; Petrović, A.; Puvača, N.; Puvača, D.; Konstantinović, B. Plant Protection Products Residues Assessment in the Organic and Conventional Agricultural Production. Sustainability 2021, 13, 1075. [Google Scholar] [CrossRef]
- Varikou, K.; Garantonakis, N.; Marketaki, M.; Charalampous, A.; Anagnostopoulos, C.; Bempelou, E. Residual degradation and toxicity of insecticides against Bactrocera oleae. Environ. Sci. Pollut. Res. 2018, 25, 479–489. [Google Scholar] [CrossRef]
- Comission Implementing Regulation (EU). 2019/1090 of 26 June 2019 Concerning the Non-Renewal of Approval of the Active Substance Dimethoate, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Official Journal of the European Union L 164/28-51. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32019R1090&from=EN (accessed on 24 May 2020).
- Commission Implementing Regulation (EU). 2020/892 of 29 June 2020 Concerning the Non-Renewal of the Approval of the Active Substance Beta-Cyfluthrin, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Official Journal of the European Union L 206/1-7. 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R0892&from=EN (accessed on 7 December 2020).
- Varikou, K.; Kasiotis, K.; Bempelou, E.; Manea-Karga, E.; Anagnostopoulos, C.; Charalampous, A.; Garantonakis, N.; Birouraki, A.; Hatjina, F.; Machera, K. A Pesticide Residues Insight on Honeybees, Bumblebees and Olive fruits after Cover and Bait Spray Applications against the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae). Insects 2020, 11, 855. [Google Scholar] [CrossRef]
- Sanz-Cortés, F.; Martínez-Calvo, J.; Badenes, M.L.; Bleiholder, H.; Hack, H.; Llacer, G.; Meier, U. Phenological growth stages of olive trees (Olea europea). Ann. Appl. Biol. 2002, 140, 151–157. [Google Scholar] [CrossRef]
- EPA—Environmental Protection Agency. Guidance for the Determination of Dislodgeable Foliar Residue. Report HS-1600. 27 November 1990. Revision No.1 20 February 2002. Available online: http://pesticidetruths.com/wp-content/uploads/2017/10/Reference-Dislodgeable-Residues-1990-11-27-Guidance-For-Dewtermination-California-EPA.pdf (accessed on 8 June 2019).
- Goh, K.S.; Edmiston, S.; Maddy, K.T.; Meinders, D.D.; Margetich, S. Dissipation of dislodgeable residue of chlorpyrifos and dichlorvos on turf. Bull. Environ. Contam. Toxicol. 1986, 37, 27–32. [Google Scholar] [CrossRef]
- European Commission. Guidance SANTE/11312/2021—Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed; SANTE. SANTE/11312/2021; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Utture, S.C.; Banerjee, K.; Dasgupta, S.; Patil, S.H.; Jadhav, M.R.; Wagh, S.S.; Kolekar, S.S.; Anuse, M.A.; Adsule, P.G. Dissipation and distribution behavior of azoxystrobin, carbendazim, and difenoconazole in pomegranate fruits. J. Agric. Food Chem. 2011, 59, 7866–7873. [Google Scholar] [CrossRef]
- Hwang, K.W.; Bang, W.S.; Jo, H.W.; Moon, J.K. Dissipation and removal of the etofenprox residue during processing in spring onion. J. Agric. Food Chem. 2015, 63, 6675–6680. [Google Scholar] [CrossRef]
- Vapa Tankosić, J.; Puvača, N.; Giannenas, I.; Tufarelli, V.; Ignjatijević, S. Food Safety Policy in the European Union. J. Agron. Technol. Eng. Manag. 2022, 5, 712–717. [Google Scholar] [CrossRef]
- IUPAC—International Union of Pure and Applied Chemistry. Agrochemicals, Residue Analytical Methods; IUPAC: Zurich, Switzerland, 2010. [Google Scholar]
- Fenner, K.; Canonica, S.; Wackett, L.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [Green Version]
- EFSA (European Food Safety Authority). Peer review of the pesticide risk assessment of the activesubstance beta-cyfluthrin. EFSA J. 2020, 18, 6058, Erratum in EFSA J. 2020, 16, 5348. [Google Scholar] [CrossRef]
- Bissel, S.; Grahham, E.; Marade, J.; Troiano, J. Comparison of Sampling Methods for Determination of Pesticide Residue on Leaf Surfaces; Report No. HS-1583; California Department of Food and Agriculture: Sacramento, CA, USA, 1990.
- Evaristo, A.; Casadei De Baptista, G. Dislodgeable residues of methamidophos in staked tomatoes. Sci. Agric. 2002, 59, 469–473. [Google Scholar] [CrossRef] [Green Version]
- Krieger, R.; Vega, H.; Chen, Z.; Chen, L.; Lopez, T.; Sankaran, G.; Wong, Y.; Gee, D.; Coehlo, J.; Meyer, G.; et al. Green Risk Reduction! Enhancing Consumer Confidence in the Production and Marketing of California Strawberries; 2009–2010 Research Projects; University of California: Berkeley, CA, USA, 2010. [Google Scholar]
- Yang, T.; Doherty, J.; Zhao, B.; Kinchla, A.J.; Clark, J.A.; He, L. Effectiveness of Commercial and Homemade Washing Agents in Removing Pesticide Residues on and in Apples. J. Agric. Food Chem. 2017, 65, 9744–9752. [Google Scholar] [CrossRef] [PubMed]
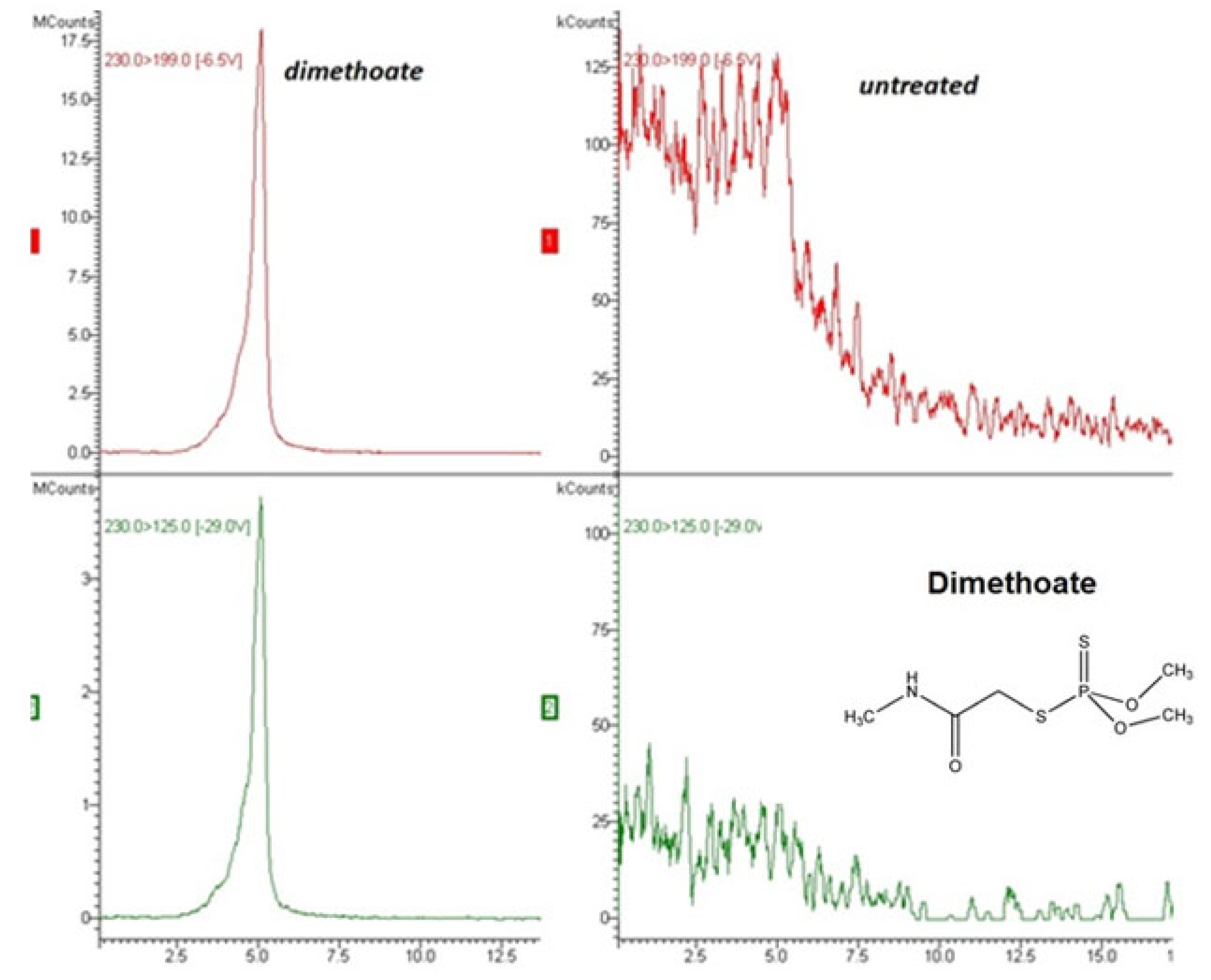
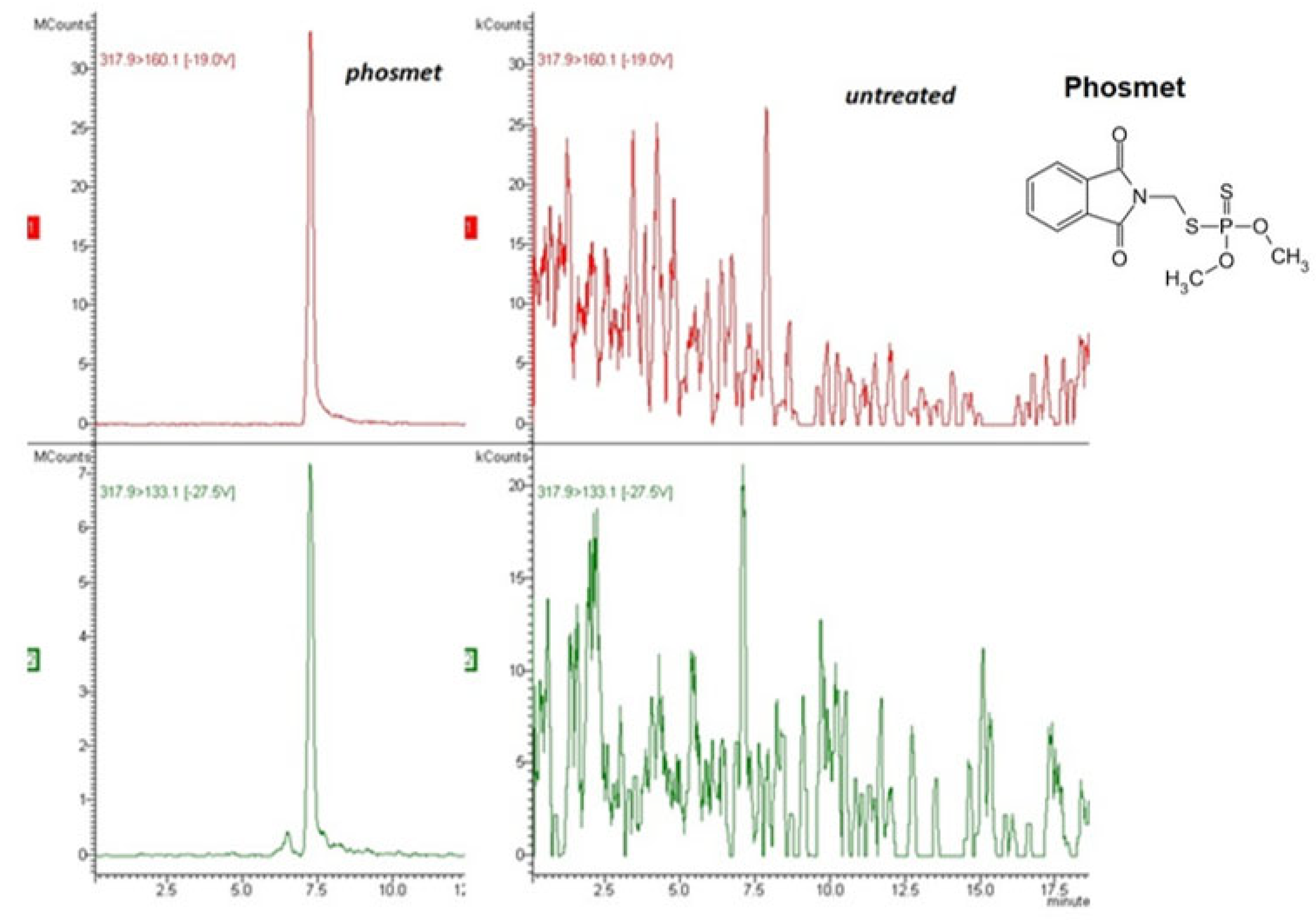


| Compound | Fortification Level (mg/kg) | Mean Recovery (%) (n = 6) | RSD (%) (n = 6) | Equation of the Calibration Curve | Correlation Coefficient Squared (R2) |
|---|---|---|---|---|---|
| Dimethoate | 0.05 | 114 | 5.67 | y = 5 × 108 x + 6 × 106 | 0.999 |
| 0.1 | 72 | 7.42 | |||
| 0.5 | 112 | 2.23 | |||
| 1 | 100 | 8.65 | |||
| Phosmet | 0.05 | 99 | 6.7 | y = 4 × 107 x + 534,035 | 0.996 |
| 0.1 | 113 | 11.22 | |||
| 0.5 | 97 | 17.4 | |||
| 1 | 119 | 6.21 | |||
| Beta-cyfluthrin | 0.05 | 74 | 6.44 | y = 4 × 108 x + 643 | 0.998 |
| 0.1 | 87 | 3.76 | |||
| 0.5 | 94 | 7.01 | |||
| 1 | 79 | 8.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bempelou, E.; Varikou, K.; Anagnostopoulos, C.; Charalampous, A.; Samari, N.; Economou, L.; Garantonakis, N.; Liapis, K. Toxicity of Insecticides in the Adult and Larva Olive Fruit Fly, after Estimation of the Dislodgeable Foliar and Fruit Residues in Olive Trees by LC-MS/MS and GC-MS/MS. Agriculture 2023, 13, 543. https://doi.org/10.3390/agriculture13030543
Bempelou E, Varikou K, Anagnostopoulos C, Charalampous A, Samari N, Economou L, Garantonakis N, Liapis K. Toxicity of Insecticides in the Adult and Larva Olive Fruit Fly, after Estimation of the Dislodgeable Foliar and Fruit Residues in Olive Trees by LC-MS/MS and GC-MS/MS. Agriculture. 2023; 13(3):543. https://doi.org/10.3390/agriculture13030543
Chicago/Turabian StyleBempelou, Eleftheria, Kyriaki Varikou, Chris Anagnostopoulos, Angeliki Charalampous, Nikolia Samari, Leonidas Economou, Nikolaos Garantonakis, and Konstantinos Liapis. 2023. "Toxicity of Insecticides in the Adult and Larva Olive Fruit Fly, after Estimation of the Dislodgeable Foliar and Fruit Residues in Olive Trees by LC-MS/MS and GC-MS/MS" Agriculture 13, no. 3: 543. https://doi.org/10.3390/agriculture13030543





