Unravelling the Efficient Applications of Zinc and Selenium for Mitigation of Abiotic Stresses in Plants
Abstract
:1. Introduction
2. Zinc (Zn) and Selenium (Se) Absorption and Transport in Plants
3. Importance of Zinc and Selenium and the Effects of Their Deficiency on Plants
4. Process of Zn and Se Application, Its Downstream Effects on Plants
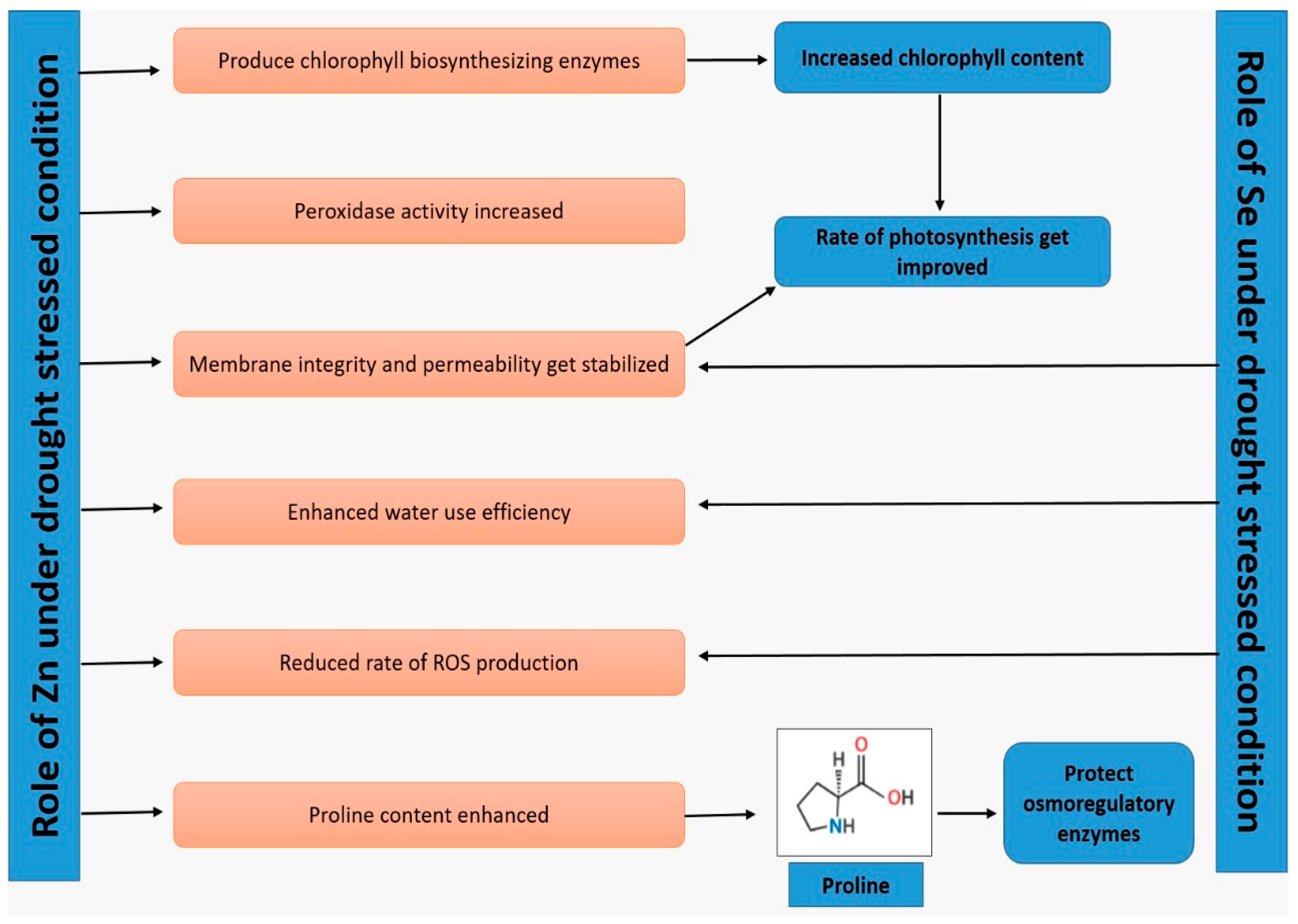
5. Role of Zn and Se in Plants under Drought Stress
6. Role of Zn and Se in Plants under Salinity Stress
7. Role of Zn and Se in Plants under Heavy Metal Stress
8. Role of Se in Plants to Overcome Heat Stress
9. Conclusions and Future Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sardhara, K.; Mehta, K. Impact of Abiotic and Biotic Strain on the Plant. Acad. J. Bot. Sci. 2019, 1, 6–10. [Google Scholar]
- Imran, Q.M.; Falak, N.; Hussain, A.; Mun, B.-G.; Yun, B.-W. Abiotic Stress in Plants; Stress Perception to Molecular Response and Role of Biotechnological Tools in Stress Resistance. Agronomy 2021, 11, 1579. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-P.; Shan, L.; Inanaga, S.; Inoue, M. Water-Saving Approaches for Improving Wheat Production. J. Sci. Food Agric. 2005, 85, 1379–1388. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, C.; Chu, L.-Y.; Shao, H.-B. Responses of Higher Plants to Abiotic Stresses and Agricultural Sustainable Development. J. Plant Interact. 2007, 2, 135–147. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of Zinc in Plant Nutrition-A Review. Am. J. Exp. Agric. 2013, 3, 374. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in Agriculture: A Nutrient or Contaminant for Crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Hassan, M.; Aamer, M.; Chattha, M.; Tang, H.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The Critical Role of Zinc in Plants Facing the Drought Stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Al-Zahrani, H.S.; Alharby, H.F.; Hakeem, K.R.; Rehman, R.U. Exogenous Application of Zinc to Mitigate the Salt Stress in Vigna radiata (L.) Wilczek—Evaluation of Physiological and Biochemical Processes. Plants 2021, 10, 1005. [Google Scholar] [CrossRef]
- Hussein, M.M.; Abou-Baker, N.H. The Contribution of Nano-Zinc to Alleviate Salinity Stress on Cotton Plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef]
- ul Hassan, Z.; Ali, S.; Rizwan, M.; Hussain, A.; Akbar, Z.; Rasool, N.; Abbas, F. Role of Zinc in Alleviating Heavy Metal Stress. In Essential Plant Nutrients: Uptake, Use Efficiency, and Management; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 351–366. [Google Scholar]
- Morkunas, I.; Woźniak, A.; Mai, V.C.; Rucińska-Sobkowiak, R.; Jeandet, P. The Role of Heavy Metals in Plant Response to Biotic Stress. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 2320. [Google Scholar] [CrossRef] [Green Version]
- Fageria, N.K. Dry Matter Yield and Nutrient Uptake by Lowland Rice at Different Growth Stages. J. Plant Nutr. 2004, 27, 947–958. [Google Scholar] [CrossRef]
- Lerdau, M. Mineral Nutrition of Plants: Principles and Perspectives. Second Edition. By Emanuel Epstein and Arnold J Bloom. Q. Rev. Biol. 2005, 80, 359. [Google Scholar] [CrossRef]
- Alloway, B.J. Zinc in Soils and Crop Nutrition. Available online: https://www.topsoils.co.nz/wp-content/uploads/2014/09/Zinc-in-Soils-and-Crop-Nutrition-Brian-J.-Alloway.pdf (accessed on 10 August 2022).
- Gunes, A.; Cicek, N.; Inal, A.; Alpaslan, M.; Eraslan, F.; Güneri, E.; Guzelordu, T. Genotypic Response of Chickpea (Cicer arietinum L.) Cultivars to Drought Stress Implemented at Pre- and Post-Anthesis Stages and Its Relations with Nutrient Uptake and Efficiency. Plant Soil Environ. 2011, 52, 368–376. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Selenium in Higher Plants: Physiological Role, Antioxidant Metabolism and Abiotic Stress Tolerance. J. Plant Sci. 2010, 5, 354–375. [Google Scholar] [CrossRef]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; dos Reis, A.R. Selenium Protects Rice Plants from Water Deficit Stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; dos Reis, A.R. Selenate and Selenite Affect Photosynthetic Pigments and ROS Scavenging through Distinct Mechanisms in Cowpea (Vigna unguiculata (L.) Walp) Plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Vadlamudi, K.; Upadhyay, H.; Singh, A.; Reddy, M. Influence of Zinc Application in Plant Growth: An Overview. Eur. J. Mol. Clin. Med. 2020, 7, 2321–2327. [Google Scholar]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc Absorption in Plants: Uptake, Transport, Translocation and Accumulation. Rev. Environ. Sci. Biotechnol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Palmer, C.M.; Guerinot, M.L. Facing the Challenges of Cu, Fe and Zn Homeostasis in Plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef]
- Demidchik, V.; Davenport, R.J.; Tester, M. Nonselective Cation Channels in Plants. Annu. Rev. Plant Biol. 2002, 53, 67–107. [Google Scholar] [CrossRef]
- Sondergaard, T.E.; Schulz, A.; Palmgren, M.G. Energization of Transport Processes in Plants. Roles of the Plasma Membrane H+-ATPase. Plant Physiol. 2004, 136, 2475–2482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium Speciation from Food Source to Metabolites: A Critical Review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Harris, J.; Schneberg, K.A.; Pilon-Smits, E.A.H. Sulfur-Selenium-Molybdenum Interactions Distinguish Selenium Hyperaccumulator Stanleya Pinnata from Non-Hyperaccumulator Brassica Juncea (Brassicaceae). Planta 2014, 239, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Mazej, D.; Osvald, J.; Stibilj, V. Selenium Species in Leaves of Chicory, Dandelion, Lamb’s Lettuce and Parsley. Food Chem. 2008, 107, 75–83. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Mahmood, A.; Chattha, M.U.; Nawaz, M.; Subhani, M.N.; Kharal, M.; Khan, S. Biofortification of Wheat Cultivars to Combat Zinc Deficiency. Front. Plant Sci. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Chattha, M.U.; Ullah, A.; Khan, I.; Qadeer, A.; Aamer, M.; Khan, A.U.; Nadeem, F.; Khan, T.A. Agronomic Biofortification to Improve Productivity and Grain Zn Concentration of Bread Wheat. Int. J. Agric. Biol. 2019, 21, 615–620. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium Supplementation and Crop Plant Tolerance to Metal/Metalloid Toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Ahmadvand, G. Effect of Zinc and Manganese Foliar Application on Yield, Quality and Enrichment on Potato (Solanum tuberosum L.). Asian J. Plant Sci. 2007, 6, 1256–1260. [Google Scholar] [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in Physiological and Biochemical Processes in Wheat Supplied with Zinc and Potassium under Saline Condition. J. Plant Nutr. 2018, 41, 19–28. [Google Scholar] [CrossRef]
- Sharma, P.N.; Kumar, N.; Bisht, S.S. Effect of Zinc Deficiency on Chlorophyll Content, Photosynthesis and Water Relations of Cauliflower Plants. Photosynth. Prague 1994, 30, 353–359. [Google Scholar]
- Diao, M.; Ma, L.; Wang, J.; Cui, J.; Fu, A.; Liu, H. Selenium Promotes the Growth and Photosynthesis of Tomato Seedlings under Salt Stress by Enhancing Chloroplast Antioxidant Defense System. J. Plant Growth Regul. 2014, 33, 671–682. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilizers; Macmillan Publishing Company: New York, NY, USA, 1985. [Google Scholar]
- Marschner, H. Mineral Nutrition of Higher Plants; Academic Press, Elsevier Ltd.: Cambridge, MA, USA, 1995. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Dang, H.; Li, R.; Sun, Y.; Zhang, X.; Li, Y. Absorption, Accumulation and Distribution of Zinc in Highly-Yielding Winter Wheat. Agric. Sci. China 2010, 9, 965–973. [Google Scholar] [CrossRef]
- Disante, K.B.; Fuentes, D.; Cortina, J. Response to Drought of Zn-Stressed Quercus Suber L. Seedlings. Environ. Exp. Bot. 2011, 70, 96–103. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrients and Crop Production: An Introduction. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–39. [Google Scholar]
- Welch, R.M. The Impact of Mineral Nutrients in Food Crops on Global Human Health. Plant Soil 2002, 247, 83–90. [Google Scholar] [CrossRef]
- Snowball, K.; Robson, A.D. Symptoms of Nutrient Deficiencies: Lupins; Soil Science and Plant Nutrition, School of Agriculture, University of Western Australia: Nedlands, Australia, 1986. [Google Scholar]
- Neue, H.U.; Lantin, R.S. Micronutrient Toxicities and Deficiencies in Rice. In Soil Mineral Stresses: Approaches to Crop Improvement; Yeo, A.R., Flowers, T.J., Eds.; Monographs on Theoretical and Applied Genetics; Springer: Berlin/Heidelberg, Germany, 1994; pp. 175–200. [Google Scholar]
- Van Breemen, N.; Quijano, C.C.; Sen, L.N. Zinc Deficiency in Wetland Rice Along a Toposequence of Hydromorphic Soils in the Philippines: I. Soil Conditions and Hydrology. Plant Soil 1980, 57, 203–214. [Google Scholar] [CrossRef]
- Yoshida, S.; Tanaka, A. Zinc Deficiency of the Rice Plant in Calcareous Soils. Soil Sci. Plant Nutr. 1969, 15, 75–80. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111: Possible Roles of Zinc in Protecting Plant Cells from Damage by Reactive Oxygen Species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Grzebisz, W.; Wronska, M.; Diatta, J.B.; Szczepaniak, W. Effect of Zinc Foliar Application at an Early Stage of Maize Growth on Patterns of Nutrients and Dry Matter Accumulation by the Canopy. Part II. Nitrogen Uptake and Dry Matter Accumulation Patterns. J. Elem. 2008, 13, 29–40. [Google Scholar]
- Leach, K.A.; Hameleers, A. The Effects of a Foliar Spray Containing Phosphorus and Zinc on the Development, Composition and Yield of Forage Maize. Grass Forage Sci. 2001, 56, 311–315. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Boldrin, K.V.F.; Ávila, F.W.; Guilherme, L.R.G. Soil and Foliar Application of Selenium in Rice Biofortification. J. Food Compos. Anal. 2013, 31, 238–244. [Google Scholar] [CrossRef]
- Javadi, F.; Kalatejari, S.; Diyanat, M. Effect of Foliar or Soil Application of Selenium on Some Morphological and Physiological Traits of Garden Pansy (Viola x Wittrockiana Gams) Grown under Salinity Stress. Acta Agric. Slov. 2020, 115, 357–368. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Guo, A.; Yang, S.; Chen, J.; Qiao, Y.; Anwar, S.; Wang, K.; Yang, Z.; Gao, Z.; et al. Combined Foliar and Soil Selenium Fertilizer Improves Selenium Transport and the Diversity of Rhizosphere Bacterial Community in Oats. Environ. Sci. Pollut. Res. Int. 2021, 28, 64407–64418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Duan, Y.; Zhang, Y.; Wang, H.; Huang, D.; Zhang, M. Effects of Foliar Selenium Application on Growth and Rhizospheric Soil Micro-Ecological Environment of Atractylodes Macrocephala Koidz. S. Afr. J. Bot. 2021, 137, 98–109. [Google Scholar] [CrossRef]
- Thalooth, A.; Tawfik, M.; Mohamed, H. A Comparative Study on the Effect of Foliar Application of Zinc, Potassium and Magnesium on Growth, Yield and Some Chemical Constituents of Mungbean Plants Grown under Water Stress Conditions. World J. Agric. Sci. 2006, 2, 37–46. [Google Scholar]
- Yadavi, A.; Aboueshaghi, R.; Movahhedi-Dehnavi, M.; Balouchi, H. Effect of micronutrients foliar application on grain qualitative characteristics and some physiological traits of bean (Phaseolus vulgaris L.) Under drought stress. Indian J. Fundam. Appl. Life Sci. 2014, 4, 124–131. [Google Scholar]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. Review: Biofortification of Durum Wheat with Zinc and Iron. Cereal Chem. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Ehsanullah; Tariq, A.; Randhawa, M.A.; Anjum, S.A.; Nadeem, M.; Naeem, M. Exploring the Role of Zinc in Maize (Zea mays L.) through Soil and Foliar Application. Univers. J. Agric. Res. 2015, 3, 69–75. [Google Scholar] [CrossRef]
- Khan, H.; Maitlo, A.A. Yield and Micronutrients Content of Bread Wheat (Triticum aestivum L.) under a Multinutrient Fertilizer–Hal-Tonic. Int. J. Agric. Biol. 2006, 8, 5. [Google Scholar]
- Farooq, M.; Wahid, A.; Siddique, K.H.M. Micronutrient Application through Seed Treatments: A Review. J. Soil Sci. Plant Nutr. 2012, 12, 125–142. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M. Zinc Seed Coating Improves the Growth, Grain Yield and Grain Biofortification of Bread Wheat. Acta Physiol. Plant. 2016, 38, 238. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Q.; Sun, P.; Song, C. Impact of Droughts on Winter Wheat Yield in Different Growth Stages during 2001–2016 in Eastern China. Int. J. Disaster Risk Sci. 2018, 9, 376–391. [Google Scholar] [CrossRef]
- Kapoor, D.; Bhardwaj, S.; Landi, M.; Sharma, A.; Ramakrishnan, M.; Sharma, A. The Impact of Drought in Plant Metabolism: How to Exploit Tolerance Mechanisms to Increase Crop Production. Appl. Sci. 2020, 10, 5692. [Google Scholar] [CrossRef]
- Shokat, S.; Großkinsky, D.K.; Roitsch, T.; Liu, F. Activities of Leaf and Spike Carbohydrate-Metabolic and Antioxidant Enzymes Are Linked with Yield Performance in Three Spring Wheat Genotypes Grown under Well-Watered and Drought Conditions. BMC Plant Biol. 2020, 20, 400. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Emam, Y.; Ashraf, M.; Arvin, M.J. Alleviation of Field Water Stress in Wheat Cultivars by Using Silicon and Salicylic Acid Applied Separately or in Combination. Crop Pasture Sci. 2019, 70, 36–43. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of Ascorbate-Glutathione Pathway in Mitigating Oxidative Damage in Plants under Abiotic Stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Mehla, N.; Sindhi, V.; Josula, D.; Bisht, P.; Wani, S. An Introduction to Antioxidants and Their Roles in Plant Stress Tolerance. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017; pp. 1–23. [Google Scholar]
- Ge, T.; Sui, F.; Bai, L.; Tong, C.; Sun, N. Effects of Water Stress on Growth, Biomass Partitioning, and Water-Use Efficiency in Summer Maize (Zea mays L.) throughout the Growth Cycle. Acta Physiol. Plant. 2012, 34, 1043–1053. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Chen, F.; Yang, S.; Chen, X. Soil Water Dynamics and Water Use Efficiency in Spring Maize (Zea mays L.) Fields Subjected to Different Water Management Practices on the Loess Plateau, China. Agric. Water Manag. 2010, 97, 769–775. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of Drought-Induced Oxidative Stress in Maize (Zea mays L.) Plants by Dual Application of 24-Epibrassinolide and Spermine. Environ. Exp. Bot. 2015, 113, 47–58. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Mao, Q.; Wan, H.; Zhou, G.; Cheng, Y. The SlWRKY81 Transcription Factor Inhibits Stomatal Closure by Attenuating Nitric Oxide Accumulation in the Guard Cells of Tomato under Drought. Physiol. Plant. 2021, 172, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.A.; Men, S.; Hussain, S.; Zhang, Q.; Ashraf, U.; Anjum, S.A.; Ali, I.; Wang, L. Maize Tolerance against Drought and Chilling Stresses Varied with Root Morphology and Antioxidative Defense System. Plants 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Jan, S.; Abbas, N.; Ashraf, M.; Ahmad, P. Roles of Potential Plant Hormones and Transcription Factors in Controlling Leaf Senescence and Drought Tolerance. Protoplasma 2019, 256, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Effects of Drought, Heat and Their Interaction on the Growth, Yield and Photosynthetic Function of Lentil (Lens Culinaris Medikus) Genotypes Varying in Heat and Drought Sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.M.; Nayyar, H. Individual and Combined Effects of Transient Drought and Heat Stress on Carbon Assimilation and Seed Filling in Chickpea. Funct. Plant Biol. FPB 2014, 41, 1148–1167. [Google Scholar] [CrossRef]
- Raheleh, R.; Ramazanali, K.-N.; Ali, G.; Abdolreza, B.; Farzaneh, N. Drought Stress Effects on Photosynthesis, Chlorophyll Fluorescence and Water Relations in Tolerant and Susceptible Chickpea (Cicer arietinum L.) Genotypes. Acta Biol. Cracoviensia 2011, 53, 47–56. [Google Scholar]
- Samarah, N.H.; Alqudah, A.M.; Amayreh, J.A.; McAndrews, G.M. The Effect of Late-Terminal Drought Stress on Yield Components of Four Barley Cultivars. J. Agron. Crop Sci. 2009, 195, 427–441. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.; Javed, B.; Mashwani, Z.-R.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T.; Akram, A. Foliar Applications of Bio-Fabricated Selenium Nanoparticles to Improve the Growth of Wheat Plants under Drought Stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Blum, A. Drought Resistance, Water-Use Efficiency, and Yield Potential—Are They Compatible, Dissonant, or Mutually Exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Kiani, S.P.; Talia, P.; Maury, P.; Grieu, P.; Heinz, R.; Perrault, A.; Nishinakamasu, V.; Hopp, E.; Gentzbittel, L.; Paniego, N.; et al. Genetic Analysis of Plant Water Status and Osmotic Adjustment in Recombinant Inbred Lines of Sunflower under Two Water Treatments. Plant Sci. 2007, 172, 773–787. [Google Scholar] [CrossRef]
- Luo, X.; Bai, X.; Zhu, D.; Li, Y.; Ji, W.; Cai, H.; Wu, J.; Liu, B.; Zhu, Y. GsZFP1, a New Cys2/His2-Type Zinc-Finger Protein, Is a Positive Regulator of Plant Tolerance to Cold and Drought Stress. Planta 2012, 235, 1141–1155. [Google Scholar] [CrossRef]
- Xu, D.-Q.; Huang, J.; Guo, S.-Q.; Yang, X.; Bao, Y.-M.; Tang, H.-J.; Zhang, H.-S. Overexpression of a TFIIIA-Type Zinc Finger Protein Gene ZFP252 Enhances Drought and Salt Tolerance in Rice (Oryza sativa L.). FEBS Lett. 2008, 582, 1037–1043. [Google Scholar] [CrossRef]
- Sadoogh, F.S.; Shariatmadari, H.; Khoshgoftarmanesh, A.H.; Mosaddeghi, M.R. Adjusted nutrition of tomato with potassium and zinc in drought stress conditions induced by polyethylene glycol 6000 in hydroponic culture. J. Sci. Technol. Greenh. Cult. 2014, 5, 67–80. [Google Scholar]
- Ahmed, N.; Ahmad, F.; Abid, M.; Ullah, M.A. Impact of Zinc Fertilization on Gas Exchange Characteristics and Water Use Efficiency of Cotton Crop under Arid Environment. Pak. J. Bot. 2009, 41, 2189–2197. [Google Scholar]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological Responses and Yield of Wheat Plants in Zinc-Mediated Alleviation of Drought Stress. Front. Plant Sci. 2017, 8, 860. [Google Scholar] [CrossRef]
- Wang, H.; Jin, J. Effects of Zinc Deficiency and Drought on Plant Growth and Metabolism of Reactive Oxygen Species in Maize (Zea mays L). Agric. Sci. China 2007, 6, 988–995. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H. Assessment of Selenium Role in Promoting or Inhibiting Potato Plants under Water Stress. J. Hortic. Sci. Ornam. Plants 2016, 8, 125–139. [Google Scholar]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the Effect of Drought on Growth and Yield of Pomegranates by Foliar Spraying of Different Sizes of Selenium Nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Germ, M.; Stibilj, V.; Kreft, I. Metabolic Importance of Selenium for Plants. Eur. J. Plant Sci. Biotechnol. 2007, 1, 91–97. [Google Scholar]
- Babaeian, M.; Piri, I.; Tavassoli, A.; Esmaeilian, Y.; Gholami, H. Effect of Water Stress and Micronutrients (Fe, Zn and Mn) on Chlorophyll Fluorescence, Leaf Chlorophyll Content and Sunflower Nutrient Uptake in Sistan Region. Afr. J. Agric. Res. 2011, 6, 3526–3531. [Google Scholar]
- Sun, L.; Song, F.; Guo, J.; Zhu, X.; Liu, S.; Liu, F.; Li, X. Nano-ZnO-Induced Drought Tolerance Is Associated with Melatonin Synthesis and Metabolism in Maize. Int. J. Mol. Sci. 2020, 21, 782. [Google Scholar] [CrossRef] [Green Version]
- Danielson, J.Å.; Johanson, U. Unexpected Complexity of the Aquaporin Gene Family in the Moss Physcomitrella Patens. BMC Plant Biol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity Tolerance of Crops–What Is the Cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High Concentrations of Na+ and Cl– Ions in Soil Solution Have Simultaneous Detrimental Effects on Growth of Faba Bean under Salinity Stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Exogenous Selenium Pretreatment Protects Rapeseed Seedlings from Cadmium-Induced Oxidative Stress by Upregulating Antioxidant Defense and Methylglyoxal Detoxification Systems. Biol. Trace Elem. Res. 2012, 149, 248–261. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Mineral Element Acquisition and Growth Response of Plants Grown in Saline Environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Parker, D.R.; Aguilera, J.J.; Thomason, D.N. Zinc-Phosphorus Interactions in Two Cultivars of Tomato (Lycopersicon esculentum L.) Grown in Chelator-Buffered Nutrient Solutions. Plant Soil 1992, 143, 163–177. [Google Scholar] [CrossRef]
- Arough, Y.K.; Sharifi, R.S.; Sedghi, M.; Barmaki, M. Effect of Zinc and Bio Fertilizers on Antioxidant Enzymes Activity, Chlorophyll Content, Soluble Sugars and Proline in Triticale Under Salinity Condition. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 116–124. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Shome, S.; Tewari, S.; Bhattacharya, M.K.; Panda, S. Effect of Zn Nano-Particles on Growth Responses of Rice; Mc Graw Hill PVT.: Delhi, India, 2015. [Google Scholar]
- Torabian, S.; Zahedi, M.; Khoshgoftarmanesh, A. Effect of Foliar Spray of Zinc Oxide on Some Antioxidant Enzymes Activity of Sunflower under Salt Stress. J. Agric. Sci. Technol. 2016, 18, 1013–1025. [Google Scholar]
- Alharby, H.; Hasanuzzaman, M.; Al-Zahrani, H.; Hakeem, K. Exogenous Selenium Mitigates Salt Stress in Soybean by Improving Growth, Physiology, Glutathione Homeostasis and Antioxidant Defense. Phyton-Int. J. Exp. Bot. 2021, 90, 373–388. [Google Scholar] [CrossRef]
- El-Badri, A.M.; Batool, M.; Wang, C.; Hashem, A.M.; Tabl, K.M.; Nishawy, E.; Kuai, J.; Zhou, G.; Wang, B. Selenium and Zinc Oxide Nanoparticles Modulate the Molecular and Morpho-Physiological Processes during Seed Germination of Brassica Napus under Salt Stress. Ecotoxicol. Environ. Saf. 2021, 225, 112695. [Google Scholar] [CrossRef]
- Zengin, F.K.; Munzuroglu, O. Toxic Effects of Cadmium (Cd++) on Metabolism of Sunflower (Helianthus annuus L.) Seedlings. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2006, 56, 224–229. [Google Scholar]
- Soydam Aydin, S.; Gökçe, E.; Büyük, I.; Aras, S. Characterization of Stress Induced by Copper and Zinc on Cucumber (Cucumis sativus L.) Seedlings by Means of Molecular and Population Parameters. Mutat. Res. 2012, 746, 49–55. [Google Scholar] [CrossRef]
- Koç, E.; Üstün, A.S.; Arıcı, Y.K. Effect of different zinc concentrations on total protein, hydrogen peroxide content and peroxidase activity in pepper (Capsicum annuum L.) seedlings. Artvin Çoruh Üniversitesi Orman Fakültesi Derg. 2012, 13, 205–212. [Google Scholar]
- Yadav, S.K. Heavy Metals Toxicity in Plants: An Overview on the Role of Glutathione and Phytochelatins in Heavy Metal Stress Tolerance of Plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef]
- Ghosh, M.; Singh, S. A Review on Phytoremediation of Heavy Metals and Utilization of Its By-Products. Appl. Ecol. Environ. Res. 2005, 3, 1–18. [Google Scholar] [CrossRef]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The Genetic Basis of Metal Hyperaccumulation in Plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Alloway, B. Heavy Metals and Metalloids as Micronutrients for Plants and Animals. In Heavy Metals in Soils-Trace Metals and Metalloids in Soils and Their Bioavailability; Springer: Singapore, 2013; Volume 22, pp. 195–209. [Google Scholar]
- Appenroth, K.-J. Definition of “Heavy Metals” and Their Role in Biological Systems. In Soil Heavy Metals; Soil Biology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 19–29. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Camares, O.; Veisseire, P. Effects of Zinc and Influence of Acremonium Lolii on Growth Parameters, Chlorophyll a Fluorescence and Antioxidant Enzyme Activities of Ryegrass (Lolium perenne L. Cv Apollo). J. Exp. Bot. 2000, 51, 945–953. [Google Scholar] [PubMed]
- Brennan, R. Zinc Application and Its Availability to Plants. Ph.D. Thesis, Murdoch University, Perth, Australia, 2005. [Google Scholar]
- Filek, M.; Keskinen, R.; Hartikainen, H.; Szarejko, I.; Janiak, A.; Miszalski, Z.; Golda, A. The Protective Role of Selenium in Rape Seedlings Subjected to Cadmium Stress. J. Plant Physiol. 2008, 165, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liu, Y.; Gong, X.; Zeng, G.; Zheng, B.; Wang, D.; Sun, Z.; Zhou, L.; Zeng, X. Effects of Selenium and Silicon on Enhancing Antioxidative Capacity in Ramie (Boehmeria nivea (L.) Gaud.) under Cadmium Stress. Environ. Sci. Pollut. Res. Int. 2015, 22, 9999–10008. [Google Scholar] [CrossRef]
- Thounaojam, T.C.; Panda, P.; Choudhury, S.; Patra, H.K.; Panda, S.K. Zinc Ameliorates Copper-Induced Oxidative Stress in Developing Rice (Oryza sativa L.) Seedlings. Protoplasma 2014, 251, 61–69. [Google Scholar] [CrossRef]
- Hassan, M.J.; Zhang, G.; Wu, F.; Wei, K.; Chen, Z. Zinc Alleviates Growth Inhibition and Oxidative Stress Caused by Cadmium in Rice. J. Plant Nutr. Soil Sci. 2005, 168, 255–261. [Google Scholar] [CrossRef]
- Zhao, A.Q.; Tian, X.H.; Lu, W.H.; Gale, W.J.; Lu, X.C.; Cao, Y.X. Effect of Zinc on Cadmium Toxicity in Winter Wheat. J. Plant Nutr. 2011, 34, 1372–1385. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Modulation of Cadmium-Induced Oxidative Stress in Ceratophyllum demersum by Zinc Involves Ascorbate-Glutathione Cycle and Glutathione Metabolism. Plant Physiol. Biochem. PPB 2005, 43, 107–116. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Cadmium-Zinc Interactions in a Hydroponic System Using Ceratophyllum demersum L.: Adaptive Ecophysiology, Biochemistry and Molecular Toxicology. Braz. J. Plant Physiol. 2005, 17, 3–20. [Google Scholar] [CrossRef]
- Cherif, J.; Mediouni, C.; Ben Ammar, W.; Jemal, F. Interactions of Zinc and Cadmium Toxicity in Their Effects on Growth and in Antioxidative Systems in Tomato Plants (Solanum lycopersicum). J. Environ. Sci. China 2011, 23, 837–844. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Zinc Alleviates Cadmium-Induced Oxidative Stress in Ceratophyllum demersum L.: A Free Floating Freshwater Macrophyte. Plant Physiol. Biochem. 2003, 41, 391–397. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Kochian, L.V. Transport Interactions between Cadmium and Zinc in Roots of Bread and Durum Wheat Seedlings. Physiol. Plant. 2002, 116, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S. Toxic Metal Accumulation, Responses to Exposure and Mechanisms of Tolerance in Plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Clabeaux, B.L.; Navarro, D.A.; Aga, D.S.; Bisson, M.A. Combined Effects of Cadmium and Zinc on Growth, Tolerance, and Metal Accumulation in Chara Australis and Enhanced Phytoextraction Using EDTA. Ecotoxicol. Environ. Saf. 2013, 98, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, N.K.; Akoumianaki, I.A.; Barouchas, P.E. The Effects of Cadmium and Zinc Interactions on the Concentration of Cadmium and Zinc in Pot Marigold (“Calendula officinalis” L.). Aust. J. Crop Sci. 2011, 5, 277–282. [Google Scholar]
- Köleli, N.; Eker, S.; Cakmak, I. Effect of Zinc Fertilization on Cadmium Toxicity in Durum and Bread Wheat Grown in Zinc-Deficient Soil. Environ. Pollut. Barking Essex 1987 2004, 131, 453–459. [Google Scholar] [CrossRef]
- Zhang, Z.-W.; Dong, Y.-Y.; Feng, L.-Y.; Deng, Z.-L.; Xu, Q.; Tao, Q.; Wang, C.-Q.; Chen, Y.-E.; Yuan, M.; Yuan, S. Selenium Enhances Cadmium Accumulation Capability in Two Mustard Family Species-Brassica Napus and B. Juncea. Plants 2020, 9, 904. [Google Scholar] [CrossRef]
- Shekari, L.; Kamelmanesh, M.M.; Mozafariyan, M.; Hasanuzzaman, M.; Sadeghi, F. Role of Selenium in Mitigation of Cadmium Toxicity in Pepper Grown in Hydroponic Condition. J. Plant Nutr. 2017, 40, 761–772. [Google Scholar] [CrossRef]
- Porter, J.R.; Gawith, M. Temperatures and the Growth and Development of Wheat: A Review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 Availability and Inactivation of Rubisco Limit Photosynthesis in Cotton Plants under Heat and Drought Stress in the Field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Seliem, M.K.; Hafez, Y.; El-Ramady, H. Using Nano-Selenium in Reducing the Negative Effects of High Temperature Stress on Chrysanthemum Morifolium Ramat. J. Sustain. Agric. Sci. 2020, 46, 47–60. [Google Scholar] [CrossRef]

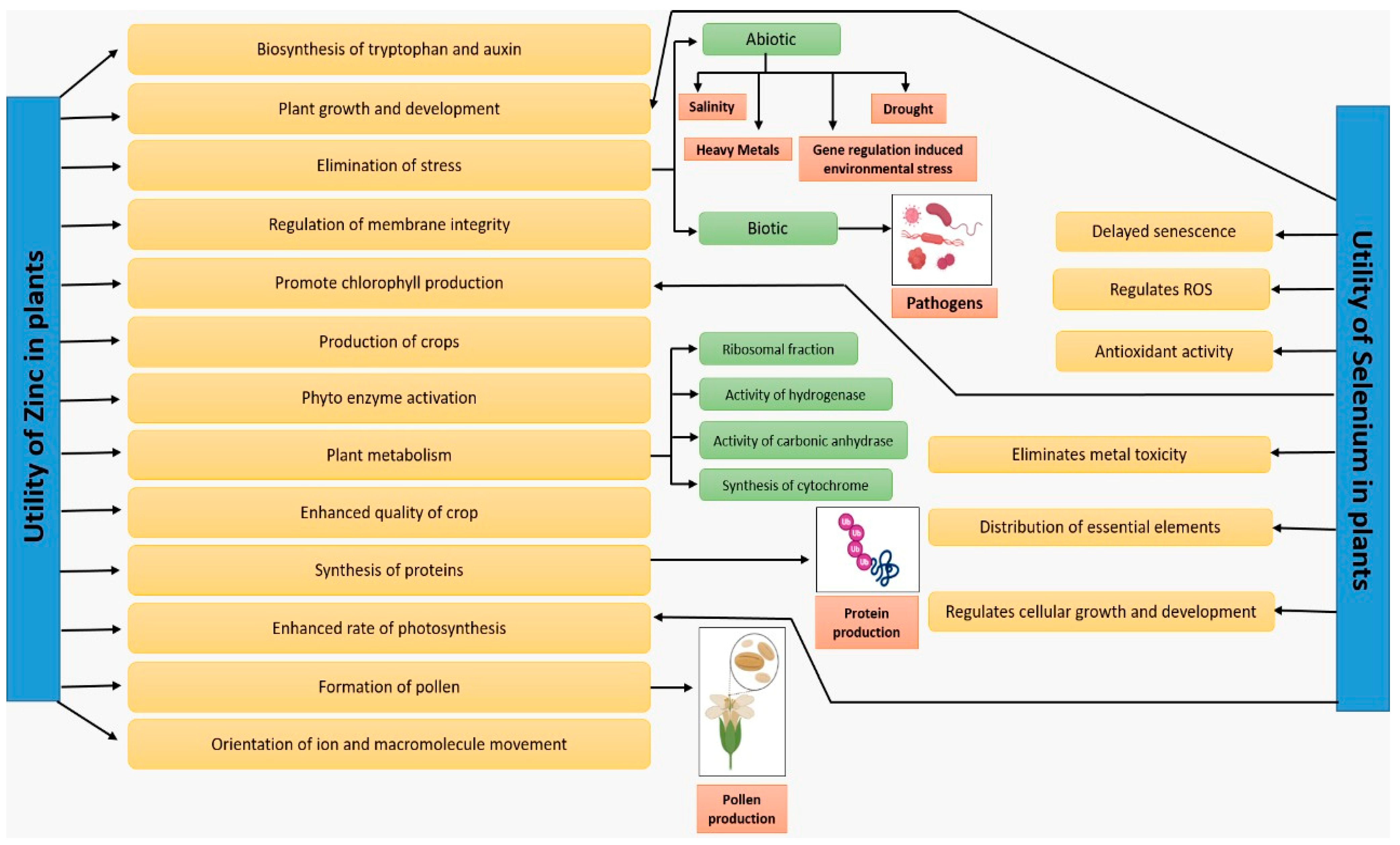


| Name of Plant. | The Part on Which Zn Is Applied | Utilities as a Result of Zn Application | References |
|---|---|---|---|
| Maize | Foliar application | Increases starch content Improves crop yield | [46] |
| Mungbean | Foliar application | Increases the growth and productivity of the crop | [48] |
| Maize and Wheat | Foliar application | Enhances the yield of both the grains | [45] |
| French Bean | Foliar application | Improves in the physiological traits Enhances crop quality Improves productivity of grains | [49] |
| Maize | Soil | Advances crop yield | [51] |
| Wheat | Soil | Increases in grain productivity | [52] |
| Wheat | Foliar + soil | remarkable boost in the yield | [27] |
| Bread wheat | Foliar + soil | Improves the growth rate Enhances crop productivity Increases Zn content in wheat | [28] |
| Garden pansy | Foliar application of Se in the form of sodium selenate | Increases fresh weight by 25.10% Increases dry weight by 25.41% | [51] |
| Rice | Foliar application of Se in the form of selenate and selenite | Enhances the productivity of rice grains | [47] |
| Rice | Soil application of Se | Produces more shoot dry matter | [47] |
| Oat | Foliar + soil application of Se in the form of Se fertilizer | Improves Se transport Enhances crop yield | [52] |
| Atractylodes macrocephala | Foliar application of Se | Increases the growth and survival rate Enhances crop yield | [53] |
| Name of Plant Affected by Drought Stress | Effect of Drought Stress on the Crop | References |
|---|---|---|
| Wheat | Affects all growth stages Affects the grain-filling stage of reproduction | [55] |
| Spring wheat | Terminates the enzyme activities responsible for sucrose and starch synthesis | [57] |
| Wheat | Damages nutrient uptake capacity Disrupts nutrient transport and absorption Affects plant-nutrient relation | [58] |
| Maize | Damages growth and crop yield significantly | [62,63,64] |
| Tomato | Prevents stomatal closure | [65] |
| Maize | Provokes several morphological changes in the crop | [66] |
| Legumes | Decreases water absorption capacity Reduces the rate of gaseous exchange Hampers crop productivity | [68] |
| Lentils | Affects stomatal movement Ultimately results in the drooping of leaves | [68,69] |
| Chickpea | Damages penetrability of the membrane, nutrient intake, rate of chlorophyll synthesis, and photosynthesis | [70,71] |
| Barley | Reduces crop yield | [72] |
| Name of Plant Species | Name of Microelement Showing Ameliorating Effects | Name of Heavy Metal | Ameliorating the Role of Zn against That Heavy Metal | References |
|---|---|---|---|---|
| Rice | Zn | Cu | Reduces Cu-induced toxicity Decreases oxidative stress | [110] |
| Rice | Zn | Cu | Reduces oxidative damage Impedes phytotoxic symptoms in rice | [113,114] |
| Rice | Zn | Cd | Enhances chlorophyll content and rate of photosynthesis Increases plant biomass Improves overall growth | [111] |
| Wheat | Zn | Cd | Reduces Cd-induced toxicity to the growth parameters | [112] |
| Tomato | Zn | Cd | Limits Cd accumulation Reduces Cd-induced toxic effects | [115] |
| Ceratophyllum demersum | Zn | Cd | Attenuates membrane damage Reduces oxidative stress | [116] |
| Bread wheat and durum wheat | Zn | Cd | Alleviates Cd toxicity | [117] |
| Angiosperms | Zn | Cd | Alleviates Cd toxicity | [118] |
| Chara australis | Zn | Cd | Diminishes Cd uptake and accumulation | [119] |
| Marigold | Zn | Cd | Diminishes Cd uptake and accumulation | [120] |
| Bread wheat and durum wheat | Zn | Cd | Treats chlorosis and necrosis induced by Cd | [121] |
| Brassica napus and B. juncea | Se | Cd | Improves Cd accumulation capacity | [122] |
| Pepper | Se | Cd | Enhances the antioxidant activities | [123] |
| Rape seedlings | Se | Cd | Reduces Cd stress | [116] |
| Ramie (Boehmeria nivea (L.) Gaud | Se | Cd | Enhances antioxidative capacity in) | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganguly, R.; Sarkar, A.; Dasgupta, D.; Acharya, K.; Keswani, C.; Popova, V.; Minkina, T.; Maksimov, A.Y.; Chakraborty, N. Unravelling the Efficient Applications of Zinc and Selenium for Mitigation of Abiotic Stresses in Plants. Agriculture 2022, 12, 1551. https://doi.org/10.3390/agriculture12101551
Ganguly R, Sarkar A, Dasgupta D, Acharya K, Keswani C, Popova V, Minkina T, Maksimov AY, Chakraborty N. Unravelling the Efficient Applications of Zinc and Selenium for Mitigation of Abiotic Stresses in Plants. Agriculture. 2022; 12(10):1551. https://doi.org/10.3390/agriculture12101551
Chicago/Turabian StyleGanguly, Retwika, Anik Sarkar, Disha Dasgupta, Krishnendu Acharya, Chetan Keswani, Victoria Popova, Tatiana Minkina, Aleksey Yu Maksimov, and Nilanjan Chakraborty. 2022. "Unravelling the Efficient Applications of Zinc and Selenium for Mitigation of Abiotic Stresses in Plants" Agriculture 12, no. 10: 1551. https://doi.org/10.3390/agriculture12101551






