Effects of Knotweed-Enriched Feed on the Blood Characteristics and Fitness of Horses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Horses and Husbandry
2.2. Experimental Design
2.3. Feed and Feeding Regimen
- (1)
- Local grass hay. The real consumption of hay per day, per horse by the 1-year-old, 2-year-old and 3-year-old warmbloods was 4, 5.5 and 8 kg hay, respectively; by the 1-year-old and 2-year-old coldbloods, it was 6 and 10 kg hay, respectively. For the nutrient content, see Table 1;
- (2)
- A regular feed supplement for foals and stallions in amounts covering their nutrient requirements with respect to their age and breed, i.e., 1.5 and 2.0 kg for 1-year old, 2.0 and 2.5 kg for 2-year old and 2.5 and 3.0 kg for 3-year old warm- and cold-blooded horses, supplied by a regional feed producer RenoFarmy (Troubky, Czech Republic), specially prepared for the horse breeding farm Tlumačov to meet the feeding requirements of the young, 1–3-year-old stallions kept there. The feed supplement was fed to them, together with grain oats. For the composition and amounts of the individual components of the feed supplement and grain oats, see Table 1;
- (3)
- 0.5 kg of the experimental mixture prepared and supplied by Dibaq (Helvíkovice, Czech Republic). For the composition and nutrient contents, see Table 1.
2.4. Measured Characteristics
2.4.1. Analysis of Resveratrol, Piceid, Astringin and Piceatannol in Knotweed Biomass
2.4.2. Blood Testing: Haematology and Biochemistry
2.5. Statistical Data Evaluation
3. Results
3.1. Blood Characteristics—Overview
3.2. Blood Characteristics of Significance
3.2.1. Total Protein
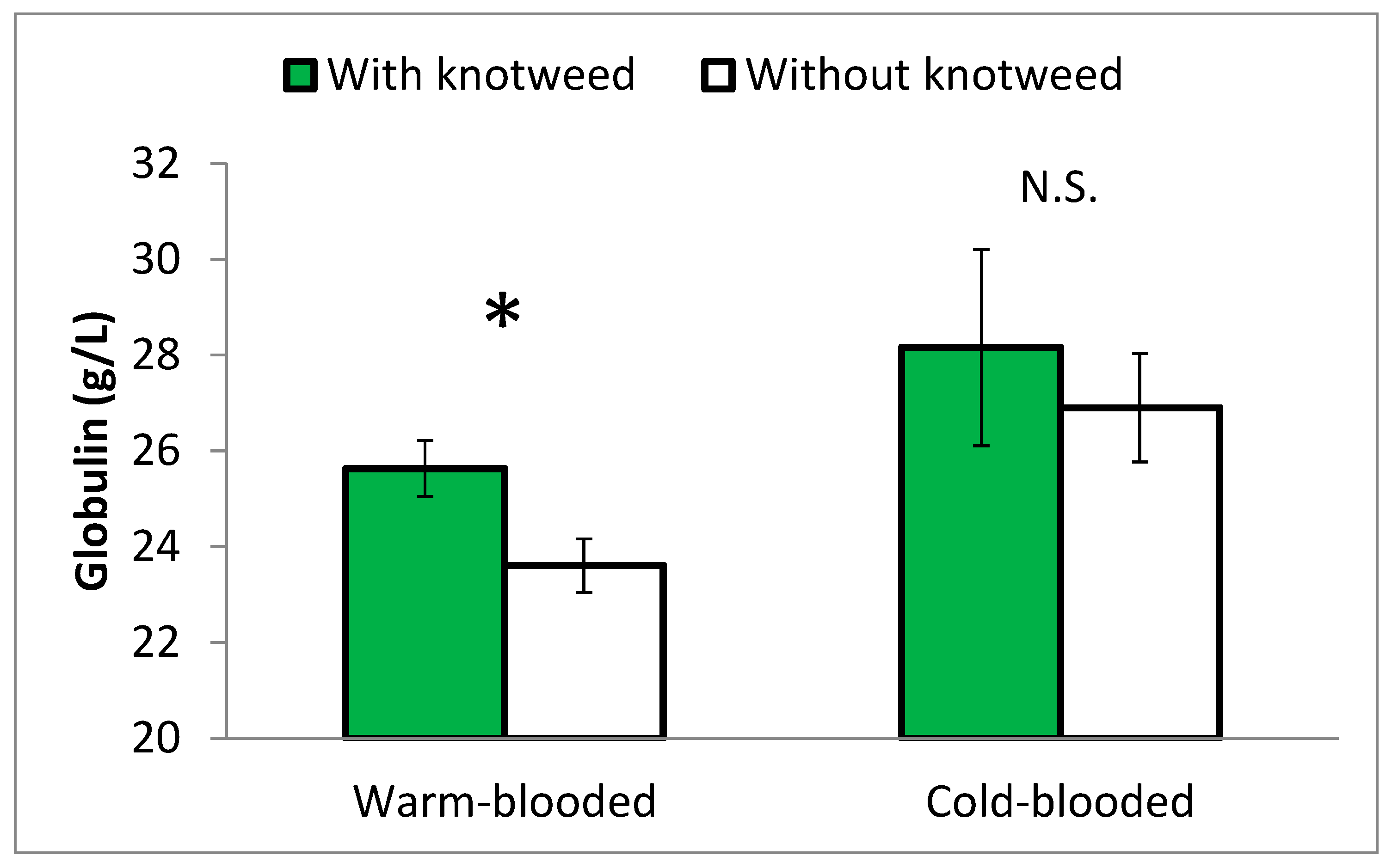
3.2.2. Globulin and Albumin/Globulin Ratio
3.2.3. Thrombocytes, Plateletcrit (PCT) and Mean Cell Hemoglobin (MCH)
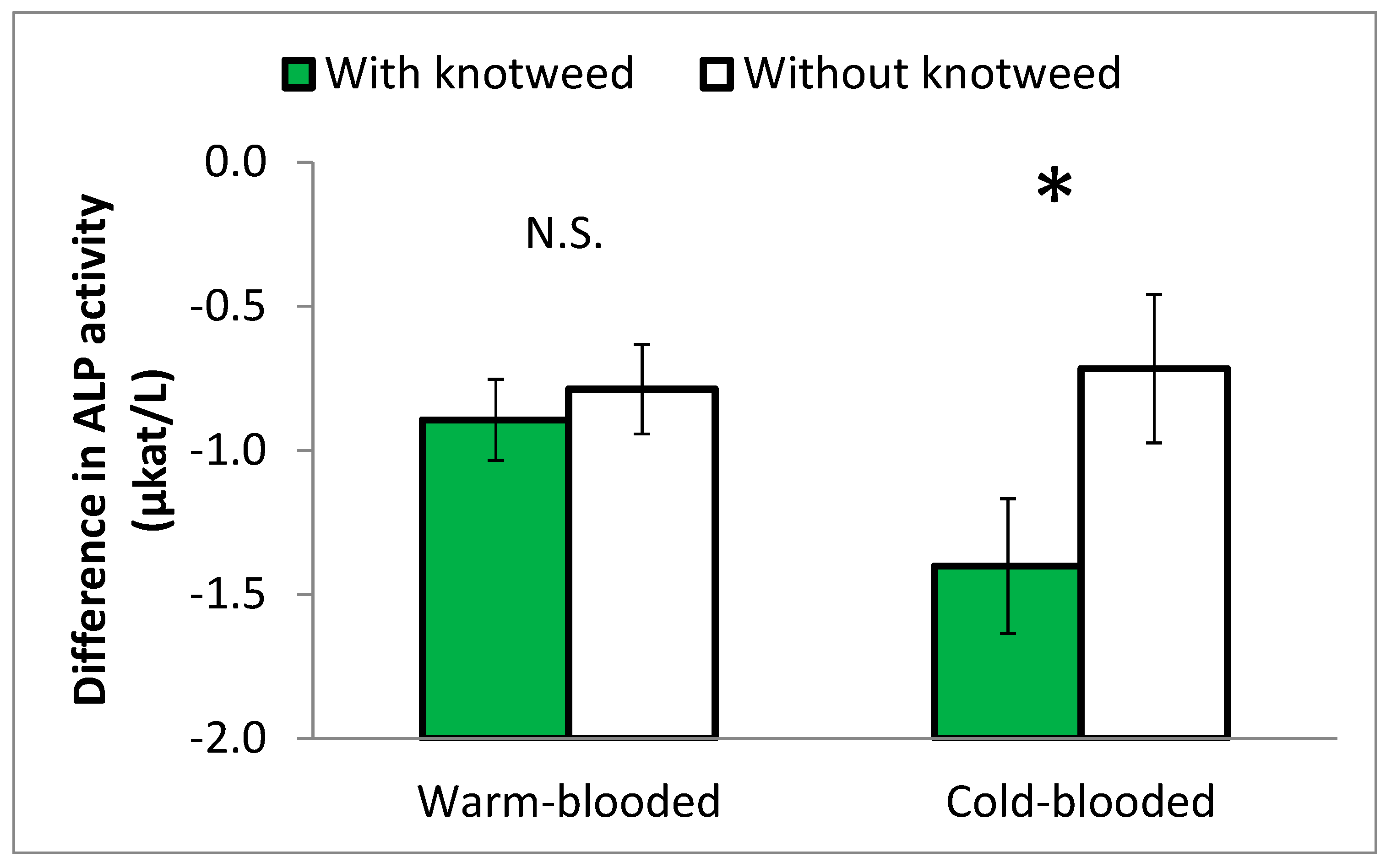
3.2.4. Activity of ALP
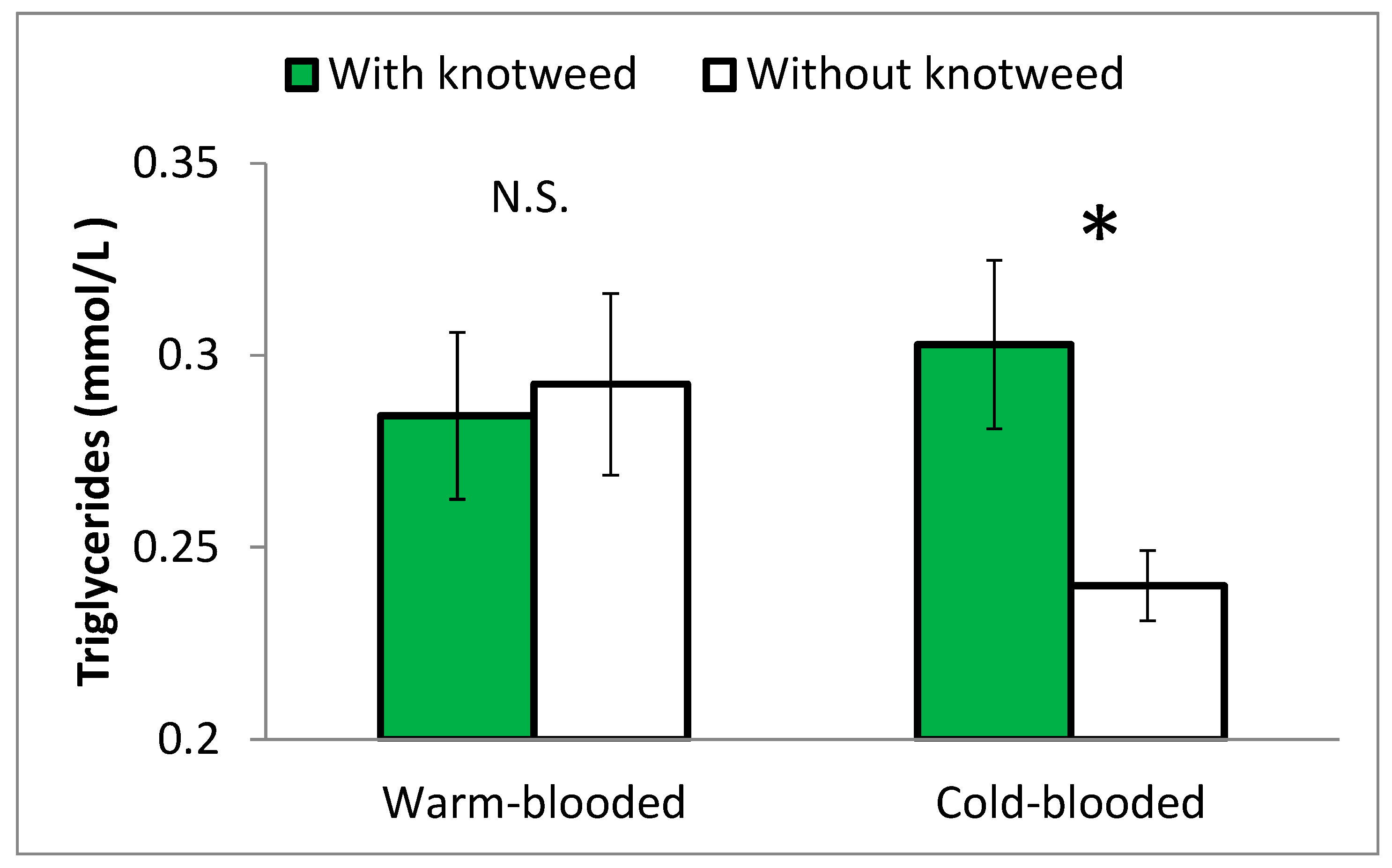
3.2.5. Triglycerides
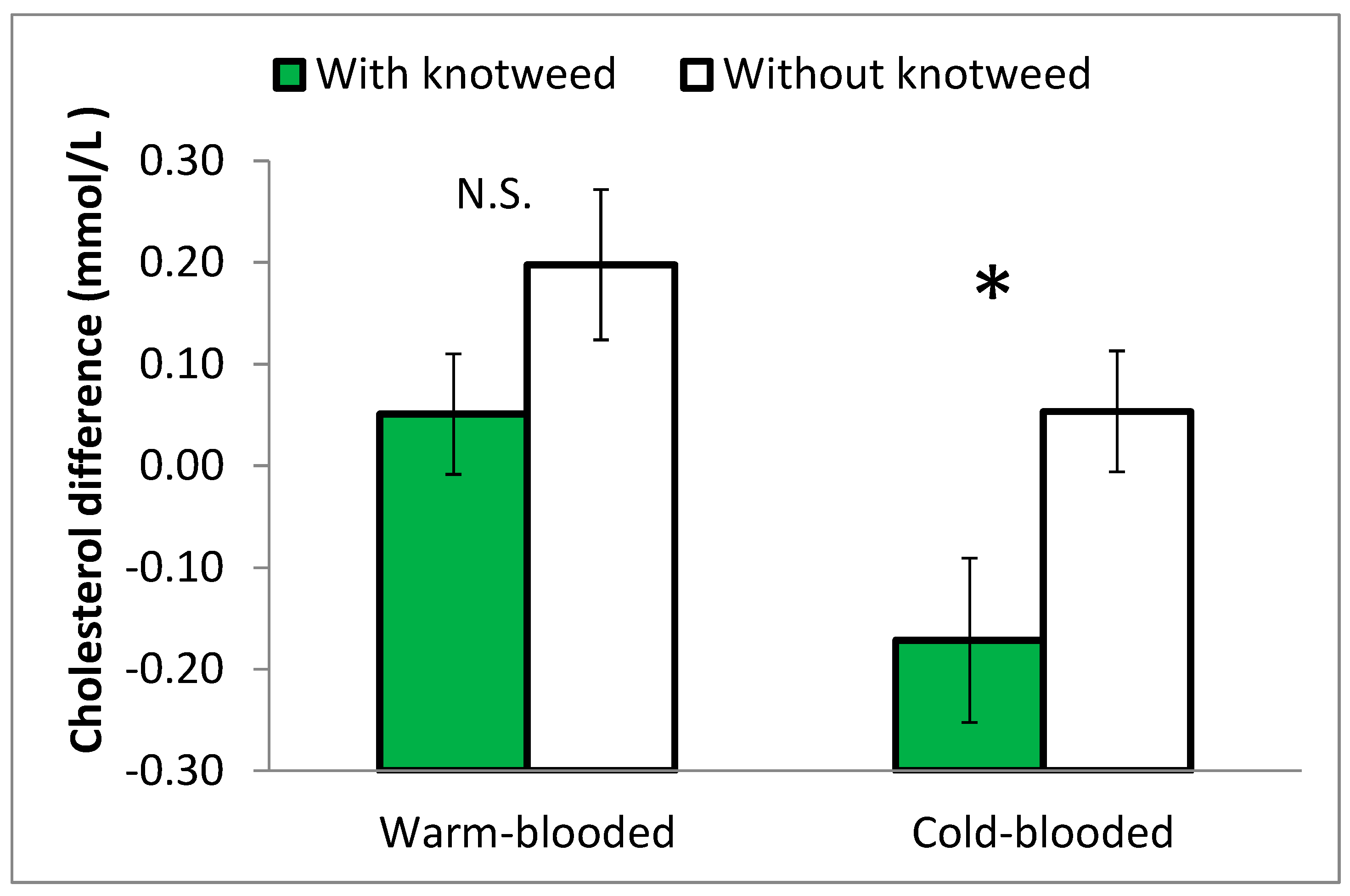
3.2.6. Cholesterol
3.2.7. Urea
3.3. Sports Carrier of the Experimental Horses
4. Discussion
4.1. Biochemistry and Haematology
4.1.1. Total Protein, Globulins and Albumin/Globulin Ratio
4.1.2. Thrombocytes
4.1.3. Activity of Alkaline Phosphatase (ALP)
4.1.4. Triglycerides
4.1.5. Cholesterol
4.1.6. Urea
4.1.7. Urea, ALP, Triglycerides and Cholesterol Interactions
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Chen, H.; Wei, W.; Ye, S.; Liao, W.; Gong, J.; Jiang, Z.; Wang, L.; Lin, S. Antiproliferative and antimetastatic effects of emodin on human pancreatic cancer. Oncol. Rep. 2011, 1257, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Kovářová, M.; Bartůňková, K.; Frantík, T.; Koblihová, H.; Prchalová, K.; Vosátka, M. Factors influencing the production of stilbenes by the knotweed, Reynoutria × bohemica. BMC Plant Biol. 2010, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Kovářová, M.; Bartůňková, K.; Frantík, T.; Koblihová, H.; Prchalová, K.; Vosátka, M. Effect of clone selection, nitrogen supply, leaf damage and mycorrhizal fungi on stilbene and emodin production in knotweed. BMC Plant Biol. 2011, 11, 98. [Google Scholar] [CrossRef] [Green Version]
- Kurita, S.; Kashiwagi, T.; Ebisu, T.; Shimamura, T.; Ukeda, H. Content of resveratrol and glycoside and its contribution to the antioxidative capacity of Polygonum cuspidatum (Itadori) harvested in Kochi. Biosci. Biotechnol. Biochem. 2014, 78, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Kurita, S.; Kashiwagi, T.; Ebisu, T.; Shimamura, T.; Ukeda, H. Identification of neochlorogenic acid as the predominant antioxidant in Polygonum cuspidatum leaves. Ital. J. Food Sci. 2016, 28, 25–31. [Google Scholar]
- Metličar, V.; Vovk, I.; Albreht, A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Malaguarnera, L. Influence of Resveratrol on the Immune Response. Nutrients 2019, 11, 946. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yu, C.-H.; Jiang, Y.-P.; Peng, C.; He, K.; Tang, J.-Y.; Xin, H.-L. Protective Effects of Polydatin from Polygonum cuspidatum against Carbon Tetrachloride-Induced Liver Injury in Mice. PLoS ONE 2012, 7, e46574. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.Y.; Chen, L.X.; Fang, L.; Zhang, Q. Cardioprotective effects of polydatin against myocardial injury in diabetic rats via inhibition of NADPH oxidase and NF-κB activities. BMC Complement. Med. Ther. 2020, 20, 378. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Li, X.; Luo, D.; Yan, W.; Hou, Y.; Hou, Y.; Chen, S.; Luan, J.; Zhang, Q.; Lin, D. Polydatin Attenuates OGD/R-Induced Neuronal Injury and Spinal Cord Ischemia/Reperfusion Injury by Protecting Mitochondrial Function via Nrf2/ARE Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6687212. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, T.; Lei, X.; Li, Y.; Dai, X.; Cao, Y.; Ding, Q.; Lei, X.; Li, T.; Lin, X. Neuroprotective effects of polydatin against mitochondrial-dependent apoptosis in the rat cerebral cortex following ischemia/reperfusion injury. Mol. Med. Rep. 2016, 14, 5481–5488. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Role of Polydatin: Neuropharmacological Mechanisms, Molecular Targets, Therapeutic Potentials, and Clinical Perspective. Molecules 2021, 26, 5985. [Google Scholar] [CrossRef]
- Luce, A.; Lama, S.; Millan, P.C.; Itro, A.; Sangiovanni, A.; Caputo, C.; Ferranti, P.; Cappabianca, S.; Caraglia, M.; Stiuso, P. Polydatin Induces Differentiation and Radiation Sensitivity in Human Osteosarcoma Cells and Parallel Secretion through Lipid Metabolite Secretion. Oxid. Med. Cell. Longev. 2021, 2021, 3337013. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Hou, Y.; Huang, J.; Pan, W.; Ma, Q.; Shi, Y.; Li, C.; Zhao, J.; Jia, Z.; Jiang, H.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar] [CrossRef]
- Jacob, C.; Kirsch, G.; Slusarenko, A.; Winyard, P.G.; Burkholz, T. Recent Advances in Redox Active Plant and Microbial Products: From Basic Chemistry to Widespread Applications in Medicine and Agriculture; Springer: New York, NY, USA, 2020. [Google Scholar]
- Park, S.; Lim, J.; Kim, J.R.; Cho, S. Inhibitory effects of resveratrol on hepatitis B virus X protein-induced hepatocellular carcinoma. J. Vet. Sci. 2017, 18, 419–429. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Tao, J.; Zhang, T.; Jiang, S.; Wei, W.; Han, H.; Shao, Y.; Zhou, G.; Yue, H. Resveratroloside alleviates postprandial hyperglycemia in diabetic mice by competitively inhibiting α-glucosidase. J. Agric. Food Chem. 2019, 67, 2886–2893. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Antibacterial properties of Polygonum cuspidatum roots and their major bioactive constituents. Food Chem. 2008, 109, 530–537. [Google Scholar] [CrossRef]
- Su, P.W.; Yang, C.H.; Yang, J.F.; Su, P.Y.; Chuang, L.Y. Antibacterial Activities and Antibacterial Mechanism of Polygonum cuspidatum Extracts against Nosocomial Drug-Resistant Pathogens. Molecules 2015, 20, 11119–11130. [Google Scholar] [CrossRef] [Green Version]
- Franck, T.; Kohnen, G.; Deby-Dupont, S.; Grulke, C.; Serteyn, D.D. A specific method for measurement of equine active myeloperoxidase inbiological samples and in in vitro tests. J. Vet. Diagn. Investig. 2006, 18, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Cavallaro, A.; Ainis, T.; Bottari, C.; Fimiani, V. Effect of resveratrol on some activities of isolated and in whole blood human neutrophils. Physiol. Res. 2003, 52, 555–562. [Google Scholar]
- Kohnen, S.; Franck, T.; Van Antwerpen, P.; Boudjeltia Zouaoui, K.; Mouithys-Mickalad, A.; Deby, C.; Moguilevsky, N.; Deby, G.; Lamy, N.; Serteyn, D. Resveratrol inhibits the activity of equine neutrophil myeloperoxidase by a direct interaction with the enzyme. J. Agric. Food Chem. 2007, 55, 8080–8087. [Google Scholar] [CrossRef] [PubMed]
- Zambito, J.L. Effects of Resveratrol Supplementation on Glycemic Response and Oxidant Status in Moderately Exercised Mature Quarter Horse Geldings. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2011; 96p. Available online: https://researchrepository.wvu.edu/cgi/viewcontent.cgi?article=4337&context=etd (accessed on 11 September 2021).
- Lawless, P. Two New Research Projects Study on Resveratrol’s Effect on EMS. Biological Prospects/Equithrive. 2010. Available online: https://horsenetwork.com/2017/02/resverasyn-can-help-horse/ (accessed on 13 October 2021).
- Lawless, P. What is Resverasyn and How Can It Help My Horse. Available online: https://equithrive.com/blogs/news/what-is-resverasyn-and-how-can-it-help-my-horse?_pos=2&_sid=22ad88df5&_ss=r (accessed on 13 October 2021).
- Lawless, P.; Equithrive. Resverasyn, the Key Ingredient. Available online: https://equithrive.com/pages/resverasyn (accessed on 13 October 2021).
- Ememe, M.U.; Abdullahi, U.S.; Sackey, A.K.; Ayo, J.O.; Mshelia, W.P.; Edeh, R.E. Effects of a joint supplement whose main components are resveratrol and hyaluronic acid on some biochemical parameters in aged lame horses. J. Equine Sci. 2016, 27, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Ememe, M.U.; Msheliaa, W.P.; Ayo, J.O. Ameliorative Effects of Resveratrol on Oxidative Stress Biomarkers in Horses. J. Equine Vet. Sci. 2015, 35, 518–523. [Google Scholar] [CrossRef]
- Watts, A.E.; Dabareiner, R.; Marsh, C.; Carter, K.; Cummings, K.J. A randomized, controlled trial of the effects of resveratrol administration in performance horses with lameness localized to the distal tarsal joints. J. Am. Vet. Med. Assoc. 2016, 249, 650–659. [Google Scholar] [CrossRef]
- Siard, M.H.; McMurry, K.E.; Adams, A.A. Effects of polyphenols including curcuminoids, resveratrol, quercetin, pterostilbene, and hydroxypterostilbene on lymphocyte pro-inflammatory cytokine production of senior horses in vitro. Vet. Immunol. Immunopathol. 2016, 173, 50–59. [Google Scholar] [CrossRef]
- Kalughina, E.G.; Stolbova, O.A. Praziver® and Ivermek® effectiveness for horse helminthiase prevention. Eurasian J. Biosci. 2020, 14, 317–322. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012; p. 362. [Google Scholar]
- Werner, L.L.; Turnwald, G.H.; Willard, M.D. Immunologic and Plasma Protein Disorders. Small Anim. Clin. Diagn. Lab. Methods 2004, 290–305. [Google Scholar] [CrossRef]
- Gounden, V.; Vashisht, R.; Jialal, I. Hypoalbuminemia; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Tothova, C.; Nagy, O.; Kovac, G. Serum proteins and their diagnostic utility in veterinary medicine: A review. Vet. Med. 2016, 61, 475–496. [Google Scholar] [CrossRef] [Green Version]
- Harvey, J.W. Veterinary Hematology II Evaluation of Hemostasis; Elsevier: St. Louis, MO, USA, 2012; pp. 191–233. [Google Scholar] [CrossRef]
- Maděra, P.; Kovářová, M.; Frantík, T.; Filipčík, R.; Novák, J.; Vencl, Š.; Maděrová, L.; Rozkot, M.; Kuchařová, S.; Václavková, E.; et al. Effect of knotweed in diet on physiological changes in pig. Agriculture 2021, 11, 169. [Google Scholar] [CrossRef]
- Ferry-Dumazet, H.; Garnier, O.; Mamani-Matsuda, M.; Vercauteren, J.; Belloc, F.; Billiard, C.; Dupouy, M.; Thiolat, D.; Kolb, J.P.; Marit, G. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carconigenesis 2002, 23, 1323–1333. [Google Scholar] [CrossRef]
- Baker, D. Diagnosis of disorders of hemostasis. In Veterinary Hematology and Clinical Chemistry; Thrall, M., Baker, D., Campbell, T., Eds.; Blackwell Publishing: Ames, IA, USA, 2006; pp. 179–196. [Google Scholar]
- Sharma, S.; Parashar, P.; Sharma, S.; Sharma, K.P. Ameliorating role of lycopene, tomato puree, and Spirulina + tomato puree on the hematology of fluoride-exposed Swiss Albino mice. J. Diet. Suppl. 2018, 15, 827–841. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, R.; Saleem, M.; Bilal, M.; Rashid, R.; Khan, I.; Mahmood, A.; Nawaz, M. Raman spectroscopic analysis of dengue virus infection in human blood sera. Optik 2016, 127, 2086–2088. [Google Scholar] [CrossRef]
- Szulc, P.; Bauer, D.C. Biochemical markers of bone turnover in osteoporosis. In Osteoporosis, 4th ed.; Marcus, R., Feldman, D., Dempster, D.W., Luckey, M., Cauley, J.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1573–1610. [Google Scholar] [CrossRef]
- Kraft, W.; Dürr, U.M. Referenzwerte in Klinische Labordiagnostik der Tiermedizin. In Klinische Labordiagnostik in der Tiermedizin, 6th ed.; Kraft, W., Ed.; Schattauer: Stuttgart, Germany, 2005; pp. 339–357. [Google Scholar]
- Lassen, E.; Fettman, M. Laboratory evaluation of lipids. In Veterinary Hematology and Clinical Chemistry; Thrall, M.A., Weiser, G., Allison, R.W., Campbell, T.W., Eds.; Willey-Blackwell: Hoboken, NJ, USA, 2006; pp. 421–429. [Google Scholar]
- Nawrot-Hadzik, I.; Hadzik, J.; Fleischer, M.; Choromańska, A.; Sterczała, B.; Kubasiewicz-Ross, P.; Saczko, J.; Gałczyńska-Rusin, M.; Gedrange, T.; Matkowski, A. Chemical Composition of East Asian Invasive Knotweeds, their Cytotoxicity and Antimicrobial Efficacy Against Cariogenic Pathogens: An In-Vitro Study. Med. Sci. Monit. 2019, 25, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Arichi, H.; Kimura, Y.; Okuda, H.; Baba, K.; Kozawa, M.; Arichi, S. Effects of Stilbene Components of the roots of Polygonum cuspidatum Sieb. et Zucc. on Lipid Metabolism. Chem. Pharm. Bull. 1982, 30, 1766–1770. [Google Scholar] [CrossRef] [Green Version]
- Tung-Ting, S.; Meng-Heng, L.; Chi-On, C.; Huan, Z.; Shun-Wan, C.; Kam-Wah Mok, D. Cholesterol-lowering effects of piceatannol, a stilbene from wine, using untargeted metabolomics. J. Funct. Foods 2017, 28, 127–137. [Google Scholar] [CrossRef]
- Du, J.; Sun, L.N.; Xing, W.W.; Huang, B.K.; Jia, M.; Wu, J.Z.; Zhang, H.; Qin, L.P. Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine 2009, 16, 652–658. [Google Scholar] [CrossRef]
- Patocka, J.; Navratilova, Z.; Ovando, M. Biologically Active Compounds of Knotweed (Reynoutria spp.). Mil. Med. Sci. Lett. 2017, 86, 17–31. [Google Scholar] [CrossRef] [Green Version]
- Reece, W.O. Fyziologie A Funkční Anatomie Domácích Zvířat, 2nd ed.; Grada Publishing: Prague, Czech Republic, 2011; pp. 313–360. [Google Scholar]
- Kusano, R.; Andou, H.; Fujieda, M.; Tanaka, T.; Matsuo, Y.; Kouno, I. Polymer-like Polyphenols of Black Tea and Their Lipase and Amylase Inhibitory Activities. Chem. Pharm. Bull. 2008, 56, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiang-Wen, L.; Feng-Lin, Y.; Haw-Wei, H.; Tzu-Hui, W.; Horng-Huey, K.; Wen-Sheng, T.; Chun-Ching, L. Resveratrol Nanoparticle System Improves Dissolution Properties and Enhances the Hepatoprotective Effect of Resveratrol through Antioxidant and Anti-Inflammatory Pathways. J. Agric. Food Chem. 2012, 60, 4662–4671. [Google Scholar] [CrossRef]
- Xu, L.; Huang, G.; Guo, X.; Zhou, Q.; He, S. Total flavonoids extracted from Polygonum knotweed L, exerts beneficial hepatoprotection against liver injury. J. Cell. Biochem. 2019, 120, 12677–12683. [Google Scholar] [CrossRef] [PubMed]
- Fonnesbeck, P.V.; Linda, D.; Symons, L.U. Effect of Diet on Concentration of Protein, Urea Nitrogen, Sugar and Cholesterol of Blood Plasma of Horses. J. Anim. Sci. 1969, 28, 216–219. [Google Scholar] [CrossRef]
- Owen, J.M.; McCullagh, K.G.; Crook, D.H.; Hinton, M. Seasonal Variations in the Nutrition of Horses at Grass. Equine Vet. J. 1978, 10, 260–266. [Google Scholar] [CrossRef]
- Morris, D.M.; Zilversmit, B.D.; Hintz, F.H. Hyperlipoproteinaemia in fasting ponies. J. Lipid Res. 1972, 13, 383–389. [Google Scholar] [CrossRef]
- Watson, T.D.; Burns, L.; Love, S.; Packard, C.J.; Shepherd, J. The isolation, characterisation and quantification of the equine plasma lipoproteins. Equine Vet. J. 1991, 23, 353–359. [Google Scholar] [CrossRef]
- Watson, T.D.; Packard, C.J.; Shepherd, J. Plasma lipid transport in the horse (Equus caballus). Comp. Biochem. Physiol. B 1993, 106, 27–34. [Google Scholar] [CrossRef]
- Wensing, T.H.; Van Gent, C.M.; Schotman, A.J.H.; Kroneman, J. Hyperlipoproteinaemia in ponies: Mechanisms and response to therapy. Clin. Chim. Acta 1975, 58, 1–15. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, H.C., III. Equine hyperlipidemias. Vet. Clin. Equine Pract. 2011, 27, 59–72. [Google Scholar] [CrossRef] [PubMed]



| Unit | Grass Hay | Feed Supplement | Experimental Mixture for Control Horses | Experimental Mixture for Knotweed-Fed Horses | Salt | Control Horses, Total | Knotweed Fed Horses, Total | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Oat Grain | Supplementary Feed | KnotWeed | Rest of Mixture | |||||||
| INTAKE | kg/day | 8.00 | 0.25 | 2.50 | 0.50 | 0.15 | 0.35 | 0.03 | 11.28 | 11.28 |
| ENERGY | MJ/day | 56.90 | 3.10 | 28.20 | 6.50 | 1.18 | 4.63 | 0.00 | 94.70 | 94.01 |
| Protein | g/day | 752.00 | 29.50 | 432.50 | 51.50 | 18.97 | 35.6 | 0.00 | 1265.50 | 1268.57 |
| Lysine | g/day | 24.00 | 1.00 | 17.50 | 1.70 | 0.73 | 1.19 | 0.00 | 44.20 | 44.42 |
| Calcium | g/day | 26.40 | 0.20 | 22.50 | 0.22 | 1.2 | 0.15 | 0.00 | 49.32 | 50.45 |
| Phosphorus | g/day | 25.60 | 0.80 | 15.00 | 1.97 | 0.37 | 1.37 | 0.00 | 43.37 | 43.14 |
| Magnesium | g/day | 12.80 | 0.30 | 5.00 | 0.42 | 0.39 | 0.29 | 0.00 | 18.52 | 18.78 |
| Sodium | g/day | 1.60 | 0.10 | 4.00 | 0.04 | 0.07 | 0.03 | 10.00 | 15.74 | 15.80 |
| Selenium | mg/day | 0.80 | 0.05 | 0.71 | 0.01 | 0.01 | 0.01 | 0.00 | 1.57 | 1.58 |
| Copper | mg/day | 56.00 | 1.50 | 18.30 | 1.78 | 0.77 | 1.25 | 0.00 | 77.58 | 77.82 |
| Zinc | mg/day | 206.40 | 8.80 | 255.00 | 10.73 | 5.86 | 7.46 | 0.00 | 480.93 | 483.52 |
| Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|
| Biochemical Characteristic | Unit | Control Horses | Knotweed-Fed Horses | Control Horses | Knotweed-Fed Horses |
| Total protein | g/L | 60.696 ± 0.581 | 61.787 ± 0.710 | 61.281 ± 0.754 | 60.743 ± 1.274 |
| Albumin | g/L | 36.083 ± 0.313 | 35.405 ± 0.377 | 34.452 ± 0.668 | 33.823 ± 0.605 |
| Globulin | g/L | 24.612 ± 0.573 | 26.381 ± 0.744 | 26.829 ± 0.703 | 26.920 ± 1.258 |
| Urea | mmol/L | 5.500 ± 0.141 | 5.324 ± 0.169 | 5.171 ± 0.162 | 5.537 ± 0.279 |
| Creatinine | µmol/L | 110.482 ± 1.981 | 108.787 ± 2.264 | 87.484 ± 1.734 | 88.467 ± 1.718 |
| Cholesterol | mmol/L | 2.307 ± 0.045 | 2.307 ± 0.054 | 2.061 ± 0.053 | 2.110 ± 0.056 |
| Glucose | mmol/L | 6.344 ± 0.192 | 6.399 ± 0.226 | 5.842 ± 0.207 | 5.804 ± 0.182 |
| Triglyceride | mmol/L | 0.328 ± 0.019 | 0.337 ± 0.018 | 0.274 ± 0.016 | 0.291 ± 0.016 |
| AST activity | µkat/L | 5.168 ± 0.062 | 5.053 ± 0.092 | 5.755 ± 0.189 | 5.703 ± 0.319 |
| ALT activity | µkat/L | 0.213 ± 0.006 | 0.215 ± 0.011 | 0.202 ± 0.010 | 0.210 ± 0.009 |
| ALP activity | µkat/L | 3.042 ± 0.148 | 3.077 ± 0.153 | 3.575 ± 0.219 | 3.774 ± 0.292 |
| Haematological Characteristic | Unit | Control Horses | Knotweed-Fed Horses | Control Horses | Knotweed-Fed Horses |
| Haemoglobin | g/L | 108.500 ± 2.015 | 108.649 ± 2.178 | 113.194 ± 2.940 | 111.233 ± 2.721 |
| PCT | % | 0.084 ± 0.003 b | 0.100 ± 0.004 a | ||
| MCH | pg | 13.150 ± 0.139 | 13.095 ± 0.148 | 14.074 ± 0.117 | 13.910 ± 0.148 |
| Erythrocyte | 1012/L | 10.471 ± 2.278 | 8.267 ± 0.124 | 8.001 ± 0.173 | 7.950 ± 0.152 |
| Thrombocyte | 109/L | 161.839 ± 5.665 b | 189.367 ± 7.769 a | ||
| Leucocyte | 109/L | 8.228 ± 0.227 | 8.749 ± 0.293 | 8.781 ± 0.319 | 9.047 ± 0.351 |
| Lymphocyte | 109/L | 4.604 ± 0.188 | 4.680 ± 0.186 | 5.591 ± 0.286 | 5.756 ± 0.317 |
| Neutrophil | 109/L | 3.362 ± 0.130 | 3.771 ± 0.223 | 2.830 ± 0.261 | 2.871 ± 0.223 |
| Monocyte | 109/L | 0.028 ± 0.007 b | 0.055 ± 0.007 a | 0.187 ± 0.022 | 0.157 ± 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovářová, M.; Maděra, P.; Frantík, T.; Novák, J.; Vencl, Š. Effects of Knotweed-Enriched Feed on the Blood Characteristics and Fitness of Horses. Agriculture 2022, 12, 109. https://doi.org/10.3390/agriculture12010109
Kovářová M, Maděra P, Frantík T, Novák J, Vencl Š. Effects of Knotweed-Enriched Feed on the Blood Characteristics and Fitness of Horses. Agriculture. 2022; 12(1):109. https://doi.org/10.3390/agriculture12010109
Chicago/Turabian StyleKovářová, Marcela, Petr Maděra, Tomáš Frantík, Jan Novák, and Štěpán Vencl. 2022. "Effects of Knotweed-Enriched Feed on the Blood Characteristics and Fitness of Horses" Agriculture 12, no. 1: 109. https://doi.org/10.3390/agriculture12010109