3.2. Effects of Treatments on the Chemical Properties of Yellow Latosol
The chemical analyses of the Yellow Latosol after incubation revealed that increasing doses of biomass ash (8.75, 17.5, 35, and 70 Mg ha−1) promoted significant and positive changes (p < 0.05) in the soil chemical attributes, such as increases in the soil pH values and P, K, Ca, and Mg contents. These increases subsequently caused increases in the soil base saturation and reduced the Al3+ levels and potential acidity (H + Al).
The increasing ash levels conferred significant effects on the pH values in water (r > 0.85) and in CaCl
2 (r > 0.88) at the three soil incubation times (
Table 3). The applications of 35 and 70 Mg ha
−1 of ash contributed most to the increase in soil pH, with increases between 1.17 and 1.60 pH units when compared to the soil that did not receive ash input. Among the three incubation times, these two treatments did not differ significantly from each other, which caused a reduction in the linearity between the increasing ash input and the increase in pH values. Thus, the quadratic polynomial regression model adequately fit the results (
Figure 3a,b). To determine the pH values in H
2O, Equations (1)–(3) were used (
Figure 3a), and for the pH values in CaCl
2, Equations (4)–(6) were used (
Figure 3b). The greatest increases in pH due to ash applications were observed at 40 days of incubation (
Figure 3a,b). Equations (2) and (5) (
Figure 3a,b) were used to determine the point of maximum neutralization of the soil acidity, with the results indicating that with the addition of 58 Mg ha
−1 of ash, pH values of 5.71 in H
2O and 4.84 in CaCl
2 were obtained. It is important to note that although the ash applications caused positive effects on the soil pH, the variation in the pH values between the ash treatments and control soil was low, with coefficients of variation (CV) lower than 15.7% (
Table 3).
Even the high-dose ash treatments (35 and 70 Mg ha
−1) did not result in pH values or exchangeable cation (Ca
2+ and Mg
2+) contents that were significantly similar to those obtained in the lime treatment (
Table 3 and
Figure 3c). However, the treatment with the lowest ash concentration (8.75 Mg ha
−1) showed higher pH values than those obtained in the treatment with phosphate fertilizer (
Table 3 and
Figure 3c). As expected, the higher pH values obtained with the application of lime are due to its high total neutralizing value (92.4%) and relatively high concentrations of Ca and Mg carbonates. In contrast, biomass ash and phosphate fertilizer have low total neutralizing values (20.5% and 6.7%, respectively) and lower carbonate concentrations.
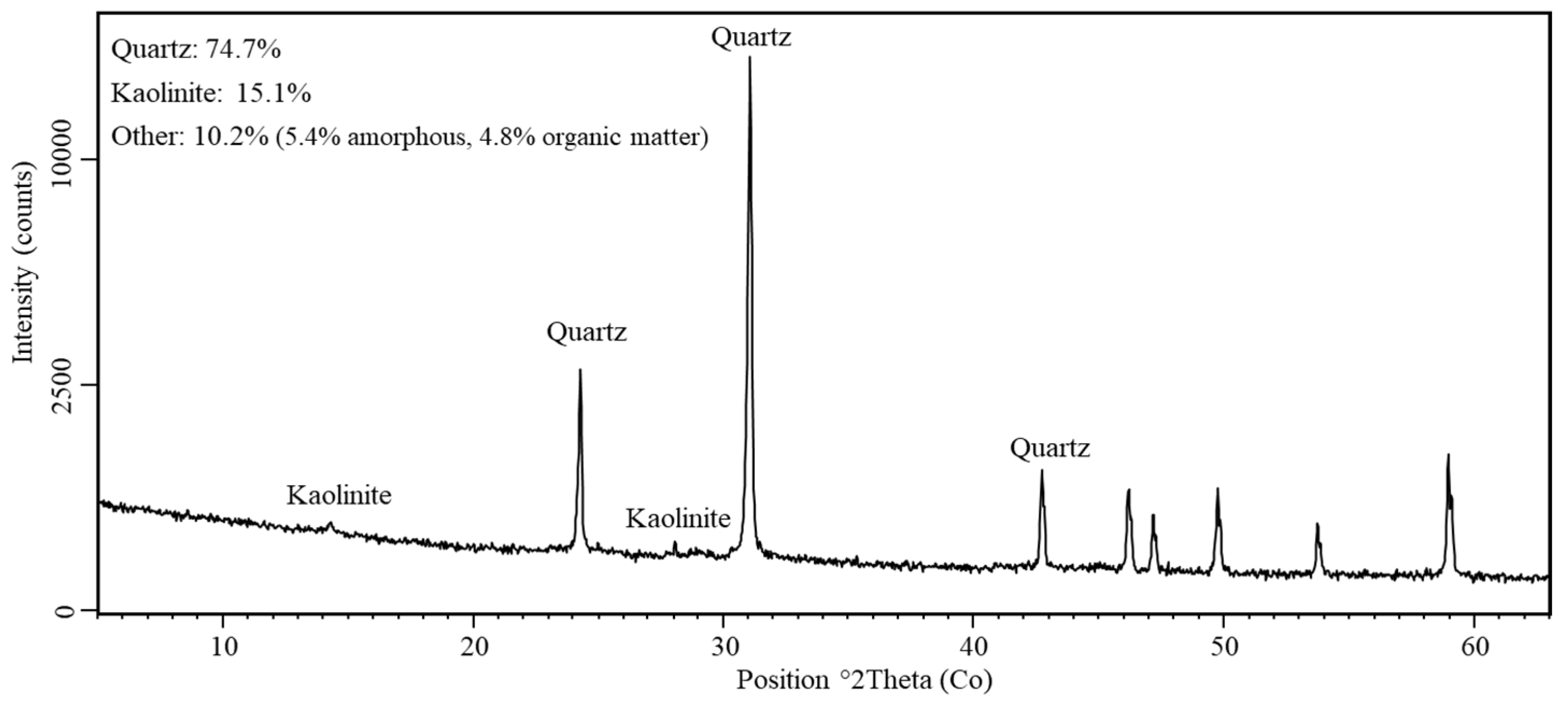
The increase in soil pH caused by the application of ash was possibly attributed to the large number of amorphous components in this material, accounting for more than 50% of the total content [
14]. These amorphous compounds host Ca, Mg, and K oxides, which can form carbonates and bicarbonates when in contact with atmospheric conditions [
16]. When exposed to soil solutions, these minerals dissociate into OH
− or HCO
3−, and these ions, in turn, neutralize H
+ and Al
+3 ions that are mainly responsible for soil acidity [
9].
The release of OH
− or HCO
3− ions and increases in the pH values of the soils were also accompanied by release of the exchangeable cations Ca
2+ and Mg
2+ in the soil solutions. As shown in
Figure 3 and
Table 3, the increases in the levels of these nutrients were caused by the increasing input of ash in the soil, with significant and positive correlation coefficients (r > 0.92).
The Ca and Mg levels showed significant and positive differences among the ash treatments and control soil, with moderate coefficients of variation (CV between 57.7 and 65.9%) (
Table 3). The highest concentrations of these nutrients were obtained at 20 days of incubation, with a slight decrease at 40 and 60 days (
Figure 3d,e). As expected, the treatment with 70 Mg ha
−1 of ash provided the highest amounts of Ca and Mg, with moderate to high levels of Ca (2.70–4.17 cmol
c dm
−3) and Mg (1.30–1.40 cmol
c dm
−3), while the concentrations of these nutrients in the control soil were only 0.33 and 0.20 cmol
c dm
−3, respectively (
Table 3). The polynomial regression model best fit these results, obtaining, from Equations (7) and (10) (
Figure 3d,e), the maximum Ca (4.34 cmol
c dm
−3) and Mg (1.62 cmol
c dm
−3) contents with 64 and 69 Mg ha
−1 of ash, respectively. According to the recommendations for the liming and fertilization of the soils of the state of Pará [
20], the sums of the Ca and Mg contents obtained by ash applications were within the mean (2–5 cmol
c dm
−3) and high (>5 cmol
c dm
−3) values required for most crops in the region. Notably, moderate levels of Ca + Mg were observed in the treatment with the lowest ash level (8.75 Mg ha
−1).
The Ca and Mg values obtained in the soils with increasing ash doses were lower than the levels obtained in the lime treatment (
Figure 3f and
Table 3). This effect was expected because lime has Ca and Mg concentrations of 240 and 83 g dm
−3, respectively, while ash contains 74 g dm
−3 of Ca and 12 g dm
−3 of Mg. On the other hand, the treatment with the lowest dose of ash (8.75 t ha
−1) showed Ca + Mg contents higher than those observed in the treatment with phosphate fertilizer (
Figure 3f).
Saarsalmi et al. [
21], in a study on the application of wood ash to soil in which wild pine (
Pinus sylvestris L.) was grown, also demonstrated that ash input can reduce soil acidity and increase the levels of exchangeable cations (Ca
2+ and Mg
2+). In addition, these authors demonstrated that these effects persisted even after 16 years of ash application. Hansen et al. [
7], in a study on the application of a mixture of heavy ash and fly ash, showed that the application of even high doses of ash (30 t ha
−1) did not cause heavy metal toxicity in the soil.
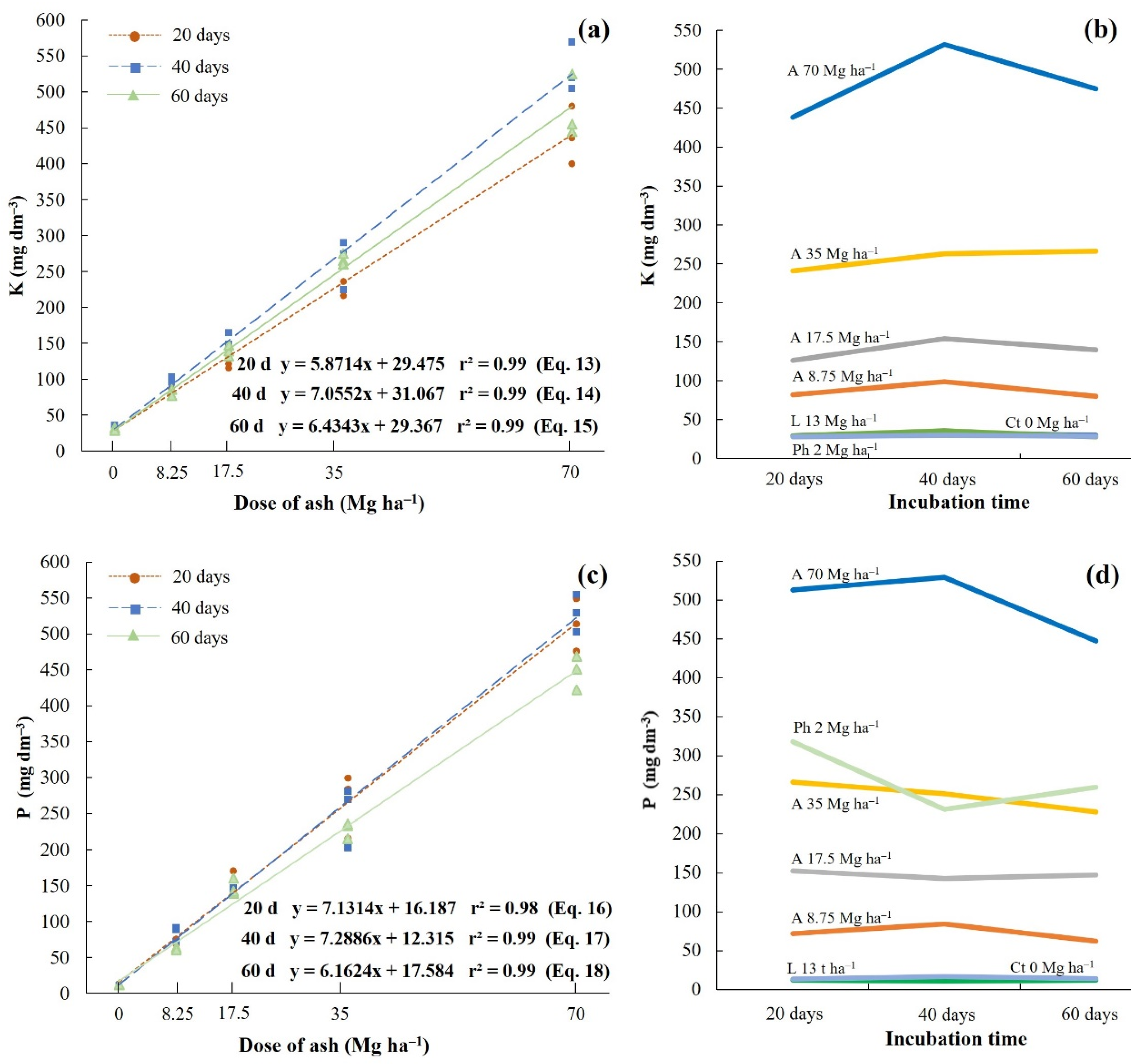
Increasing doses of ash also had positive effects on the release and increase of exchangeable K
+ levels in the soil. The concentrations of this nutrient showed significant differences between the ash treatments and the control soil, with coefficients of variation above 88.5% (
Table 4). As shown in
Figure 4a and
Table 4, the three incubation times showed linear relationships between the increasing ash doses and the nutrient K levels in the soil, demonstrating a high correlation coefficient between these two variables (r > 0.99). The highest concentrations of K were obtained after 40 days of incubation, and as with the other cations (Ca
2+ and Mg
2+), the dose of 70 Mg ha
−1 of ash was responsible for the greatest input of this nutrient (531.7 g dm
−3) (
Figure 4a,b). According to the liming and fertilization recommendations [
20], this content is considered very high for the soils of the State of Pará, especially Latosols. It is important to note that the smallest ash addition (8.75 Mg ha
−1) increased the low level of soil K (from an initial value of 33.7 g dm
−3) to a high level (80 to 98.7 g dm
−3). As shown in
Figure 4b, the K levels obtained in the treatments with ash were higher and, in general, showed significant differences from the levels obtained in the treatments with lime and phosphate fertilizer.
The concentration of exchangeable cations, such as K
+, Ca
2+, and Mg
2+, increases in soils treated with ash because these elements are present mainly in the form of highly reactive oxides and/or hydroxides, making K, Ca, and Mg the most soluble elements in ashes [
14,
22].
Similar to K, the extractable P contents increased linearly with increasing ash doses (
Figure 4c), with a high correlation coefficient between these two variables (r > 0.99) (
Table 4). The concentration of this nutrient in the soil also showed significant changes among the treatments with ash, with a coefficient of variation above 95%. The highest
p levels were obtained within 40 days of incubation and at a dose of 70 Mg ha
−1 of ash, which is directly related to better soil pH conditions (
Figure 3a,b) [
23,
24]. The average content obtained in this treatment was 529 g dm
−3, which is considered very high for a Latosol from the state of Pará, Brazil [
20]. It is important to note that the lowest ash dose (8.75 Mg ha
−1) also provided very high levels of available P in the soil (84.4 g dm
−3). In addition, the P levels obtained in the treatment with 35 Mg ha
−1 of ash (228.07–266.50 mg dm
−3) showed significant similarities with the levels obtained in the treatment with phosphate fertilizer (231.23–318.20 mg dm
−3) (
Table 4 and
Figure 4d).
Another important factor that contributed to the increase in P availability in the soil treated with ash was a reduction in the concentration of exchangeable Al
3+, which is one of the main elements responsible for P immobilization and its precipitation in the form of Al-phosphates in acidic soils from the Amazon region [
25].
As shown in
Table 5, increases in the concentrations of exchangeable cations in the soil (K
+, Ca
2+, and Mg
2+), conferred by the increases in ash doses, produced significant and positive effects on the base saturation of CEC at a soil pH of 7.0 (V), increasing from ~3.6% in the control soil to ~40% in the treatment with 70 Mg h
−1 of ash. Consequently, the increasing base saturation of CEC contributed significantly to a reduction in the CEC of aluminum saturation (m), reducing from ~81.6% in the control soil to ~1.7% in the treatment with 70 Mg h
−1 ash, thus resulting in an inversely proportional relationship between these two attributes. A base saturation (V) of ~20%, obtained at 17.5 Mg ha
−1, reduced the aluminum saturation (m) to less than 4% at 40 and 60 days of incubation, which is considered adequate for most crops of the state of Pará [
20].
The application of ash to the soil, even in large quantities, did not increase the potential acidity (H + Al) of the soil or increase the concentration of exchangeable Al
+ (
Table 5). As seen in
Table 5, increasing ash doses caused significant reductions in Al
3+ levels and moderate changes in potential acidity. The lower concentration of exchangeable Al
+3 probably resulted from the release of OH
− or HCO
3− ions or from the presence of soluble Si in the ash, which may have captured Al to form aluminum silicates. Regarding potential acidity, moderate reduction was mainly promoted by the immobilization of Al
3+, which favors the absorption of micronutrients by plants [
11].
The application of ash generally did not have significant effects on the levels of OM and CEC (
Table 6). This behavior was expected because OM contents are oxidized and transformed into gaseous constituents during biomass combustion [
10,
16]. Low levels of CEC are associated with the absence of clay minerals and reduced concentrations of OM in the ash.
The benefits of ash on soil fertility, such as reduced acidity and increased nutrient input, were also demonstrated by [
7,
12,
26] and other studies. However, its application to soil requires attention because high doses can result in high soil salinity, promote toxicity by micronutrients and heavy metals, and compromise soil aeration [
27,
28,
29].
Therefore, due to the large amount of ash necessary to promote increases in pH and increase the availability of P and exchangeable cations, the reuse of the mineral waste studied here is more suitable for the cultivation of undemanding agricultural species or for silviculture purposes [
14,
30]. A second alternative is its reuse as a nutritional additive/supplement to phosphate fertilizer. In addition to representing a solution to the waste disposal problem produced by the industry, this approach could also improve the agronomic efficiency of phosphate fertilizer because ash can raise the soil pH, increase the availability of P, K, Ca, and Mg, and reduce the toxicity of exchangeable Al
3+.