The Effects of Suaeda salsa/Zea mays L. Intercropping on Plant Growth and Soil Chemical Characteristics in Saline Soil
Abstract
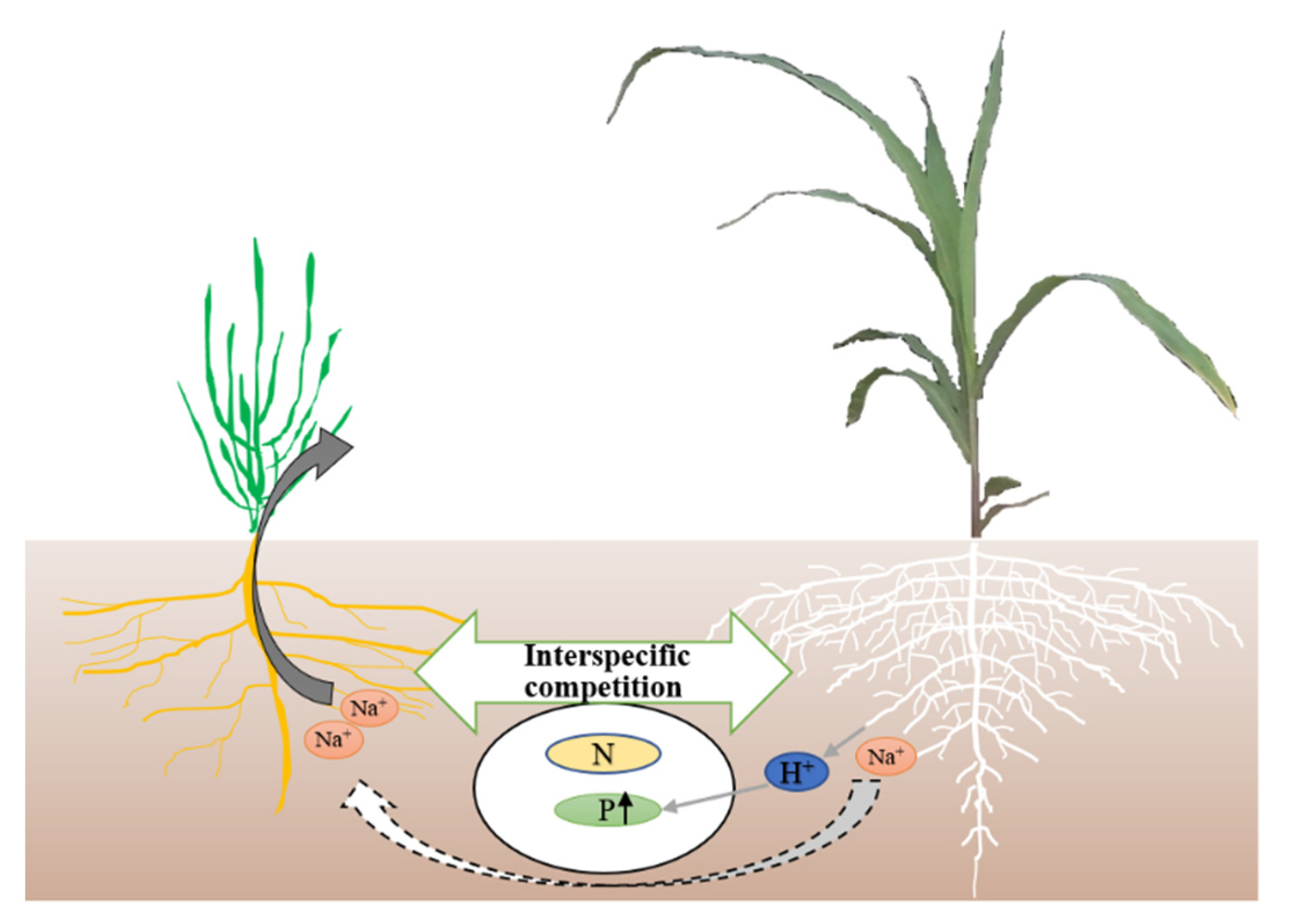
:1. Introduction
2. Materials and Methods
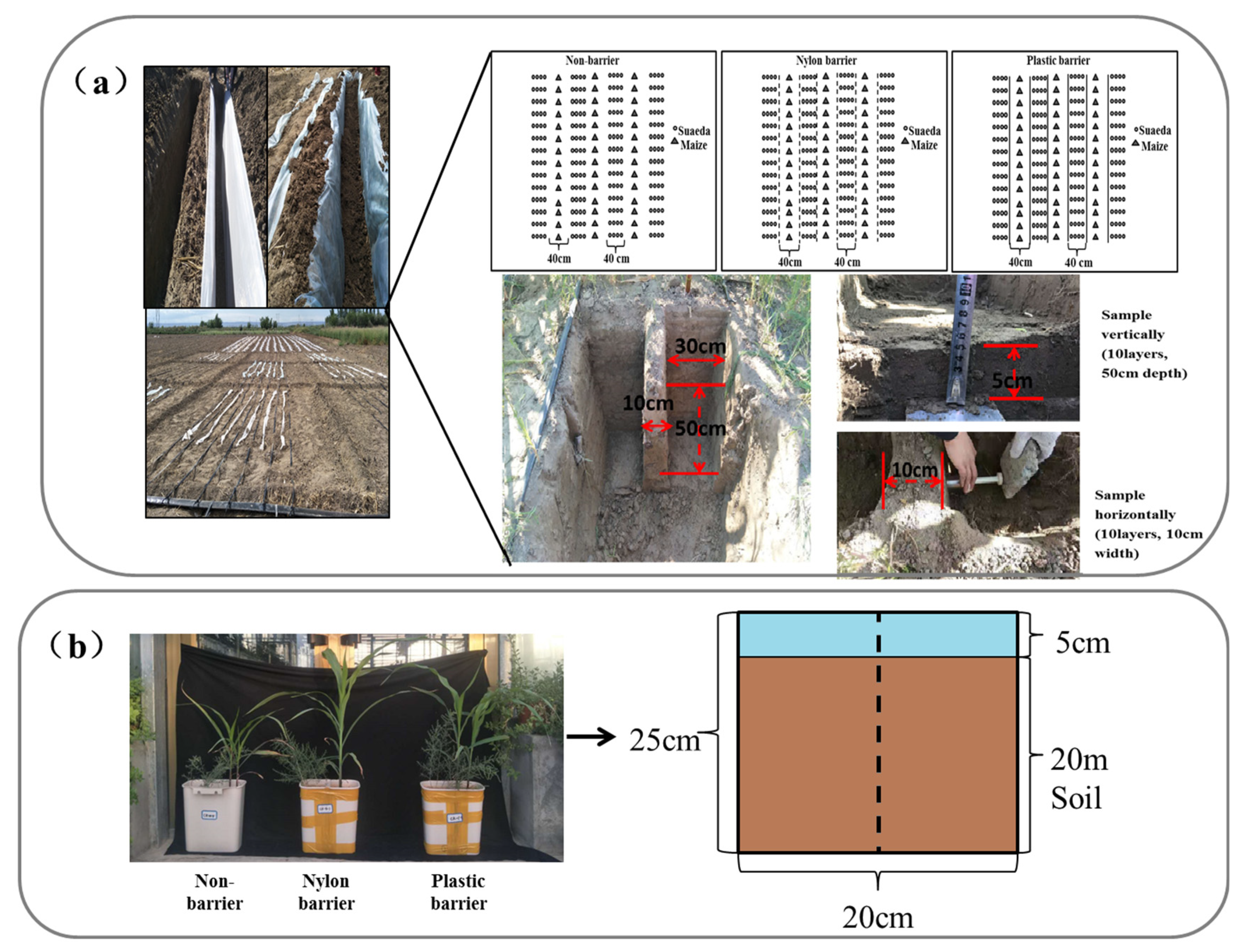
2.1. Field Experiment
2.2. Pot Experiment
2.3. Statistical Analyses
3. Results
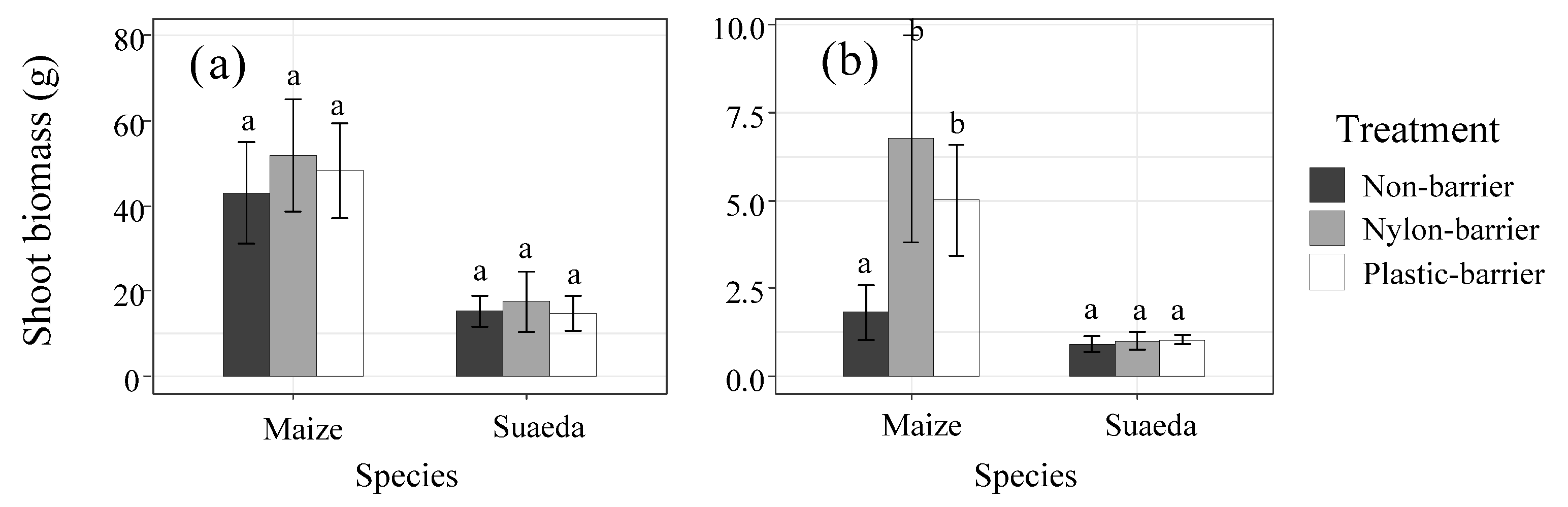
3.1. Plant Biomasses
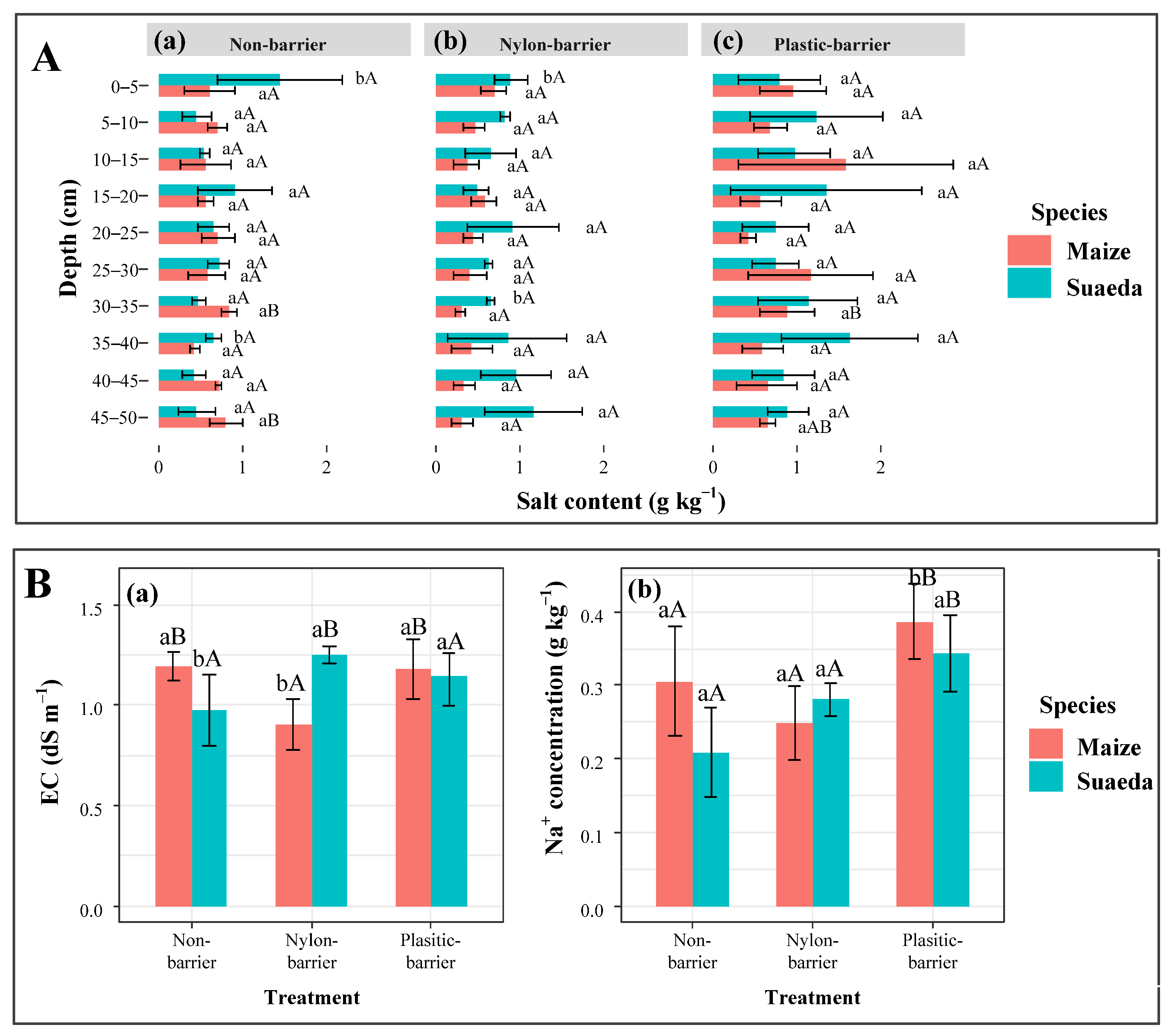
3.2. Soil EC, Salt, and Na+ Contents
3.3. Soil Available N and Olsen-P Contents
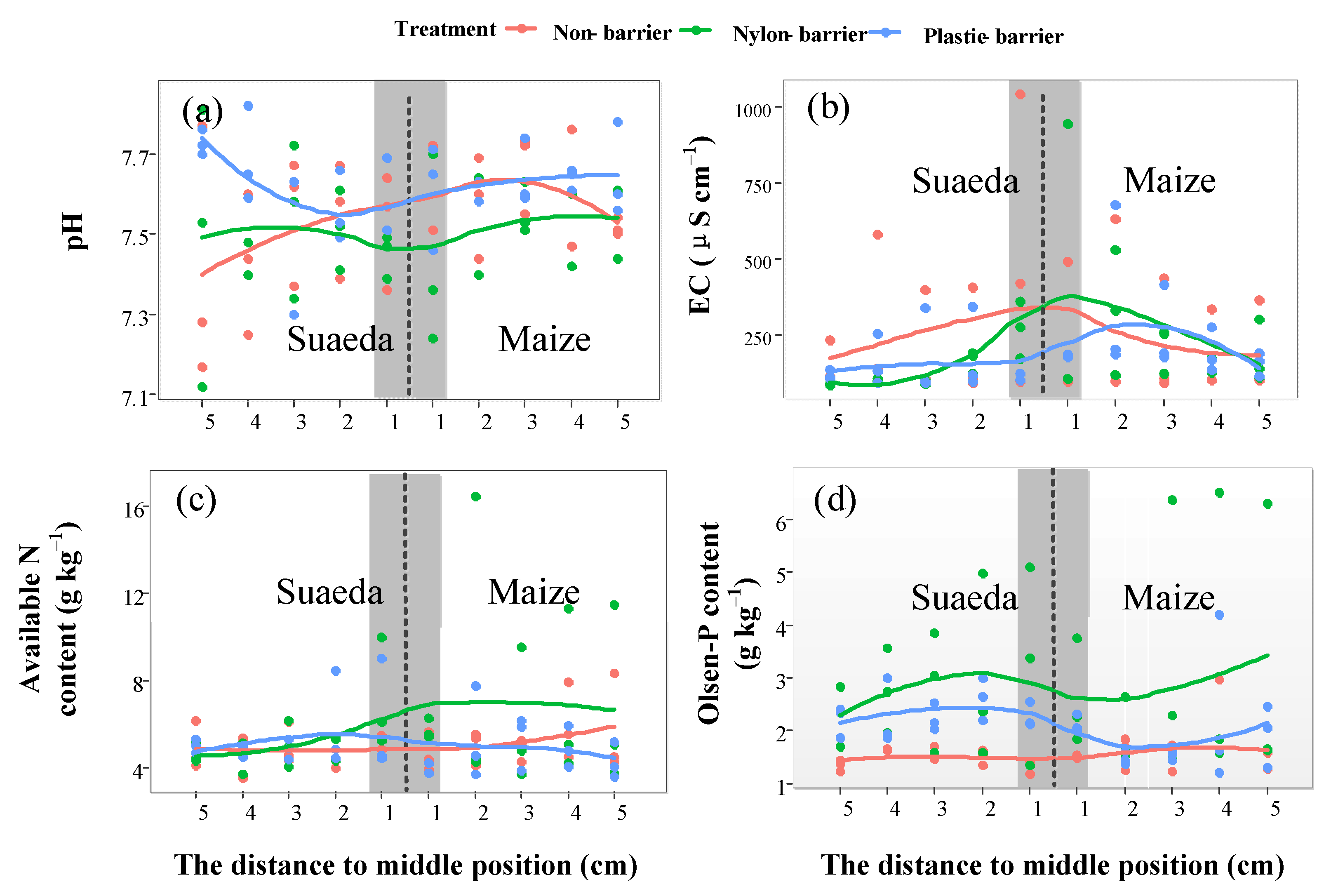
3.4. Horizontal Variations in Soil Properties
4. Discussion
4.1. The Effects of Intercropping Systems on Biomass
4.2. The Soil Salt’s Spatial Variations in The Intercropping Systems
4.3. Soil Nutrients’ Variations in The Suaeda/Maize Intercropping Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bennett, T.H.; Flowers, T.J.; Bromham, L. Repeated evolution of salt-tolerance in grasses. Biol. Lett. 2013, 9, 0029. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Bose, J.; Hedrich, R. Salt bladders: Do they matter? Trends Plant Sci. 2014, 19, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Leng, B.Y.; Wang, B.S. Progress in studying salt secretion from the salt glands in recretohalophytes: How do plants secrete salt? Front. Plant Sci. 2016, 7, 977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Suhono, G.B.F.; Li, Y.; Jiang, M.Y.; Chen, Y.; Siddique, K.H.M. Salt-tolerance in castor bean (Ricinus communis L.) is associated with thicker roots and better tissue K+/Na+ Distribution. Agriculture 2021, 11, 821. [Google Scholar] [CrossRef]
- Chaudhary, D.R.; Kim, J.; Kang, H. Influences of different halophyte vegetation on soil microbial community at Temperate Salt Marsh. Microb. Ecol. 2018, 75, 729–738. [Google Scholar] [CrossRef]
- Song, J.; Feng, G.; Tian, C.Y.; Zhang, F.S. Strategies for adaptation of Suaeda physophora, Haloxylon ammodendron and Haloxylon persicum to a saline environment during seed-germination stage. Ann. Bot. 2005, 96, 399–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munns, R.; Greenway, H.; Kirst, G.O. Halotolerant eukaryotes. In Encyclopedia of Plant Physiology; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin, Germany, 1983; Volume 12C, pp. 59–135. [Google Scholar]
- Matsushita, N.; Matoh, I. Characterization of Na^ exclusion mechanisms of salt-tolerant reed plants in comparison with salt-sensitive rice plants. Physiol. Plant. 1991, 83, 170–176. [Google Scholar] [CrossRef]
- Byrt, C.S.; Xu, B.; Krishnan, M.; Lightfoot, D.J.; Athman, A.; Jacobs, A.K.; Watson-Haigh, N.S.; Plett, D.; Munns, R.; Tester, M. The Na+ transporter, TaHKT1;5-D, limits shoot Na+ accumulation in bread wheat. Plant J. 2014, 80, 516–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, X.; Jiang, L.; Zhang, K.; Tanveer, M.; Tian, C.Y.; Zhao, Z.Y. Reclamation of saline soil by planting annual euhalophyte Suaeda salsa with drip irrigation: A three-year field experiment in arid northwestern China. Ecol. Eng. 2021, 159, 106090. [Google Scholar] [CrossRef]
- Etesami, H.; Beattie, G.A. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 2018, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Shultana, R.; Kee Zuan, A.T.; Yusop, M.R.; Saud, H.M. Characterization of salt-tolerant plant growth-promoting rhizobacteria and the effect on growth and yield of saline-affected rice. PLoS ONE 2020, 15, e0238537. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, J.H.; Zhang, F.S.; Li, X.L.; Yang, S.C.; Rengel, Z. Wheat/maize or wheat/soybean strip intercropping I. Yield advantage and interspecific interactions on nutrients. Field Crops Res. 2001, 71, 123–137. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F.S.; Li, X.L.; Christie, P.; Sun, J.H.; Yang, S.C.; Tang, C.X. Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr. Cycl. Agroecosyst. 2003, 65, 61–71. [Google Scholar] [CrossRef]
- Bi, Y.X.; Zhou, P.; Li, S.J.; Wei, Y.Q.; Xiong, X.; Shi, Y.H.; Liu, N.; Zhang, Y.J. Interspecific interactions contribute to higher forage yield and are affected by phosphorus application in a fully-mixed perennial legume and grass intercropping system. Field Crops Res. 2019, 244, 107636. [Google Scholar] [CrossRef]
- Salgado, G.C.; Ambrosano, E.J.; Rossi, F.; Otsuk, I.P.; Ambrosano, G.M.B.; Santana, C.A.; Muraoka, T.; Trivelin, P.C.O. Biological N fixation and N transfer in an intercropping system between legumes and organic cherry tomatoes in succession to green corn. Agriculture 2021, 11, 690. [Google Scholar] [CrossRef]
- Vandermeer, J.H. The Ecology of Intercropping; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Lv, J.X.; Dong, Y.; Dong, K.; Zhao, Q.; Yang, Z.X.; Chen, L. Intercropping with wheat suppressed Fusarium wilt in faba bean and modulated the composition of root exudates. Plant Soil 2020, 448, 153–164. [Google Scholar] [CrossRef]
- Tian, J.; Tang, M.; Xu, X.; Luo, S.; Condron, L.M.; Lambers, H.; Cai, K.; Wang, J. Soybean (Glycine max (L.) Merrill) intercropping with reduced nitrogen input influences rhizosphere phosphorus dynamics and phosphorus acquisition of sugarcane (Saccharum officinarum). Biol. Fertil. Soils 2020, 56, 1063–1075. [Google Scholar] [CrossRef]
- Sashidhar, B.; Podile, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J. Appl. Microbiol. 2010, 109, 1–12. [Google Scholar] [CrossRef]
- Lamers, L.P.; van Diggelen, J.M.; Op den Camp, H.J.; Visser, E.J.; Lucassen, E.C.; Vile, M.A.; Jetten, M.S.; Smolders, A.J.; Roelofs, J.G. Microbial transformations of nitrogen, sulfur, and iron dictate vegetation composition in wetlands: A review. Front. Microbiol. 2012, 3, 156. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Shi, W.J. Cotton/halophytes intercropping decreases salt accumulation and improves soil physicochemical properties and crop productivity in saline-alkali soils under mulched drip irrigation: A three-year field experiment. Field Crops Res. 2021, 262, 108027. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2014, 115, 541–553. [Google Scholar] [CrossRef] [Green Version]
- Barhoumi, Z.; Trabelsi, N.; Atia, A.; Djebali, W.; Chaïbi, W.; Abdelly, C.; Smaoui, A. Salt stress response in the halophyte Limoniastrum guyonianum Boiss. Flora 2015, 217, 1–9. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Guo, J.R.; Du, M.; Lu, C.X.; Wang, B.S. NaCl improves reproduction by enhancing starch accumulation in the ovules of the euhalophyte Suaeda salsa. BMC Plant Biol. 2020, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Qin, Y.Z.; Chai, Q.; Feng, F.X.; Zhao, C.; Yu, A.Z. Interspecies interactions in relation to root distribution across the rooting profile in wheat-maize intercropping under different plant densities. Front. Plant Sci. 2018, 9, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Shen, J.B.; Mao, D.R. Research Methods of Plant Nutrition; China Agricultural University Press: Beijing, China, 2011. [Google Scholar]
- Wang, Q.T.; Xiao, J.; Ding, J.X.; Zou, T.T.; Zhang, Z.L.; Liu, Q.; Yin, H.J. Differences in root exudate inputs and rhizosphere effects on soil N transformation between deciduous and evergreen trees. Plant Soil 2021, 458, 277–289. [Google Scholar] [CrossRef]
- Latati, M.; Aouiche, A.; Tellah, S.; Laribi, A.; Benlahrech, S.; Kaci, G.; Ouarem, F.; Ounane, S.M. Intercropping maize and common bean enhances microbial carbon and nitrogen availability in low phosphorus soil under Mediterranean conditions. Eur. J. Soil Biol. 2017, 80, 9–18. [Google Scholar] [CrossRef]
- Ling, Q.; Zhao, X.N.; Wu, P.T.; Gao, X.D.; Sun, W.H. Effect of the fodder species canola (Brassica napus L.) and daylily (Hemerocallis fulva L.) on soil physical properties and soil water content in a rainfed orchard on the semiarid Loess Plateau, China. Plant Soil 2019, 453, 209–228. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Baek, K.H. Physiological and biochemical perspectives of non-salt tolerant plants during bacterial interaction against soil salinity. Plant Physiol. Biochem. 2017, 116, 116–126. [Google Scholar] [CrossRef]
- Song, D.; Tariq, A.; Pan, K.; Khan, S.U.; Saleh, T.A.; Gong, S.; Zhang, A.; Wu, X. Influence of planting distance and density on the yield and photosynthetic traits of sweet potato (Ipomoea balatas L.) under an intercropping system with walnut (Juglans regia) saplings. Soil Tillage Res. 2020, 196, 104484. [Google Scholar] [CrossRef]
- McDonald, G.K.; Taylor, J.D.; Verbyla, A.; Kuchel, H. Assessing the importance of subsoil constraints to yield of wheat and its implications for yield improvement. Crop Pasture Sci. 2013, 63, 1043–1065. [Google Scholar] [CrossRef]
- Bian, A.N.; Lin, M.; Wang, W.Q.; Bin, W.S. Effects of root salt stress on growth and allocation of mineral elements in halophyte and glycophyte seedlings. J. Trop. Subtrop. Bot. 2015, 23, 405–412. [Google Scholar]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef]
- Alharby, H.F.; Colmer, T.D.; Barrett-Lennard, E.G. Salinization of the soil solution decreases the further accumulation of salt in the root zone of the halophyte Atriplex nummulariaLindl. growing above shallow saline groundwater. Plant Cell Environ. 2018, 41, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.R.; Ma, Y.J.; Hai, Y. Jujube is at a competitiveness disadvantage to cotton in intercropped system. Agron. J. 2021, 113, 3475–3488. [Google Scholar] [CrossRef]
- Nafi, E.; Webber, H.; Danso, I.; Naab, J.B.; Frei, M.; Gaiser, T. Soil tillage, residue management and site interactions affecting nitrogen use efficiency in maize and cotton in the Sudan Savanna of Africa. Field Crops Res. 2019, 244, 107629. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Zhang, F.S.; Guo, T.; Bao, X.; Smith, F.A.; Smith, S.E. Root distribution and interactions between intercropped species. Oecologia 2005, 147, 280–290. [Google Scholar] [CrossRef]
- Liu, G.C.; Li, L.; Huang, G.B.; Sun, J.H.; Guo, T.W.; Zhang, F.S. Intercropping advantage and contribution of above-ground and below-ground interactions in the barley-maize intercropping. Sci. Agric. Sin. 2005, 38, 1787–1795. [Google Scholar]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.B.; Tang, X.Y.; Zhang, F.S. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.L.; Almvik, M.; Clarke, N.; Eich-Greatorex, S.; Ogaard, A.F.; Krogstad, T.; Lambers, H.; Clarke, J.L. Contrasting responses of root morphology and rootexuded organic acids to low phosphorus availability in three important food crops with divergent root traits. AoB Plants 2015, 7, plv097. [Google Scholar] [CrossRef]
- Gitari, H.I.; Karanja, N.N.; Gachene, C.K.K.; Kamau, S.; Sharma, K.; Schulte-Geldermann, E. Nitrogen and phosphorous uptake by potato (Solanum tuberosum L.) and their use efficiency under potato-legume intercropping systems. Field Crops Res. 2018, 222, 78–84. [Google Scholar] [CrossRef]
- Mai, W.X.; Xue, X.R.; Feng, G.; Yang, R.; Tian, C.Y. Can optimization of phosphorus input lead to high productivity and high phosphorus use efficiency of cotton through maximization of root/mycorrhizal efficiency in phosphorus acquisition? Field Crops Res. 2018, 216, 100–108. [Google Scholar] [CrossRef]
- Li, H.G.; Shen, J.B.; Zhang, F.S.; Marschner, P.; Cawthray, G.; Rengel, Z. Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol. Fertil. Soils 2010, 46, 79–91. [Google Scholar] [CrossRef]
- Song, Y.N.; Zhang, F.S.; Marschner, P.; Fan, F.L.; Gao, H.M.; Bao, X.G.; Sun, J.H.; Li, L. Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol. Fertil. Soils 2007, 43, 565–574. [Google Scholar] [CrossRef]


| Soil Depth (cm) | pH | EC (dS m−1) | Total Salt (g kg−1) | Available N (mg kg−1) | Olsen-P (mg kg−1) | Available K (mg kg−1) |
|---|---|---|---|---|---|---|
| 0–10 | 7.247 | 1.260 | 1.308 | 7.556 | 5.282 | 2.858 |
| 10–20 | 7.403 | 1.027 | 2.567 | 13.208 | 5.369 | 3.099 |
| 20–30 | 7.383 | 0.616 | 1.833 | 7.032 | 4.876 | 2.336 |
| 30–50 | 7.373 | 0.691 | 2.025 | 6.308 | 4.110 | 2.448 |
| 50–100 | 7.490 | 0.582 | 1.570 | 4.964 | 4.683 | 2.691 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhao, Z.; Ge, S.; Zhang, K.; Tian, C.; Mai, W. The Effects of Suaeda salsa/Zea mays L. Intercropping on Plant Growth and Soil Chemical Characteristics in Saline Soil. Agriculture 2022, 12, 107. https://doi.org/10.3390/agriculture12010107
Wang S, Zhao Z, Ge S, Zhang K, Tian C, Mai W. The Effects of Suaeda salsa/Zea mays L. Intercropping on Plant Growth and Soil Chemical Characteristics in Saline Soil. Agriculture. 2022; 12(1):107. https://doi.org/10.3390/agriculture12010107
Chicago/Turabian StyleWang, Shoule, Zhenyong Zhao, Shaoqing Ge, Ke Zhang, Changyan Tian, and Wenxuan Mai. 2022. "The Effects of Suaeda salsa/Zea mays L. Intercropping on Plant Growth and Soil Chemical Characteristics in Saline Soil" Agriculture 12, no. 1: 107. https://doi.org/10.3390/agriculture12010107





