The Electrical Conductivity of Nutrient Solution Influenced the Growth, Centellosides Content and Gene Expression of Centella asiatica in a Hydroponic System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growing Conditions
2.3. Treatment
2.4. Analysis of Growth
2.5. High-Performance Liquid Chromatography (HPLC) Analysis
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results and Discussions
3.1. Plant Growth Parameters
3.2. Centelloside Content
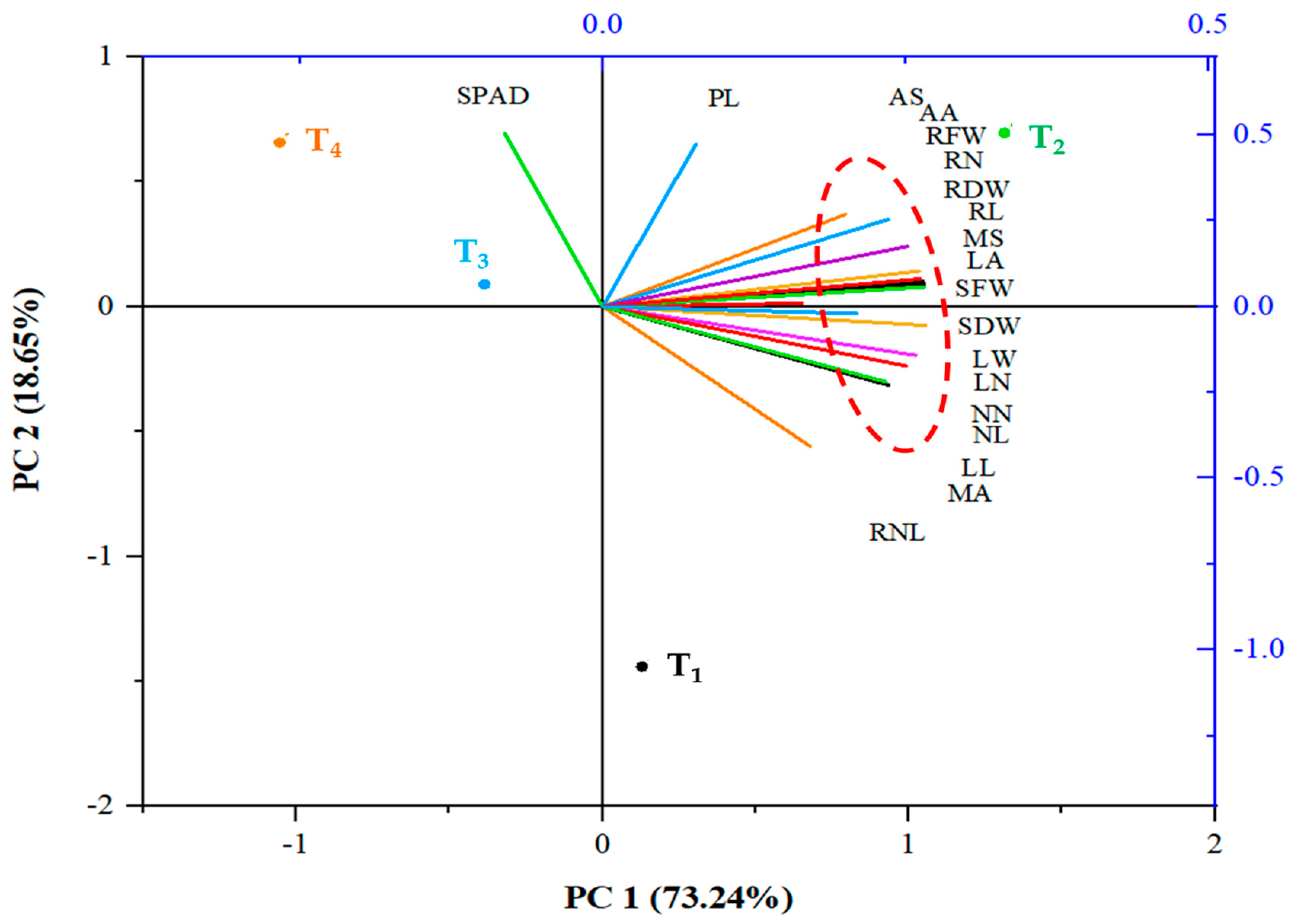
3.3. Correlation
3.4. Expression of Genes Involved in Centelloside Biosynthetic Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buraphaka, H.; Putalun, W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Prod. 2020, 146, 112171. [Google Scholar] [CrossRef]
- Zainol, M.K.; Abd-Hamid, A.; Yusof, S.; Muse, R. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) urban. Food Chem. 2003, 81, 575–581. [Google Scholar] [CrossRef]
- Biswas, T.; Parveen, O.; Pandey, V.P.; Mathur, A.; Dwivedi, U.N. Heavy metal accumulation efficiency, growth and centelloside production in the medicinal herb Centella asiatica (L.) urban under different soil concentrations of cadmium and lead. Ind. Crops Prod. 2020, 157, 112948. [Google Scholar] [CrossRef]
- Kamble, S.M.; Patil, C.R. Asiatic acid ameliorates doxorubicin-induced cardiac and hepato-renal toxicities with Nrf2 transcriptional factor activation in rats. Cardiovasc. Toxicol. 2018, 18, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Nagoor, M.M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological properties, molecular mechanisms, and pharmaceutical development of asiatic acid: A pentacyclic triterpenoid of therapeutic promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Pragadheesh, V.S.; Mathur, A.; Srivastava, N.K.; Singh, M.; Mathur, A.K. Growth and centelloside production in hydroponically established medicinal plant-Centella asiatica (L.). Ind. Crops Prod. 2012, 35, 309–312. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef] [PubMed]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the electrical conductivity of a nutrient solution for plant growth and bioactive compounds of Agastache rugosa in a plant factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef]
- Sonneveld, C.; Voogt, W.; Sonneveld, C.; Voogt, W. Nutrient solutions for soilless cultures. J. Plant. Nutr. Soil Sci. 2009, 257–275. [Google Scholar] [CrossRef]
- Kimura, Y.; Sumiyoshi, M.; Samukawa, K.I.; Satake, N.; Sakanaka, M. Facilitating action of asiaticoside at low doses on burn wound repair and its mechanism. Eur. J. Pharmacol. 2008, 584, 415–423. [Google Scholar] [CrossRef]
- Wan, J.; Gong, X.; Jiang, R.; Zhang, Z.; Zhang, L. Antipyretic and anti-inflammatory effects of asiaticoside in lipopolysaccharide-treated rat through up-regulation of heme oxygenase-1. Phytother. Res. 2013, 27, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Kraft, O.; Hartmann, A.K.; Hoenke, S.; Serbian, I.; Csuk, R. Madecassic Acid—A new scaffold for highly cytotoxic agents. Int. J. Mol. Sci. 2022, 23, 4362. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, T.C.; Mong, M.C. Antibacterial effects and action modes of asiatic acid. Biomedicine 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Mathur, A.K.; Mathur, A. Advances and emerging research trends for modulation of centelloside biosynthesis in Centella asiatica (L.) Urban-A review. Ind. Crops Prod. 2019, 141, 111768. [Google Scholar] [CrossRef]
- Alcalde, M.A.; Cusido, R.M.; Moyano, E.; Palazon, J.; Bonfill, M. Metabolic gene expression and centelloside production in elicited Centella asiatica hairy root cultures. Ind. Crops Prod. 2022, 184, 114988. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, J.S.; Stewart Jr, C.N. Advanced genetic tools for plant biotechnology. Nat. Rev. Genet. 2013, 14, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Phytochemical genomics-a new trend. Curr. Opin. Plant Biol. 2013, 16, 373–380. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef]
- Prasad, A.; Kumari, M.; Srivastava, N.K.; Mathur, A.K.; Mathur, A. Copper-induced modulation of biomass growth, physiological parameters, bioactive centellosides, and expression of biosynthetic pathway genes in an important medicinal herb, Centella asiatica. J. Plant Growth Regul. 2018, 37, 471–480. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Islam, T.M.; Naidu, Y.; Meon, S. The conjunctive use of compost tea and inorganic fertiliser on the growth, yield and terpenoid content of Centella asiatica (L.) urban. Sci. Hortic. 2011, 130, 289–295. [Google Scholar] [CrossRef]
- Song, J.; Chen, Z.; Zhang, A.; Wang, M.; Jahan, M.S.; Wen, Y.; Liu, X. The positive effects of increased light intensity on growth and photosynthetic performance of tomato seedlings in relation to night temperature level. Agronomy 2022, 12, 343. [Google Scholar] [CrossRef]
- Yu, G.R.; Zhuang, J.; Nakayama, K.; Jin, Y. Root water uptake and profile soil water as affected by vertical root distribution. Plant Ecol. 2007, 189, 15–30. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C. Root growth and distribution in relation to different water levels. Enhancing Underst. Quantif. Soil–Root Growth Interact. 2013, 4, 45–65. [Google Scholar]
- Monostori, I.; Árendás, T.; Hoffman, B.; Galiba, G.; Gierczik, K.; Szira, F.; Vágújfalvi, A. Relationship between SPAD value and grain yield can be affected by cultivar, environment and soil nitrogen content in wheat. Euphytica 2016, 211, 103–112. [Google Scholar] [CrossRef]
- Thomas, M.T.; Kurup, R.; Johnson, A.J.; Chandrika, S.P.; Mathew, P.J.; Dan, M.; Baby, S. Elite genotypes/chemotypes, with high contents of madecassoside and asiaticoside, from sixty accessions of Centella asiatica of south India and the Andaman Islands: For cultivation and utility in cosmetic and herbal drug applications. Ind. Crops Prod. 2010, 32, 545–550. [Google Scholar] [CrossRef]
- Plengmuankhae, W.; Tantitadapitak, C. Low temperature and water dehydration increase the levels of asiaticoside and madecassoside in Centella asiatica (L.) urban. S. Afr. J. Bot. 2015, 97, 196–203. [Google Scholar] [CrossRef]
- Kim, O.T.; Kim, M.Y.; Hong, M.H.; Ahn, J.C.; Hwang, B. Stimulation of asiaticoside accumulation in the whole plant cultures of Centella asiatica (L.) urban by elicitors. Plant Cell Rep. 2004, 23, 339–344. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Shin, Y.S.; Lee, M.J. Enhanced production of asiaticoside from hairy root cultures of Centella asiatica (L.) urban elicited by methyl jasmonate. Plant Cell Rep. 2007, 26, 1941–1949. [Google Scholar] [CrossRef]
- Yoo, N.H.; Kim, O.T.; Kim, J.B.; Kim, S.H. Enhancement of centelloside production from cultured plants of Centella asiatica by combination of thidiazuron and methyl jasmonate. Plant Biotechnol. Rep. 2011, 5, 283–287. [Google Scholar] [CrossRef]
- Gallego, A.; Ramirez-Estrada, K.; Vidal-Limon, H.R.; Hidalgo, D.; Lalaleo, L.; Khan Kayani, W.; Cusido, R.M.; Palazon, J. Biotechnological production of centellosides in cell cultures of Centella asiatica (L) urban. Eng. Life Sci. 2014, 14, 633–642. [Google Scholar] [CrossRef]
- Alqahtani, A.; Tongkao-on, W.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K.; Li, G.Q. Seasonal variation of triterpenes and phenolic compounds in Australian Centella asiatica (L.) urb. Phytochem. Anal. 2015, 26, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Borhan, M.Z.; Ahmad, R.; Rusop, M.; Abdullah, S. Green extraction: Enhanced extraction yield of asiatic acid from Centella asiatica (L.) nanopowders. J. Appl. Chem. 2013, 2013, 460168. [Google Scholar] [CrossRef]
- Devkota, A.; Jha, P. Influence of water stress on growth and yield of Centella asiatica. Int. Agrophys. 2011, 25, 211–214. [Google Scholar]
- Gao, D.; Ran, C.; Zhang, Y.; Wang, X.; Lu, S.; Geng, Y.; Guo, L.; Shao, X. Effect of different concentrations of foliar iron fertilizer on chlorophyll fluorescence characteristics of iron-deficient rice seedlings under saline sodic conditions. Plant Physiol. Biochem. 2022, 185, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Shawon, M.R.A.; Kim, J.K.; Yoon, Y.J.; Park, S.J.; Na, J.K. Effect of white LED light on the growth of apple seedlings in controlled environment system. Horticulturae 2022, 8, 692. [Google Scholar] [CrossRef]
- Ranjith, G.P.; Jisha, S.; Hemanthakumar, A.S.; Saji, C.V.; Shenoi, R.A.; Sabu, K.K. Impact of potential stimulants on asiaticoside and madecassoside levels and expression of triterpenoid-related genes in axenic shoot cultures of Centella asiatica (L.) urb. Phytochemistry 2021, 186, 112735. [Google Scholar] [CrossRef] [PubMed]
- Mangas, S.; Moyano, E.; Osuna, L.; Cusido, R.M.; Bonfill, M.; Palazón, J. Triterpenoid saponin content and the expression level of some related genes in calli of Centella asiatica. Biotechnol. Lett. 2008, 30, 1853–1859. [Google Scholar] [CrossRef]
- Kim, O.T.; Jin, M.L.; Lee, D.Y.; Jetter, R. Characterization of the asiatic acid glucosyltransferase, UGT73AH1, involved in asiaticoside biosynthesis in Centella asiatica (L.) urban. Int. J. Mol. Sci. 2017, 18, 2630. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef]



| Accession ID * | Functions | Primer ID | Primer Seq. | Tm | Size (bp) |
|---|---|---|---|---|---|
| JK517508.1 | Beta-actin (standard) | CaACT-F | AATGGTGAAGGCTGGTTTTG | 60 | 284 |
| CaACT-R | GTGGTGCCTCGGTAAGAAGA | 60 | |||
| AY787627.1 | Farnesyl diphosphate synthase (FPS) | CaAY787627.1-F | CTTTCGAATTCACCGACGAT | 60 | 248 |
| CaAY787627.1-R | GGGTTGACCTCTGCGTGTAT | 60 | |||
| AY787628.1 | Squalene synthase (SQS) | CaAY787628.1-F | ATTCGTGACCATGTCGTTGA | 60 | 259 |
| CaAY787628.1-R | TGGGTTAGGGTTGTCAAAGC | 60 | |||
| MF480551.1 | Squalene epoxidase (SQE) | CaMF480551.1-F | ACGAAACACGAGGGTACGTC | 60 | 265 |
| CaMF480551.1-R | CGGATGTAGCAGGAAGAAGC | 60 | |||
| AY520818.1 | Beta-amyrin synthase (CaABS) | CaAY520818.1-F | TGCGTTAGCTGGGTTAGCTT | 60 | 255 |
| CaAY520818.1-R | TTTGCTGCTCTATGCAATGG | 60 | |||
| KT004520.1 | Cytochrome P450 C-23 oxidase-like (CYP-450) | CaKT004520.1-F | AAAGAATCTGGCCCCCATAC | 60 | 286 |
| CaKT004520.1-R | CACTGATGCTTTTGGCTTCA | 60 | |||
| KP195716.1 | UDP-glucosyltransferase (UGT-1) | CaKP195716.1-F | CCGGGTTTACCCGATAAGAT | 60 | 280 |
| CaKP195716.1-R | CCTCTTCAACTGAGGCCTTG | 60 | |||
| MF471454.1 | UDP-glucosyltransferase 73AH1 (UGT73AH1) | CaMF471454.1-F | AACCCCAACTCTCACACCAG | 60 | 285 |
| CaMF471454.1-R | CTGAGGCCTGGGATTAAACA | 60 |
| Growth Parameter | Treatment | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Petiole length (cm) | 5.60 ± 1.17 zcy | 8.90 ± 0.75 a | 6.44 ± 1.34 bc | 8.16 ± 1.89 ab |
| Leaf number (ea) | 23.20 ± 0.84 b | 32.00 ± 2.74 a | 17.20 ± 1.48 c | 10.40 ± 1.67 d |
| Leaf length (cm) | 2.62 ± 0.20 a | 2.64 ± 0.05 a | 2.30 ± 0.16 b | 2.26 ± 0.23 b |
| Leaf width (cm) | 2.94 ± 0.17 ab | 3.04 ± 0.13 a | 2.72 ± 0.15 b | 2.84 ± 0.18 ab |
| Leaf area (cm2) | 47.15 ± 3.92 b | 95.26 ± 14.68 a | 30.91 ± 6.01 c | 18.04 ± 4.52 d |
| Node number (ea) | 3.20 ± 1.10 ab | 4.00 ± 2.12 a | 1.80 ± 1.10 bc | 0.80 ± 0.84 c |
| Node length (cm) | 6.04 ± 0.90 ab | 7.04 ± 1.10 a | 4.38 ± 2.86 b | 1.80 ± 1.75 c |
| Runner number (ea) | 1.00 ± 0.00 b | 1.80 ± 0.45 a | 1.00 ± 0.71 b | 0.60 ± 0.55 b |
| Runner length (cm) | 26.50 ± 9.66 a | 17.80 ± 6.41 ab | 13.06 ± 9.19 b | 2.30 ± 2.28 c |
| Root length (cm) | 15.48 ± 2.04 a | 16.48 ± 2.92 a | 16.76 ± 3.56 a | 14.34 ± 3.01 a |
| SPAD (value) | 40.78 ± 5.61 c | 53.96 ± 1.55 b | 53.02 ± 4.22 b | 59.46 ± 1.21 a |
| Shoot FW (g) | 2.93 ± 0.30 b | 5.50 ± 0.47 a | 2.16 ± 0.35 c | 1.34 ± 0.23 d |
| Root FW (g) | 1.23 ± 0.21 b | 1.85 ± 0.19 a | 1.18 ± 0.21 b | 1.10 ± 0.27 b |
| Shoot DW (g) | 0.37 ± 0.04 b | 0.63 ± 0.09 a | 0.28 ± 0.05 c | 0.20 ± 0.05 c |
| Root DW (g) | 0.18 ± 0.03 ab | 0.23 ± 0.04 a | 0.18 ± 0.03 ab | 0.15 ± 0.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shawon, M.R.A.; Azad, M.O.K.; Ryu, B.R.; Na, J.K.; Choi, K.Y. The Electrical Conductivity of Nutrient Solution Influenced the Growth, Centellosides Content and Gene Expression of Centella asiatica in a Hydroponic System. Agriculture 2023, 13, 2236. https://doi.org/10.3390/agriculture13122236
Shawon MRA, Azad MOK, Ryu BR, Na JK, Choi KY. The Electrical Conductivity of Nutrient Solution Influenced the Growth, Centellosides Content and Gene Expression of Centella asiatica in a Hydroponic System. Agriculture. 2023; 13(12):2236. https://doi.org/10.3390/agriculture13122236
Chicago/Turabian StyleShawon, Md Rayhan Ahmed, Md Obyedul Kalam Azad, Byeong Ryeol Ryu, Jong Kuk Na, and Ki Young Choi. 2023. "The Electrical Conductivity of Nutrient Solution Influenced the Growth, Centellosides Content and Gene Expression of Centella asiatica in a Hydroponic System" Agriculture 13, no. 12: 2236. https://doi.org/10.3390/agriculture13122236
APA StyleShawon, M. R. A., Azad, M. O. K., Ryu, B. R., Na, J. K., & Choi, K. Y. (2023). The Electrical Conductivity of Nutrient Solution Influenced the Growth, Centellosides Content and Gene Expression of Centella asiatica in a Hydroponic System. Agriculture, 13(12), 2236. https://doi.org/10.3390/agriculture13122236







