Long-Term Studies of Wheat Leaf Rust in the North-Western Region of Russia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Puccinia triticina Virulence Analysis at the Seedling Stage
2.2. Field Resistance Study
3. Results
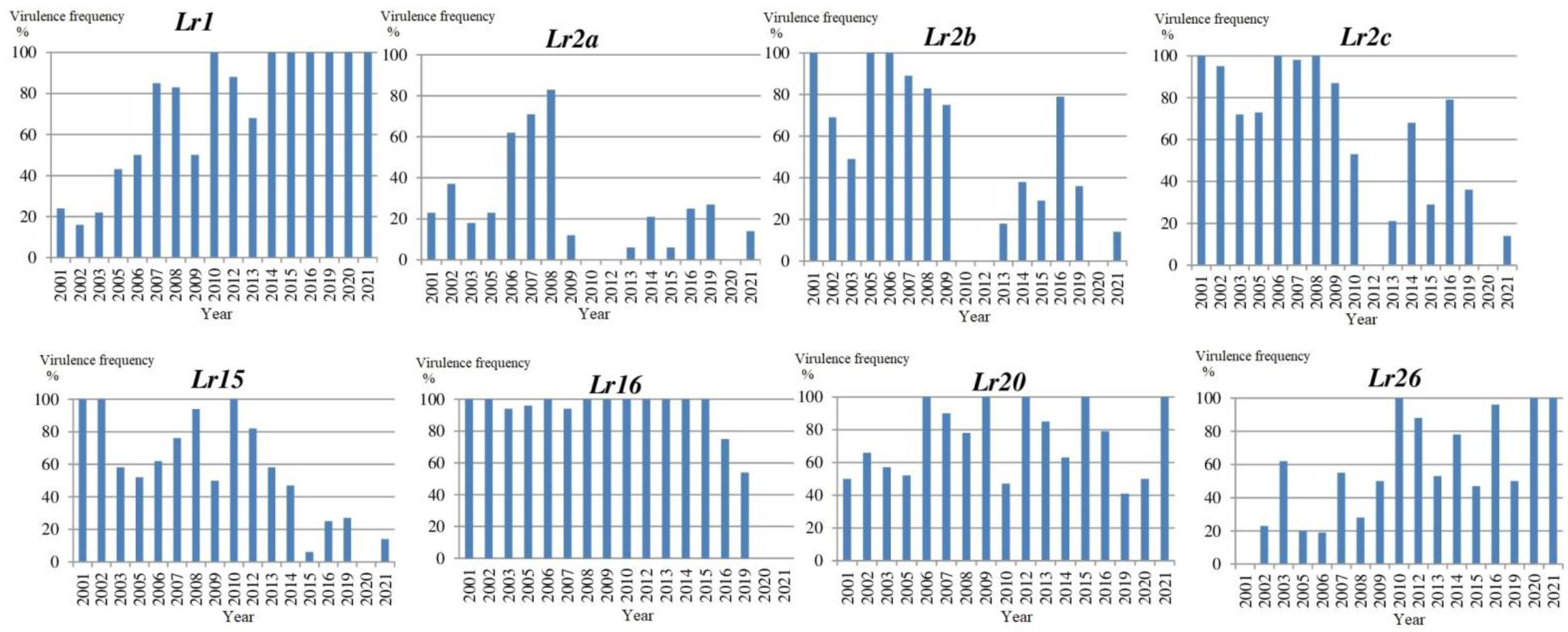
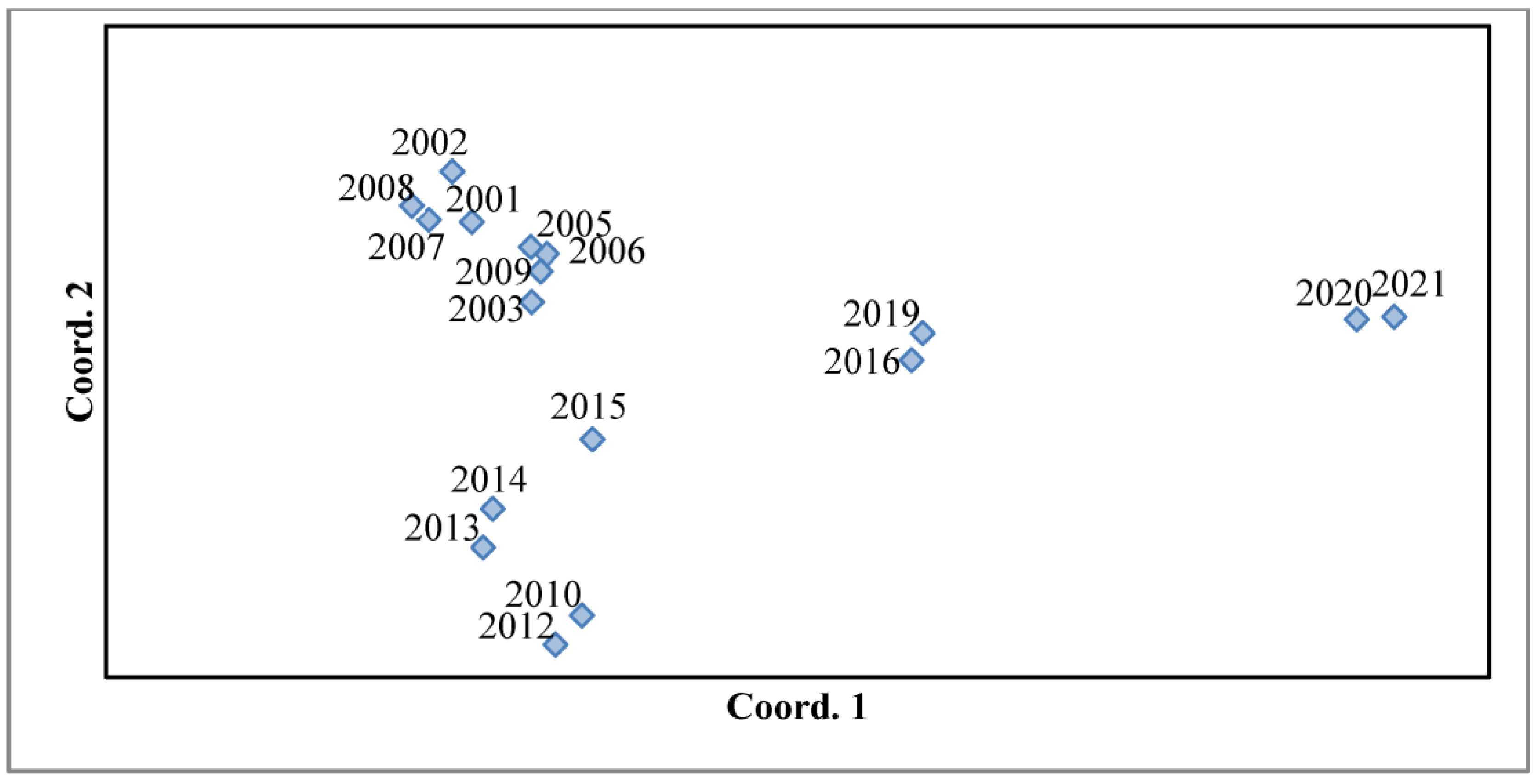
3.1. Puccinia triticina Response at the Seedling Stage
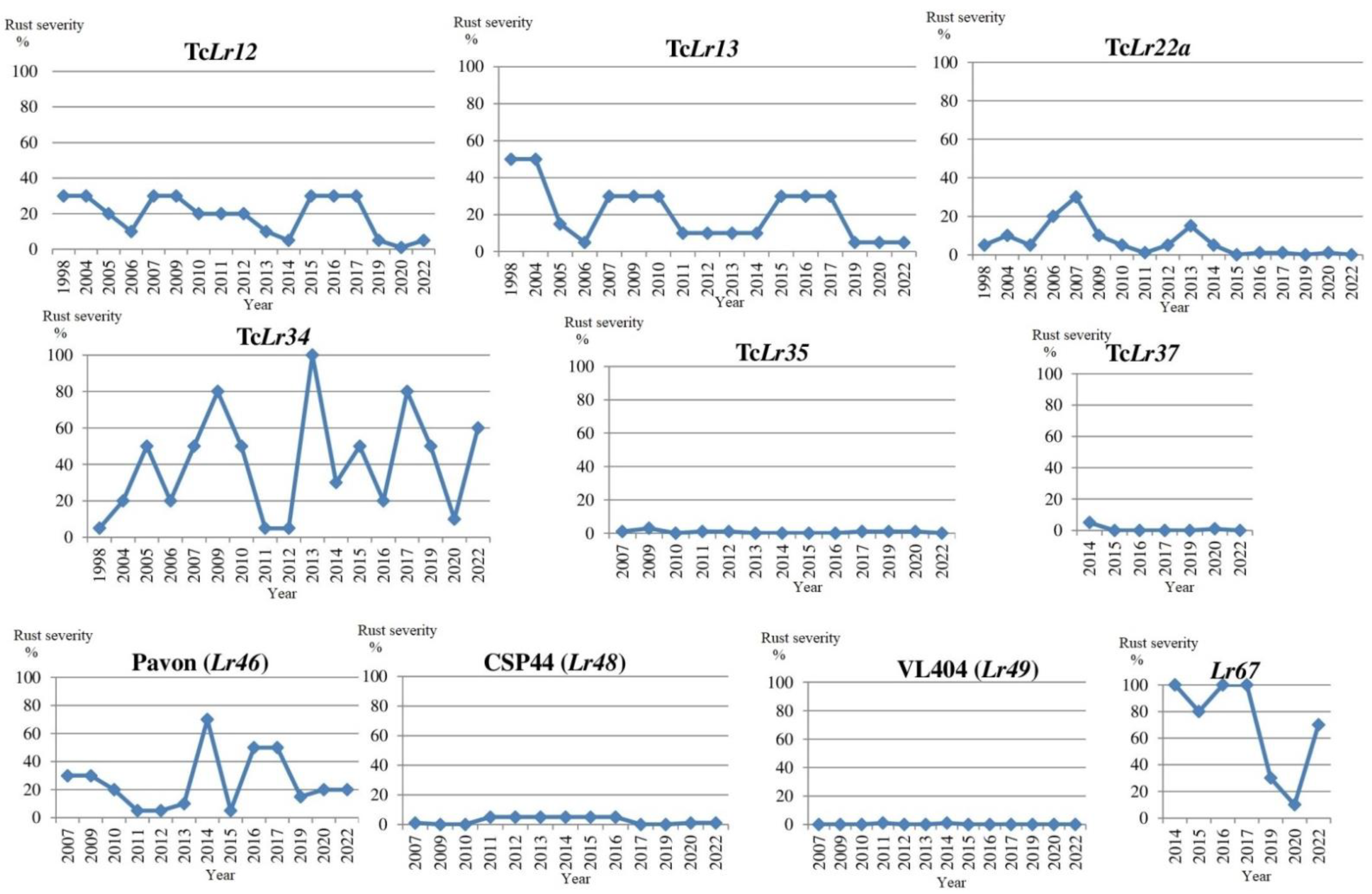
3.2. Lr Gene Effectiveness in the Field
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanin, S.S. Epiphytoties of Cereal Crop Diseases: Theory and Practice. Izbrannye trudy (Selected Works); All-Russian Research Institute of Phytopathology: Moscow, Russia, 2012; pp. 161–166. [Google Scholar]
- Sanin, S.S.; Nazarova, L.N.; Ibragimov, T.Z. The modern epidemiological situation of cereal rusts in European Russia. In Proceedings of the 11th International Cereal Rusts and Powdery Mildews Conference, Norwich, UK, 22–27 August 2004; pp. 2–25. [Google Scholar]
- McIntosh, R.A.; Dubcovsky, J.; Rogers, W.J.; Xia, X.C.; Raupp, W.J.V. Catalogue of gene symbols for wheat: 2022 supplement. Annu. Wheat Newsl. 2022, 68, 68–81. Available online: https://wheat.pw.usda.gov/ggpages/awn/68/AWN_VOL_68.pdf (accessed on 15 December 2022).
- Gultyaeva, E.; Shaydayuk, E.; Gannibal, P. Leaf Rust Resistance Genes in Wheat Cultivars Registered in Russia and Their Influence on Adaptation Processes in Pathogen Populations. Agriculture 2021, 11, 319. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Shaydayuk, E.L.; Kosman, E.G. Regional and temporal differentiation of virulence phenotypes of Puccinia triticina from common wheat in Russia during the period 2001–2018. Plant Pathol. 2020, 69, 860–871. [Google Scholar] [CrossRef]
- Lind, V.; Gultyaeva, E. Virulence of Puccinia triticina on winter wheat in Germany and the European regions of Russian Federation. J. Phytopathol. 2007, 155, 13–21. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Dmitriev, A.P.; Kosman, E. Regional diversity of Russian populations of Puccinia triticina in 2007. Can. J. Plant Pathol. 2012, 34, 213–224. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Shaidayuk, E.L.; Kazartsev, I.A.; Aristova, M.K. Structure of Russian populations of Puccinia triticina. Plant Prot. News 2015, 3, 5–10. (In Russian) [Google Scholar]
- Available online: https://www.bankgorodov.ru/econ-area/severo-zapadnyj (accessed on 17 October 2022). (In Russian).
- Agricultural Portal. Available online: https://agricultural portal.rf/analiz-posevnyh-ploshhadej (accessed on 17 October 2022). (In Russian).
- Aboukhaddour, R.; Fetch, T.; McCallum, B.D.; Harding, M.W.; Beres, B.L.; Graf, R.J. Wheat diseases on the prairies: A Canadian story. Plant Pathol. 2020, 69, 418–432. [Google Scholar] [CrossRef]
- McCallum, B.D.; Hiebert, C.W.; Cloutier, S.; Bakkeren, G.; Rosa, S.B.; Humphreys, D.G. A review of wheat leaf rust research and the development of resistant cultivars in Canada. Can. J. Plant Pathol. 2016, 38, 1–18. [Google Scholar] [CrossRef]
- Miedaner, T.; Juroszek, P. Climate change will influence disease resistance breeding in wheat in Northwestern Europe. Theor. Appl. Genet. 2021, 134, 1771–1785. [Google Scholar] [CrossRef]
- Caubel, J.; Launay, M.; Ripoche, D.; Gouache, D.; Buis, S.; Huard, F.; Huber, L.; Brun, F.; Bancale, M.O. Climate change effects on leaf rust of wheat: Implementing a coupled crop-disease model in a French regional application. Eur. J. Agron. 2017, 90, 53–166. [Google Scholar] [CrossRef]
- Launay, M.; Zurfluh, O.; Huard, F.; Buis, F.; Bourgeois, G.; Caubel, J.; Huber, L.; Bancal, M.O. Robustness of crop disease response to climate change signal under modelling uncertainties. Agri.c Syst. 2020, 178, 102733. [Google Scholar] [CrossRef]
- Gultyaeva, E.I. Genetic Structure of Puccinia triticina Populations in Russia and Its Variability under the Influence of Host Plant. Ph.D. Thesis, All-Russian Institute of Plant Protection, Pushkin-St. Petersburg, Russia, 2018; 312p. (In Russian). [Google Scholar]
- Serfling, A.; Krämer, I.; Lind, V.; Schliephake, E.; Ordon, F. Diagnostic value of molecular markers for Lr genes and characterization of leaf rust resistance of German winter wheat cultivars with regard to the stability of vertical resistance. Eur. J. Plant Pathol. 2011, 130, 559–575. [Google Scholar] [CrossRef]
- Mikhailova, L.A.; Gultyaeva, E.I.; Mironenko, N.V. Methods for studying the structure of populations of the leaf rust causative agent. In Collection of Guidelines on Plant Protection; All-Russia Institute of Plant Protection: St. Petersburg, Russian, 1998; pp. 105–126. (In Russian) [Google Scholar]
- Long, D.L.; Kolmer, J.A. A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology 1989, 79, 525–529. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Mert, Z.; Akan, K.; Demir, L.; Ünsal, R.; Şermet, C.; Keser, M.; Akin, B.; Morgounov, A. Virulence of Puccinia triticina in Turkey and leaf rust resistance in Turkish wheat cultivars. Eur. J. Plant Pathol. 2013, 135, 703–716. [Google Scholar] [CrossRef]
- McIntosh, R.A.; Wellings, C.R.; Park, R.F. Wheat Rusts: An Atlas of Resistance Genes; CSIRO: Canberra, Australia, 1995.
- Gultyaeva, E.I.; Baranova, O.A.; Dmitriev, A.P. Virulence and population structure of Puccinia triticina in Russian Federation in 2007. Plant Prot. News 2009, 4, 33–38. (In Russian) [Google Scholar]
- Gultyaeva, E.I.; Kosman, E.; Dmitriev, A.P.; Baranova, O.A. The structure of Puccinia triticina populations determined by virulence and DNA-markers in the North-West Russia in 2007. Mikol. Fitopatol. 2011, 45, 70–81. (In Russian) [Google Scholar]
- Peterson, R.F.; Campbell, A.B.; Hannah, A.E. A diagrammatic scale for estimating rust intensity on leaves and stems of cereals. Can. J. Res. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Kolmer, J.; Long, D.L.; Hughes, M.E. Physiologic specialization of Puccina triticina on wheat in the United States in 2008. Plant Dis. 2010, 94, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Fontyn, C.; Zippert, A.C.; Delestre, G.; Marcel, T.C.; Suffert, F.; Goyeau, H. Is virulence phenotype evolution driven exclusively by Lr gene deployment in French Puccinia triticina populations? Plant Pathol. 2022, 71, 1511–1524. [Google Scholar] [CrossRef]
- Mesterhazy, A.; Bartos, P.; Goyeau, H.; Niks, R.E.; Csosz, M.; Andersen, O.; Casulli, F.; Ittu, M.; Jones, E.; Manisterski, J.; et al. European virulence survey for leaf rust in wheat. Agronomie 2000, 20, 793–804. [Google Scholar] [CrossRef] [Green Version]
- Hanzalová, A.; Dumalasová, V.; Zelba, O. Virulence in the Puccinia triticina population in the Czech Republic and resistance genes in registered cultivars 1966–2019. Euphytica 2021, 217, 4. [Google Scholar] [CrossRef]
- Hanzalová, A.; Dumalasová, V.; Zelba, O. Wheat leaf rust (Puccinia triticina Eriks.) virulence frequency and detection of resistance genes in wheat cultivars registered in the Czech Republic in 2016–2018. Czech J. Genet. Plant Breed. 2020, 56, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Espino, J.; Singh, R.; Germán, S.; McCallum, B.; Park, R.; Chen, W.; Bhardwaj, S.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Kabdulova, M.G.; Mustafina, M.A.; Zhemchuzhina, N.S.; Dubovoy, V. Russian populations of Puccinia triticina in distant regions are not differentiated for virulence and molecular genotype. Plant Pathology 2015, 64, 328–336. [Google Scholar] [CrossRef]
- Manisterski, J.; Eyal, Z.; Ben-Yehuda, P.; Kosman, E. Comparative analysis of indices in the study of virulence diversity between and within populations of Puccinia recondita f. sp. tritici in Israel. Phytopathol. 2000, 90, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapuranga, J.; Zhang, N.; Zhang, L.; Liu, W.; Chang, J.; Yang, W. Harnessing genetic resistance to rusts in wheat and integrated rust management methods to develop more durable resistant cultivars. Front. Plant Sci. 2022, 13, 951095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lan, C.; Singh, R.P.; Huerta-Espino, J.; Li, Z.; Lagudah, E.; Bhavani, S. Identification and characterization of resistance loci to wheat leaf rust and stripe rust in Afghan landrace “KU3067”. Front. Plant Sci. 2022, 13, 894528. [Google Scholar] [CrossRef]
- Lagudah, E.S. Molecular genetics of race non-specific rust resistance in wheat. Euphytica 2011, 179, 81–91. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Lillemo, M.; Huerta-Espino, J.; Bhavani, S.; Singh, S.; Lan, C.; Calvo-Salazar, V.; Lagudah, E.S. Lr67/Yr46 confers adult plant resistance to stem rust and powdery mildew in wheat. Theor. Appl. Genet. 2014, 127, 781–789. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.; Crespo-Herrera, L.A.; Villaseñor-Mir, H.E.; Rodriguez-Garcia, M.F.; Dreisigacker, S.; Barcenas-Santana, D.; Lagudah, E. Adult plant slow rusting genes confer high levels of resistance to rusts in bread wheat cultivars from Mexico. Front. Plant Sci. 2020, 11, 824. [Google Scholar] [CrossRef]
- Bokore, F.E.; Knox, R.E.; Hiebert, C.W.; Cuthbert, R.D.; DePauw, R.M.; Meyer, B.; N’Diaye, A.; Pozniak, C.J.; McCallum, B.D. A Combination of leaf rust resistance genes, including Lr34 and Lr46, is the key to the Durable Resistance of the Canadian Wheat Cultivar, Carberry. Front. Plant Sci. 2022, 12, 775383. [Google Scholar] [CrossRef] [PubMed]
- McCallum, B.D.; Hiebert, C.W. Interactions between Lr67 or Lr34 and other leaf rust resistance genes in wheat (Triticum aestivum). Front. Plant Sci. 2022, 13, 871970. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Salazar, V.C.; Lagudah, E.S. First Report of Slow Rusting Gene Lr46 in Durum Wheat. In Proceedings of the Borlaug Global Rust Initiative 2011 Technical Workshop, Saint Paul, MN, USA, 13–16 June 2011; p. 191. [Google Scholar]
- Kolmer, J.A.; Su, Z.; Bernardo, A.; Bai, G.; Chao, S. Mapping and characterization of the new adult plant leaf rust resistance gene Lr77 derived from Santa Fe winter wheat. Theor Appl Genet. 2018, 131, 1553–1560. [Google Scholar] [CrossRef]
- Rauf, Y.; Lan, C.; Randhawa, R.; Singh, R.P.; Huerta-Espino, Y.; Anderson, J.A. Quantitative trait loci mapping reveals the complexity of adult plant resistance to leaf rust in spring wheat ‘Copio’. Crop Sci. 2022, 62, 1037–1050. [Google Scholar] [CrossRef]
- Sibikeev, S.N.; Krupnov, V.A. Evolution of leaf rust and protection against it of bread wheat in the Volga region. In The Bulletin Saratov State Agrarian University in Honor of N.I. Vavilov; 2007; pp. 92–94. (In Russian) [Google Scholar]
- Meshkova, L.V.; Rosseeva, L.P.; Korenyuk, E.A.; Belan, I.A. Dynamics of distribution of the wheat leaf rust pathotypes virulent to the cultivars with Lr9 gene in Omsk region. Mikol. Fitopatol. 2012, 46, 397–400. (In Russian) [Google Scholar]

| Pt code 1 | Infection type produced on near isogenic Lr lines | |||
| Host set 1: Lr1, Lr2a, Lr2c and Lr3a Host set 2: Lr9, Lr16, Lr24 and Lr26 Host set 3: Lr3ka, Lr11, Lr17 and Lr30 Host set 4: Lr2b, Lr3bg, Lr14a and Lr14b Host set 5: Lr15, Lr18, Lr19 and Lr20 | ||||
| B | L | L | L | L |
| C | L | L | L | H |
| D | L | L | H | L |
| F | L | L | H | H |
| G | L | H | L | L |
| H | L | H | L | H |
| J | L | H | H | L |
| K | L | H | H | H |
| L | H | L | L | L |
| M | H | L | L | H |
| N | H | L | H | L |
| P | H | L | H | H |
| Q | H | H | L | L |
| R | H | H | L | H |
| S | H | H | H | L |
| T | H | H | H | H |
| Parameter | 2001 | 2002 | 2003 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2012 | 2013 | 2014 | 2015 | 2016 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of isolates | 34 | 56 | 67 | 44 | 16 | 139 | 36 | 8 | 17 | 17 | 132 | 79 | 36 | 24 | 22 | 6 | 7 |
| Number of pathotypes | 3 | 12 | 42 | 32 | 6 | 33 | 11 | 5 | 2 | 5 | 24 | 18 | 5 | 3 | 8 | 1 | 2 |
| Relative richness 1 | 0.1 | 0.21 | 0.63 | 0.73 | 0.37 | 0.24 | 0.31 | 0.62 | 0.12 | 0.29 | 0.18 | 0.23 | 0.14 | 0.12 | 0.36 | 0.17 | 0.28 |
| Abundance 2 (%) | 50 | 32 | 13 | 18 | 37 | 38 | 36 | 50 | 53 | 76 | 23 | 16 | 41 | 54 | 45 | 100 | 86 |
| Number of rare pathotypes 3 | 0 | 1 | 22 | 26 | 0 | 8 | 2 | 1 | 0 | 1 | 6 | 2 | 0 | 0 | 3 | 0 | 0 |
| Complexity 4 | 14 | 14.1 | 12.9 | 12.5 | 14.9 | 15.6 | 15.4 | 14.1 | 14 | 13.3 | 13.1 | 14.2 | 13.2 | 14 | 12.7 | 12 | 12.6 |
| Kosman diversity 5 | 0.1 | 0.15 | 0.29 | 0.36 | 0.14 | 0.14 | 0.10 | 0.21 | 0.09 | 0.07 | 0.18 | 0.2 | 0.12 | 0.16 | 0.25 | 0 | 0.06 |
| Year | Pathotype Group Frequency (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B- | C- | D- | F- | K- | L- | M- | N- | P- | Q- | R- | T- | |
| 2001 | 0 | 0 | 0 | 77 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23 |
| 2002 | 0 | 6 | 0 | 56 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 16 |
| 2003 | 0 | 19 | 0 | 50 | 0 | 0 | 1 | 0 | 4 | 1 | 3 | 22 |
| 2005 | 17 | 2 | 4 | 19 | 0 | 4 | 2 | 0 | 13 | 0 | 0 | 39 |
| 2006 | 0 | 0 | 0 | 25 | 25 | 0 | 0 | 0 | 12 | 0 | 0 | 38 |
| 2007 | 0 | 0 | 0 | 13 | 3 | 0 | 3 | 0 | 14 | 0 | 0 | 67 |
| 2008 | 0 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 83 |
| 2009 | 0 | 0 | 0 | 50 | 0 | 0 | 12 | 0 | 25 | 0 | 0 | 13 |
| 2010 | 0 | 0 | 0 | 0 | 0 | 0 | 47 | 0 | 53 | 0 | 0 | 0 |
| 2012 | 6 | 6 | 0 | 0 | 0 | 0 | 88 | 0 | 0 | 0 | 0 | 0 |
| 2013 | 0 | 0 | 0 | 1 | 0 | 2 | 45 | 0 | 15 | 0 | 0 | 37 |
| 2014 | 0 | 0 | 0 | 0 | 0 | 1 | 30 | 1 | 46 | 0 | 0 | 22 |
| 2015 | 0 | 0 | 0 | 0 | 0 | 0 | 70 | 0 | 23 | 0 | 0 | 7 |
| 2016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 54 | 0 | 0 | 46 |
| 2019 | 0 | 0 | 0 | 0 | 0 | 0 | 51 | 0 | 10 | 0 | 0 | 39 |
| 2020 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 |
| 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 86 | 0 | 0 | 0 | 0 | 14 |
| Pathotype | Avirulence Lr-Gene | 2001 | 2002 | 2003 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2012 | 2013 | 2014 | 2015 | 2016 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGTTH | 1, 2a, 9, 15, 19, 24, 26 | 0 | 0 | 3 | 0 | 0 | 4 | 3 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| FGTTR | 1, 2a, 9, 19, 24, 26 | 26 | 32 | 0 | 0 | 25 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KGTTR | 1, 9, 19, 24, 26 | 0 | 5 | 0 | 0 | 12 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KHTTR | 1, 9, 19, 24 | 0 | 2 | 0 | 0 | 12 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MCTKH | 2a, 2b, 2c, 9, 15, 16 19, 24 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 100 | 86 |
| MHTKH | 2a, 2b, 2c, 9, 15, 19, 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 6 | 41 | 0 | 0 | 0 | 0 |
| MGTKG | 2a, 2b, 2c, 9, 15, 19, 20, 24, 26 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21 | 45 | 0 | 0 |
| MGTKH | 2a, 2b, 2c, 9, 15, 19, 24, 26 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 8 | 0 | 29 | 0 | 0 | 0 | 0 |
| MHTKQ | 2a, 2b, 2c, 9, 19, 20, 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 8 | 0 | 0 | 0 | 0 | 0 |
| MHTKR | 2a, 2b, 2c, 9, 19, 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 47 | 76 | 21 | 16 | 0 | 0 | 0 | 0 | 0 |
| PGTTH | 2a, 9, 15, 19, 24, 26 | 0 | 0 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 2 | 18 | 0 | 0 | 0 | 0 |
| PHTTH | 2a, 9, 15, 19, 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 8 | 6 | 54 | 0 | 0 | 0 |
| PGTTR | 2a, 9, 19, 24, 26 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| PHTTR | 2a, 9, 19, 24 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | 12 | 0 | 0 | 7 | 1 | 0 | 0 | 0 | 0 | 0 |
| PHTKR | 2a, 2b, 9, 19, 24 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 12 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| TCTTR | 9, 16, 19, 24 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 23 | 0 | 14 |
| TGTTR | 9, 19, 24, 26 | 23 | 0 | 3 | 0 | 0 | 15 | 36 | 0 | 0 | 0 | 4 | 9 | 6 | 0 | 0 | 0 | 0 |
| THTTR | 9, 19, 24 | 0 | 14 | 6 | 18 | 0 | 38 | 17 | 12 | 0 | 0 | 2 | 1 | 0 | 0 | 4 | 0 | 0 |
| TGTTH | 9, 15, 19, 20, 24, 26 | 0 | 0 | 0 | 2 | 37 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TGTTQ | 9, 19, 20, 24, 26 | 0 | 0 | 0 | 0 | 0 | 1 | 19 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| THTTQ | 9, 19, 20, 24 | 0 | 0 | 1 | 0 | 0 | 3 | 3 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gultyaeva, E.; Gannibal, P.; Shaydayuk, E. Long-Term Studies of Wheat Leaf Rust in the North-Western Region of Russia. Agriculture 2023, 13, 255. https://doi.org/10.3390/agriculture13020255
Gultyaeva E, Gannibal P, Shaydayuk E. Long-Term Studies of Wheat Leaf Rust in the North-Western Region of Russia. Agriculture. 2023; 13(2):255. https://doi.org/10.3390/agriculture13020255
Chicago/Turabian StyleGultyaeva, Elena, Philipp Gannibal, and Ekaterina Shaydayuk. 2023. "Long-Term Studies of Wheat Leaf Rust in the North-Western Region of Russia" Agriculture 13, no. 2: 255. https://doi.org/10.3390/agriculture13020255





