In Vivo Hypolipidemic Effects and Antioxidant Capacity of Pinus morrisonicola Hay Extracts by Supercritical Carbon Dioxide Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. In Vivo Treatment and Analysis
2.3.1. Treatments
2.3.2. Analysis
2.4. Statistical Analysis
3. Results and Discussion
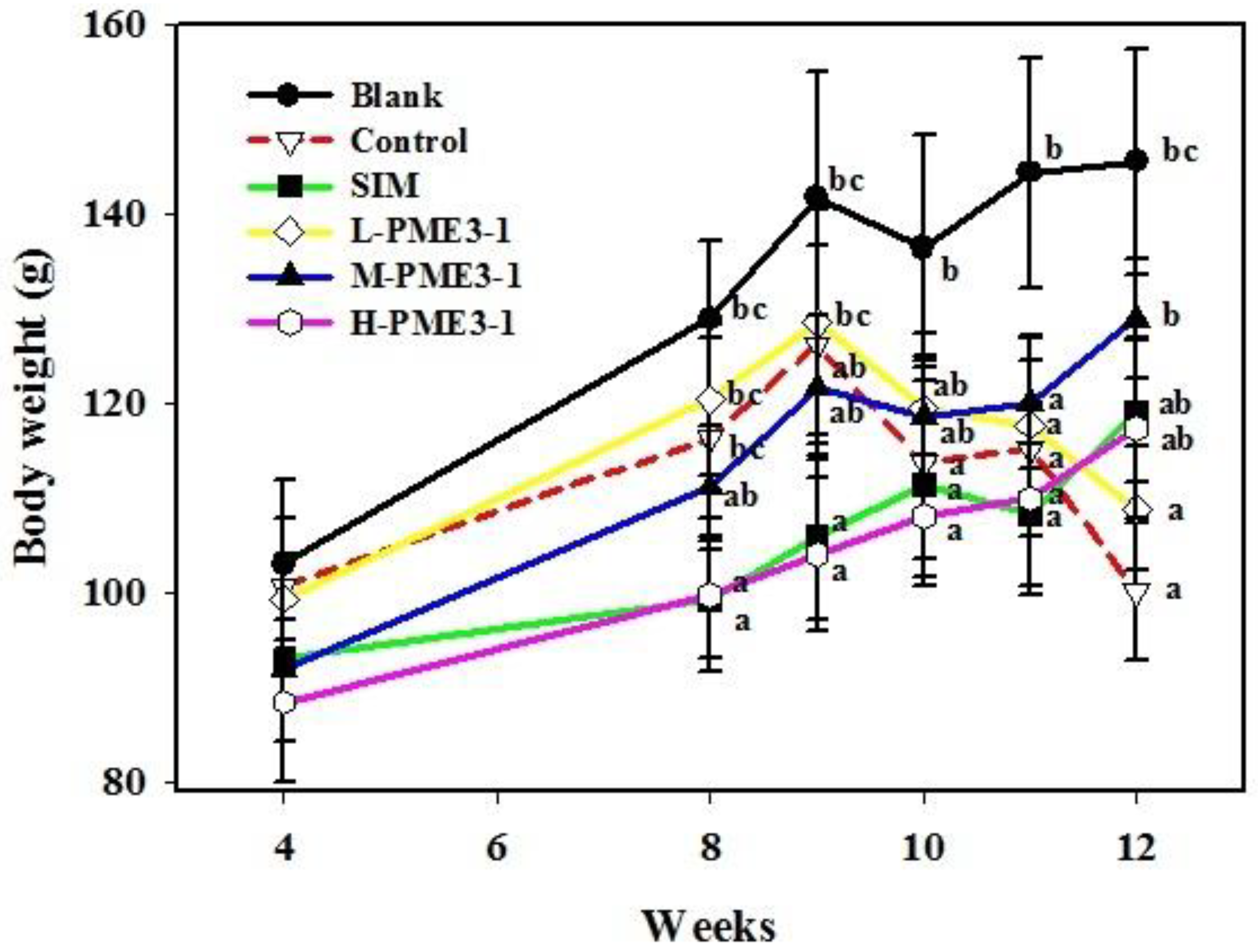
3.1. Weight Change of Hamster Bodies and Organ
3.2. Serum Analysis
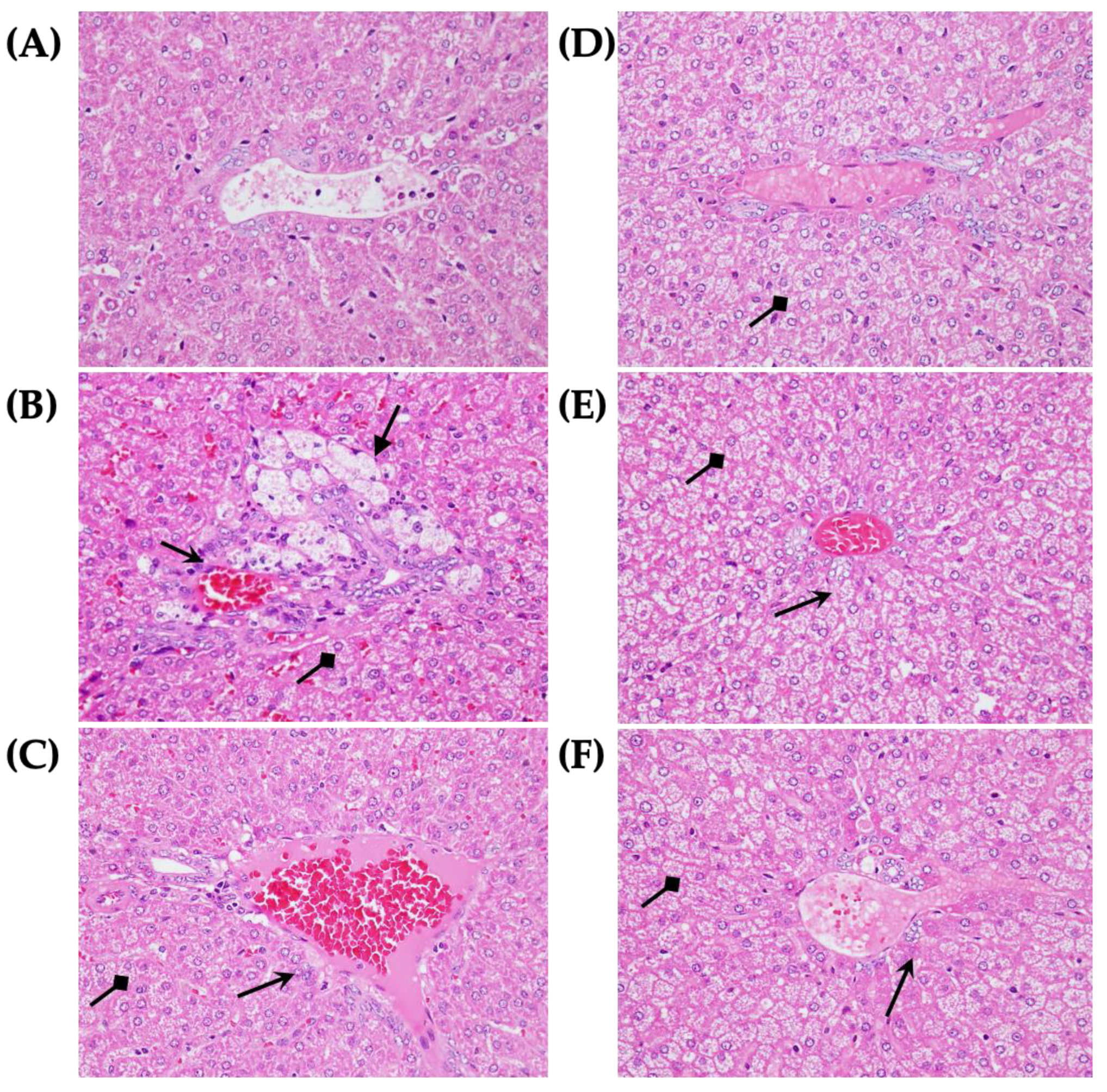
3.3. Morphological and Pathological Slices
3.4. Lipid and Cholesterol Content of Liver Tissue and Feces
3.5. Antioxidant Capacity and Cholesterol Synthesis Inhibition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Grundy, S.M. Cholesterol and coronary heart disease: A new era. JAMA 1986, 256, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.N.; Frei, B.; Vita, J.A.; Keaney, J.F., Jr. Antioxidants and atherosclerotic heart disease. N. Engl. J. Med. 1997, 337, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.J.; Xu, R.X.; Sun, J.; Tang, Y.; Li, J.J. Enhanced circulating PCSK9 concentration by berberine through SREBP-2 pathway in high fat diet-fed rats. J. Transl. Med. 2014, 12, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Maron, D.J.; Fazio, S.; Linton, M.F. Current perspectives on statins. Circulation 2000, 101, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Sudhop, T.; Reber, M.; Tribble, D.; Sapre, A.; Taggart, W.; Gibbons, P.; Musliner, T.; von Bergmann, K.; Lütjohann, D. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J. Lipid Res. 2009, 50, 2117–2123. [Google Scholar] [CrossRef] [Green Version]
- Hsu, T.Y.; Sheu, S.C.; Liaw, E.T.; Wang, T.C.; Lin, C.C. Anti-oxidant activity and effect of Pinus morrisonicola Hay. on the survival of leukemia cell line U937. Phytomedicine 2005, 12, 663–669. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Huang, D.W.; Hsu, C.L.; Fu, T.Y.C. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2008, 46, 175–185. [Google Scholar] [CrossRef]
- Chiu, H.F.; Wang, H.M.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Anti-inflammatory properties of fermented pine (Pinus morrisonicola Hay.) needle on lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells. J. Food Biochem. 2019, 43, e12994. [Google Scholar] [CrossRef]
- Nyman, B.F. Protein-proanthocyanidin interactions during extraction of Scots pine needles. Phytochemistry 1985, 24, 2939–2944. [Google Scholar] [CrossRef]
- Fang, J.M.; Chang, C.F.; Cheng, Y.S. Flavonoids from Pinus morrisonicola. Phytochemistry 1987, 26, 2559–2561. [Google Scholar] [CrossRef]
- Fang, J.M.; Su, W.C.; Cheng, Y.S. Flavonoids and stilbenes from armand pine. Phytochemistry 1988, 27, 1395–1397. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, Z.; Ding, B. Study on the antimutagenic effect of pine needle extract. Mutat. Res. Lett. 1995, 347, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Huang, T.C. Volatile and nonvolatile constituents and antioxidant capacity of oleoresins in three Taiwan citrus varieties as determined by supercritical fluid extraction. Molecules 2016, 21, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, J.A.P.; Pereira, A.P.; Mendes, R.L.; Palavra, A.M.F. Supercritical carbon dioxide extraction of Foeniculum vulgare volatile oil. Flavour Fragr. J. 2003, 18, 316–319. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Carretero, M.E.; López-Pérez, J.L.; Abad, M.J.; Bermejo, P.; Tillet, S.; Israel, A.; Noguera-P, B. Preliminary study of the anti-inflammatory activity of hexane extract and fractions from Bursera simaruba (Linneo) Sarg.(Burseraceae) leaves. J. Ethnopharmacol. 2008, 116, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Lin, L.Y.; Yu, T.H.; Peng, R.Y. Hypolipidemic and antioxidant activity of mountain celery (Cryptotaenia japonica Hassk) seed essential oils. J. Agric. Food Chem. 2008, 56, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Jung, H.A.; Kang, S.S.; Hwang, G.S.; Choi, J.S. A new abietic acid-type diterpene glucoside from the needles of Pinus densiflora. Arch. Pharmacal Res. 2009, 32, 1699–1704. [Google Scholar] [CrossRef]
- Cheng, M.C.; Chang, W.H.; Chen, C.W.; Li, W.W.; Tseng, C.Y.; Song, T.Y. Antioxidant properties of essential oil extracted from Pinus morrisonicola hay needles by supercritical fluid and identification of possible active compounds by GC/MS. Molecules 2015, 20, 19051–19065. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.Y.; Chuang, C.H.; Chen, H.C.; Yang, K.M. Lime (Citrus aurantifolia (Christm.) Swingle) essential oils: Volatile compounds, antioxidant capacity, and hypolipidemic effect. Foods 2019, 8, 398. [Google Scholar] [CrossRef] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.J. A colorimetric method for estimating serum triglycerides. Clin. Chim. Acta 1968, 22, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Sperry, W.M.; Webb, M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J. Biol. Chem. 1950, 187, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, P.J. The influence of picolines on glutathione transferase activity and subunit composition in human liver derived Hep G2 cells. Biochem. Pharmacol. 1994, 48, 1976–1978. [Google Scholar] [CrossRef]
- Mohandas, J.; Marshall, J.J.; Duggin, G.G.; Horvath, J.S.; Tiller, D.J. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: Possible implications in analgesic nephropathy. Biochem. Pharmacol. 1984, 33, 1801–1807. [Google Scholar] [CrossRef]
- Richard, M.J.; Guiraud, P.; Meo, J.; Favier, A. High-performance liquid chromatographic separation of malondialdehyde—Thiobarbituric acid adduct in biological materials (plasma and human cells) using a commercially available reagent. J. Chromatogr. B Biomed. Sci. Appl. 1992, 577, 9–18. [Google Scholar] [CrossRef]
- Ko, W.S.; Guo, C.H.; Yeh, M.S.; Lin, L.Y.; Hsu, G.S.W.; Chen, P.C.; Luo, M.C.; Lin, C.Y. Blood micronutrient, oxidative stress, and viral load in patients with chronic hepatitis C. World J. Gastroenterol. WJG 2005, 11, 4697. [Google Scholar] [CrossRef]
- Spady, D.K.; Turley, S.D.; Dietschy, J.M. Dissociation of hepatic cholesterol synthesis from hepatic low-density lipoprotein uptake and biliary cholesterol saturation in female and male hamsters of different ages. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1983, 753, 381–392. [Google Scholar] [CrossRef]
- Lee, C.L.; Tsai, T.Y.; Wang, J.J.; Pan, T.M. In vivo hypolipidemic effects and safety of low dosage Monascus powder in a hamster model of hyperlipidemia. Appl. Microbiol. Biotechnol. 2006, 70, 533–540. [Google Scholar] [CrossRef]
- Newton, J.L. Systemic symptoms in non-alcoholic fatty liver disease. Dig. Dis. 2010, 28, 214–219. [Google Scholar] [CrossRef]
- Matos, S.L.; Paula, H.D.; Pedrosa, M.L.; Santos, R.C.D.; Oliveira, E.L.D.; Chianca Júnior, D.A.; Silva, M.E. Dietary models for inducing hypercholesterolemia in rats. Braz. Arch. Biol. Technol. 2005, 48, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Hartvigsen, K.; Binder, C.J.; Hansen, L.F.; Rafia, A.; Juliano, J.; Hörkkö, S.; Steinberg, D.; Palinski, W.; Witztum, J.L.; Li, A.C. A diet-induced hypercholesterolemic murine model to study atherogenesis without obesity and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 878–885. [Google Scholar] [CrossRef]
- Gan, S.K.; Watts, G.F. Is adipose tissue lipolysis always an adaptive response to starvation?: Implications for non-alcoholic fatty liver disease. Clin. Sci. 2008, 114, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; He, Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara). J. Food Sci. 2012, 77, C824–C829. [Google Scholar] [CrossRef]
- Arslan, U.; Türkoglu, S.; Balcioglu, S.; Tavil, Y.; Karakan, T.; Çengel, A. Association between nonalcoholic fatty liver disease and coronary artery disease. Coron. Artery Dis. 2007, 18, 433–436. [Google Scholar] [CrossRef]
- Hamden, K.; Mnafgui, K.; Amri, Z.; Aloulou, A.; Elfeki, A. Inhibition of key digestive enzymes related to diabetes and hyperlipidemia and protection of liver-kidney functions by trigonelline in diabetic rats. Sci. Pharm. 2013, 81, 233–246. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.H.; Huang, M.T.; Huang, P.C. Sources of triacylglycerol accumulation in livers of rats fed a cholesterol-supplemented diet. Lipids 1995, 30, 527–531. [Google Scholar] [CrossRef]
- Guerin, M.; Dolphin, P.J.; Chapman, M.J. A new in vitro method for the simultaneous evaluation of cholesteryl ester exchange and mass transfer between HDL and apoB-containing lipoprotein subspecies. Identification of preferential cholesteryl ester acceptors in human plasma. Arterioscler. Thromb. J. Vasc. Biol. 1994, 14, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Kugiyama, K.; Doi, H.; Motoyama, T.; Soejima, H.; Misumi, K.; Kawano, H.; Nakagawa, O.; Yoshimura, M.; Ogawa, H.; Matsumura, T.; et al. Association of remnant lipoprotein levels with impairment of endothelium-dependent vasomotor function in human coronary arteries. Circulation 1998, 97, 2519–2526. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.H.; Tai, M.H.; Li, C.Y.; Chan, J.Y. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic. Biol. Med. 2006, 40, 2028–2039. [Google Scholar] [CrossRef]
- Castelli, W.P.; Garrison, R.J.; Wilson, P.W.; Abbott, R.D.; Kalousdian, S.; Kannel, W.B. Incidence of coronary heart disease and lipoprotein cholesterol levels: The Framingham Study. JAMA 1986, 256, 2835–2838. [Google Scholar] [CrossRef]
- Lee, E. Effects of powdered pine needle (Pinus densiflora seib et Zucc.) on serum and liver lipid composition and antioxidative capacity in rats fed high oxidized fat. J. Korean Soc. Food Sci. Nutr. 2003, 32, 926–930. [Google Scholar] [CrossRef]
- Park, K.J.; Kim, K.S.; Ahn, K.H.; Rhee, J.S. Pine needle oil and Korean medicinal herb complex protect hyperlipidemia and liver cell damage induced by alcohol. Korean J. Environ. Biol. 2003, 21, 410–414. [Google Scholar]
- Jeon, J.R.; Kim, J.Y. Effects of pine needle extract on differentiation of 3T3-L1 preadipocytes and obesity in high-fat diet fed rats. Biol. Pharm. Bull. 2006, 29, 2111–2115. [Google Scholar] [CrossRef] [Green Version]
- Murakami, S.; Kondo, Y.; Toda, Y.; Kitajima, H.; Kameo, K.; Sakono, M.; Fukuda, N. Effect of taurine on cholesterol metabolism in hamsters: Up-regulation of low density lipoprotein (LDL) receptor by taurine. Life Sci. 2002, 70, 2355–2366. [Google Scholar] [CrossRef]
- Yang, T.T.; Koo, M.W. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 1999, 66, 411–423. [Google Scholar] [CrossRef]
- Muramatsu, K.; Fukuyo, M.; Hara, Y. Effect of green tea catechins on plasma cholesterol level in cholesterol-fed rats. J. Nutr. Sci. Vitaminol. 1986, 32, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Sheth, S.G.; Gordon, F.D.; Chopra, S. Nonalcoholic steatohepatitis. Ann. Intern. Med. 1997, 126, 137–145. [Google Scholar] [CrossRef]
- Massy, Z.A.; Keane, W.F.; Kasiske, B.L. Inhibition of the mevalonate pathway: Benefits beyond cholesterol reduction? Lancet 1996, 347, 102–103. [Google Scholar] [CrossRef]

| Group | Blank | Control | SIM | L-PME3-1 | M-PME3-1 | H-PME3-1 |
|---|---|---|---|---|---|---|
| Feed composition (%) | ||||||
| Casein | 20 | 20 | 20 | 20 | 20 | 20 |
| Sucrose | 35 | 24.8 | 24.8 | 24.8 | 24.8 | 24.8 |
| Corn starch | 30 | 30 | 30 | 30 | 30 | 30 |
| Corn oil | 4 | 12 | 12 | 12 | 12 | 12 |
| Lard | 1 | 3 | 3 | 3 | 3 | 3 |
| Cholesterol | 0 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Ain 76 mineral | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 |
| Ain 76 vitamin | 1 | 1 | 1 | 1 | 1 | 1 |
| A-cellulose | 5 | 5 | 5 | 5 | 5 | 5 |
| D,L-methionine | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Supplementary addition (mg/kg b.w.) | ||||||
| SIM | - | - | 5.0 | 0.0 | 0.0 | 0.0 |
| PME3-1 | - | - | 0.0 | 0.2 | 1.0 | 5.0 |
| Treatment | Liver | Kidney | Spleen |
|---|---|---|---|
| Blank | 3.9 ± 0.8 c | 1.1 ± 0.3 b | 0.12 ± 0.08 a |
| Control | 6.3 ± 0.8 a | 2.1 ± 0.5 a | 0.04 ± 0.07 b |
| SIM | 3.5 ± 0.7 c | 1.2 ± 0.2 b | 0.04 ± 0.08 b |
| L-PME3-1 | 5.2 ± 1.0 b | 1.8 ± 0.6 a | 0.06 ± 0.09 b |
| M-PME3-1 | 4.0 ± 0.6 bc | 1.3 ± 0.3 b | 0.05 ± 0.07 b |
| H-PME3-1 | 3.4 ± 0.3 c | 1.4 ± 0.2 b | 0.10 ± 0.04 a |
| Treatment | TC 1 | TG 2 | HDL-C 3 | LDL-C 4 | LDL/HDL Ratio | Atherogenic Index 5 |
|---|---|---|---|---|---|---|
| Blank | 93.2 ± 18.3 c | 205.3 ± 22.7 bc | 53.7 ± 17.1 c | 11.2 ± 3.0 c | 0.20 | 0.75 |
| Control | 240.1 ± 17.8 a | 278.0 ± 90.1 a | 100.2 ± 15.9 a | 48.6 ± 9.1 a | 0.48 | 1.40 |
| SIM | 91.5 ± 23.6 c | 232.2 ± 46.1 ab | 50.5 ± 22.8 c | 13.6 ± 2.0 c | 0.26 | 0.82 |
| L-PME3-1 | 159.7 ± 12.9 b | 181.7 ± 98.2 bc | 79.3 ± 10.2 b | 20.1 ± 4.1 bc | 0.25 | 1.01 |
| M-PME3-1 | 139.0 ± 13.5 b | 184.0 ± 54.6 c | 72.4 ± 15.5 bc | 15.5 ± 3.0 c | 0.21 | 0.93 |
| H-PME3-1 | 85.3 ± 13.9 c | 181.3 ± 20.4 c | 65.0 ± 9.8 bc | 13.8 ± 3.0 c | 0.20 | 0.31 |
| Organ | Histopathology | Blank | Control | SIM | L-PME3-1 | M-PME3-1 | H-PME3-1 |
|---|---|---|---|---|---|---|---|
| Liver | Infiltration fat diffuse | 1.8 ± 0.4 b | 4.4 ± 0.7 a | 3.9 ± 0.3 a | 4.1 ± 0.6 a | 4.0 ± 0.7 a | 4.2 ± 0.4 a |
| Micro fatty change | 1.8 ± 0.4 ab | 4.4 ± 0.7 a | 3.9 ± 0.3 a | 4.1 ± 0.6 a | 4.0 ± 0.7 a | 4.2 ± 0.4 a | |
| Macro fatty change | N.D. | 0.4 ± 0.7 a | 0.4 ± 0.7 a | 0.4 ± 0.5 a | 0.1 ± 0.3 ab | 0.1 ± 0.3 ab | |
| Infiltration mononuclear cells | 0.3 ± 0.7 b | 0.8 ± 0.7 a | 1.1 ± 0.8 a | 1.0 ± 0.5 a | 0.9 ± 0.6 a | 0.7 ± 0.5 ab | |
| Necrosis | 0.6 ± 0.7 a | 0.5 ± 0.7 a | 1.0 ± 0.9 a | 0.3 ± 0.7 a | 0.2 ± 0.6 a | 0.4 ± 0.5 a | |
| Kidney | CPN 1 | 1.1 ± 0.0 c | 4.4 ± 1.3 a | 3.1 ± 2.0 bc | 4.4 ± 1.0 a | 3.8 ± 1.6 ab | 3.5 ± 1.5 ab |
| Organ | Parameter | Blank | Control | SIM | L-PME3-1 | M-PME3-1 | H-PME3-1 |
|---|---|---|---|---|---|---|---|
| Liver | TL 1 | 30.3 ± 2.9 f | 126.0 ± 4.3 a | 60.3 ± 6.9 e | 113 ± 2.0 b | 92.3 ± 3.2 c | 70.3 ± 4.6 de |
| TC 2 | 15.5 ± 0.9 c | 18.3 ± 0.4 a | 15.7 ± 0.9 c | 18.9 ± 1.2 a | 17.4 ± 0.6 bc | 16.0 ± 0.6 c | |
| TG 3 | 20.6 ± 0.6 b | 24.2 ± 0.7 a | 20.9 ± 1.0 b | 24.2 ± 1.0 a | 21.0 ± 0.6 b | 20.6 ± 0.9 b | |
| Dried fecal | TC 2 | 25.1 ± 3.0 a | 13.7 ± 0.4 e | 17.4 ± 1.6 bc | 14.5 ± 2.2 de | 14.7 ± 3.4 de | 16.1 ± 1.3 cd |
| TG 3 | 10.8 ± 1.3 d | 23.6 ± 2.0 c | 27.7 ± 1.0 ab | 27.0 ± 2.0 ab | 24.3 ± 4.9 bc | 29.0 ± 2.5 ab |
| Parameter | Blank | Control | SIM | L-PME3-1 | M-PME3-1 | H-PME3-1 |
|---|---|---|---|---|---|---|
| MDA 1 | 107.3 ± 48.1 c | 331.4 ± 93.7 ab | 206.2 ± 57.1 bc | 378.4 ± 79.0 a | 208.1 ± 56.2 abc | 269.7 ± 33.1 abc |
| GPx 2 | 144.8 ± 27.8 bc | 133.2 ± 29.6 c | 141.3 ± 29.0 bc | 138.1 ± 16.5 bc | 138.5 ± 16.3 bc | 156.3 ± 20.9 ab |
| GST 3 | 49.0 ± 6.47 a | 42.0 ± 7.03 a | 43.4 ± 7.1 a | 48.1 ± 6.3 a | 51.7 ± 8.0 a | 50.8 ± 10.7 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-J.; Cheng, M.-C.; Chen, H.-C.; Chen, C.-L.; Song, T.-Y. In Vivo Hypolipidemic Effects and Antioxidant Capacity of Pinus morrisonicola Hay Extracts by Supercritical Carbon Dioxide Extraction. Agriculture 2023, 13, 535. https://doi.org/10.3390/agriculture13030535
Lai Y-J, Cheng M-C, Chen H-C, Chen C-L, Song T-Y. In Vivo Hypolipidemic Effects and Antioxidant Capacity of Pinus morrisonicola Hay Extracts by Supercritical Carbon Dioxide Extraction. Agriculture. 2023; 13(3):535. https://doi.org/10.3390/agriculture13030535
Chicago/Turabian StyleLai, Ying-Jang, Ming-Ching Cheng, Hsin-Chun Chen, Chien-Lin Chen, and Tuzz-Ying Song. 2023. "In Vivo Hypolipidemic Effects and Antioxidant Capacity of Pinus morrisonicola Hay Extracts by Supercritical Carbon Dioxide Extraction" Agriculture 13, no. 3: 535. https://doi.org/10.3390/agriculture13030535





