Application of Ultrasound and Curing Agent during Osmotic Dehydration to Improve the Quality Properties of Freeze-Dried Yellow Peach (Amygdalus persica) Slices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Ultrasound-Assisted Osmotic Dehydration and Vacuum Freeze-Drying
2.3. Determination of Water Loss, Moisture Content and Rehydration Capacity
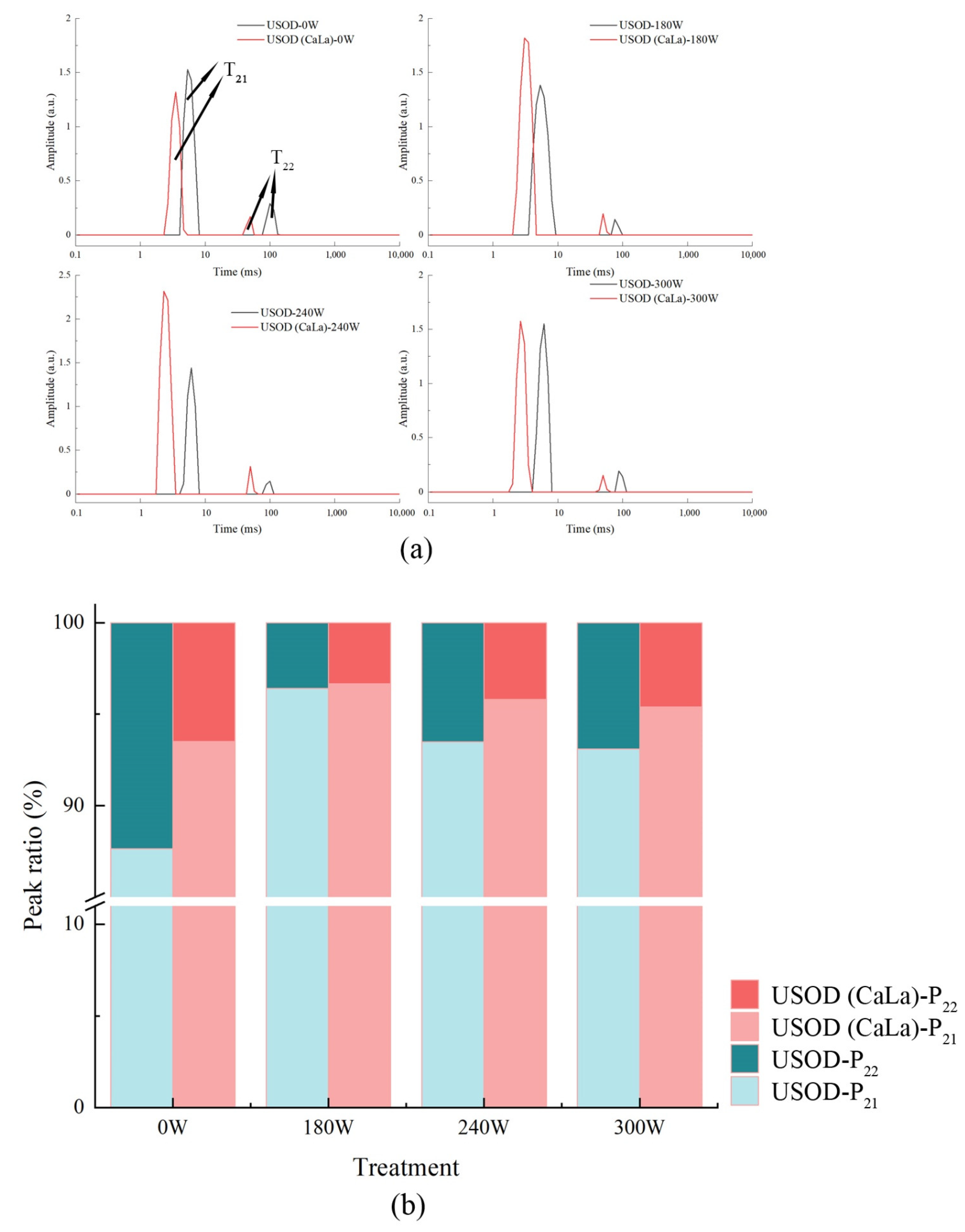
2.4. Low-Field Nuclear Magnetic Resonance (LF-NMR)
2.5. Color Measurement
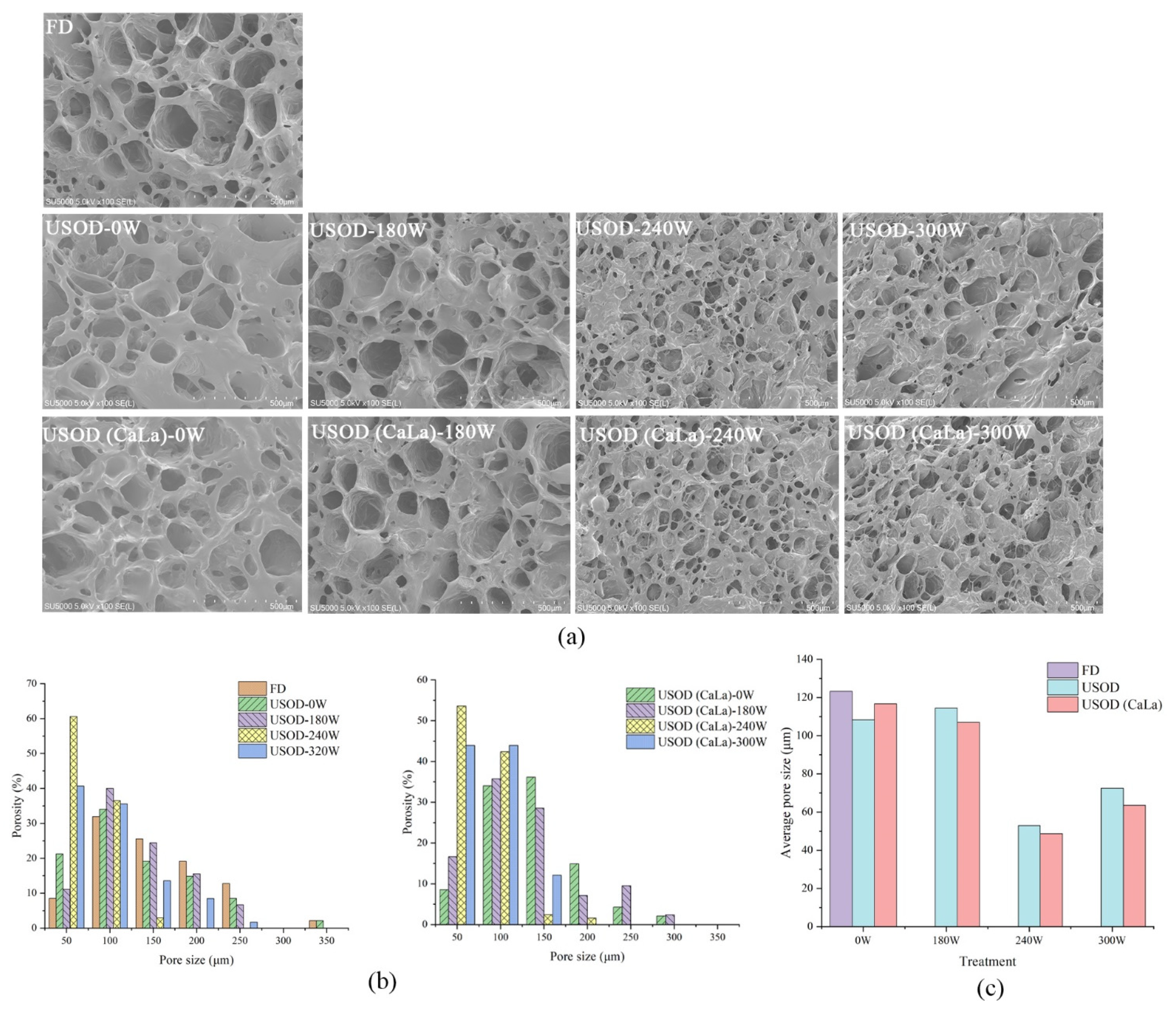
2.6. Microscopic Observation
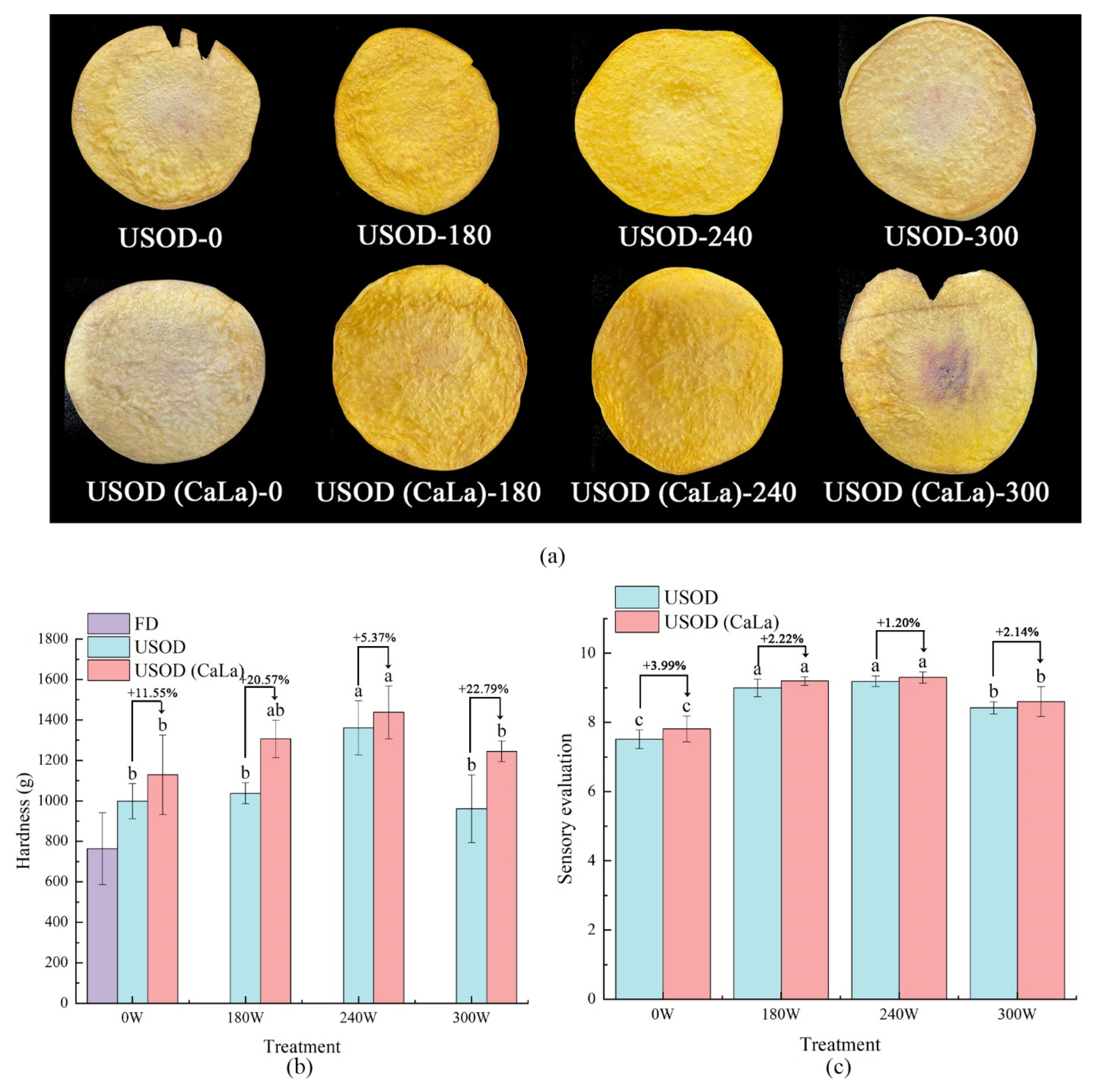
2.7. Texture Analysis
2.8. Determination of Oligomeric Proantho Cyanidins Content, Total Phenolic Content and ABTS Radical Scavenging Assay
2.9. Sensory Evaluation
2.10. Statistical Analysis
3. Results and Discussion
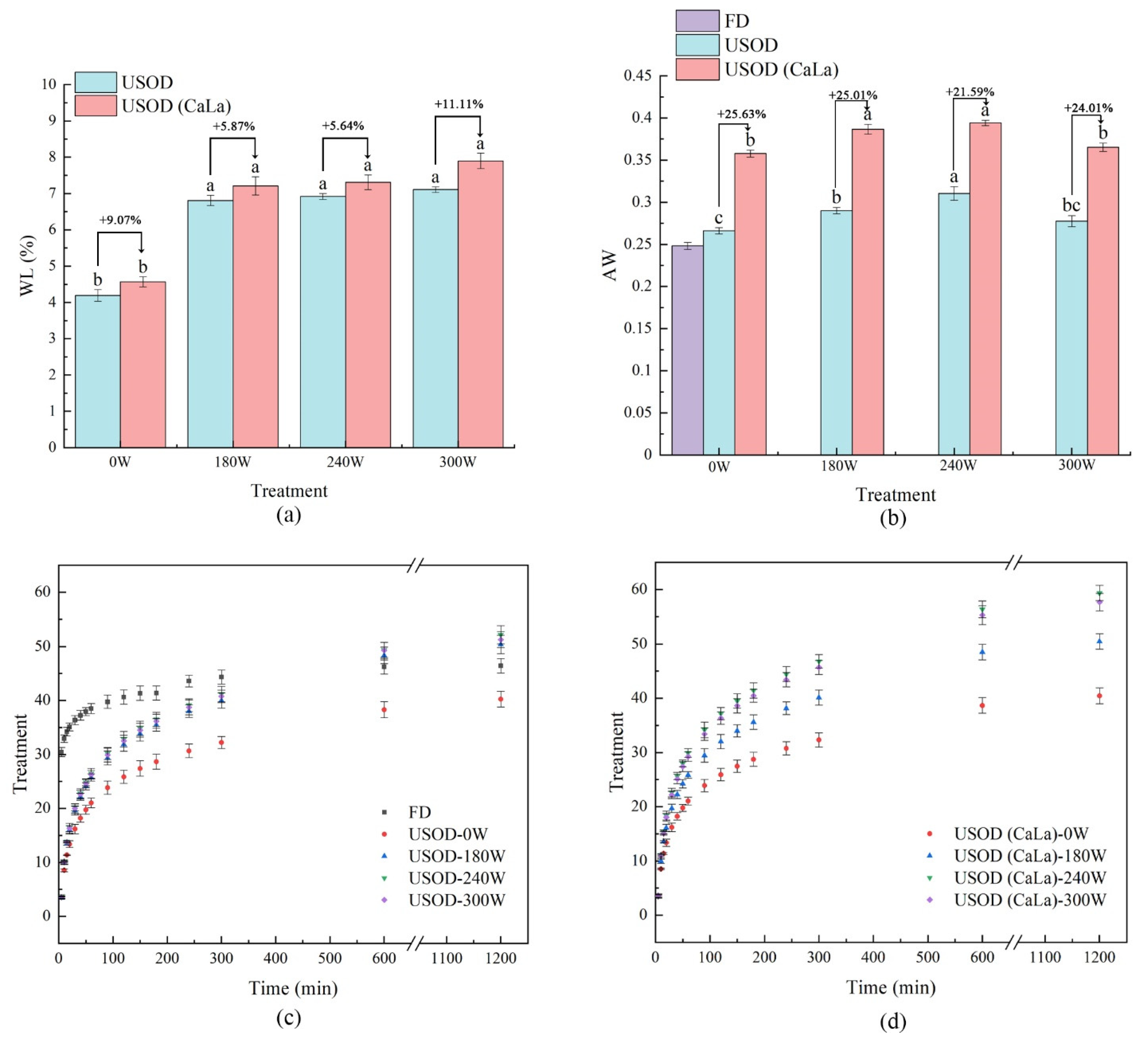
3.1. WL, Aw, RC and Water Distribution
3.2. Microstructure
3.3. Color, Texture and Sensory
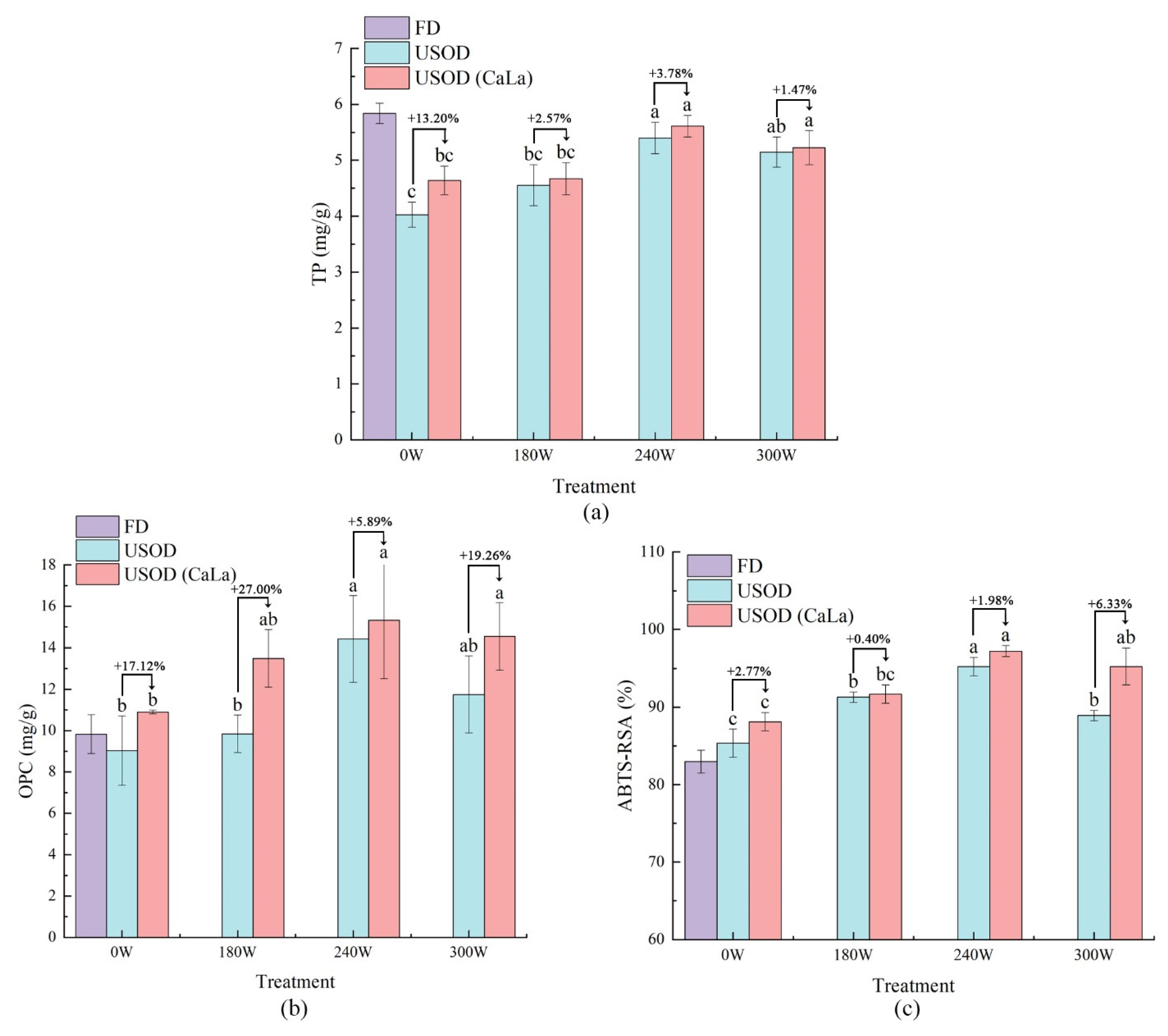
3.4. Evaluation of Nutritional Quality
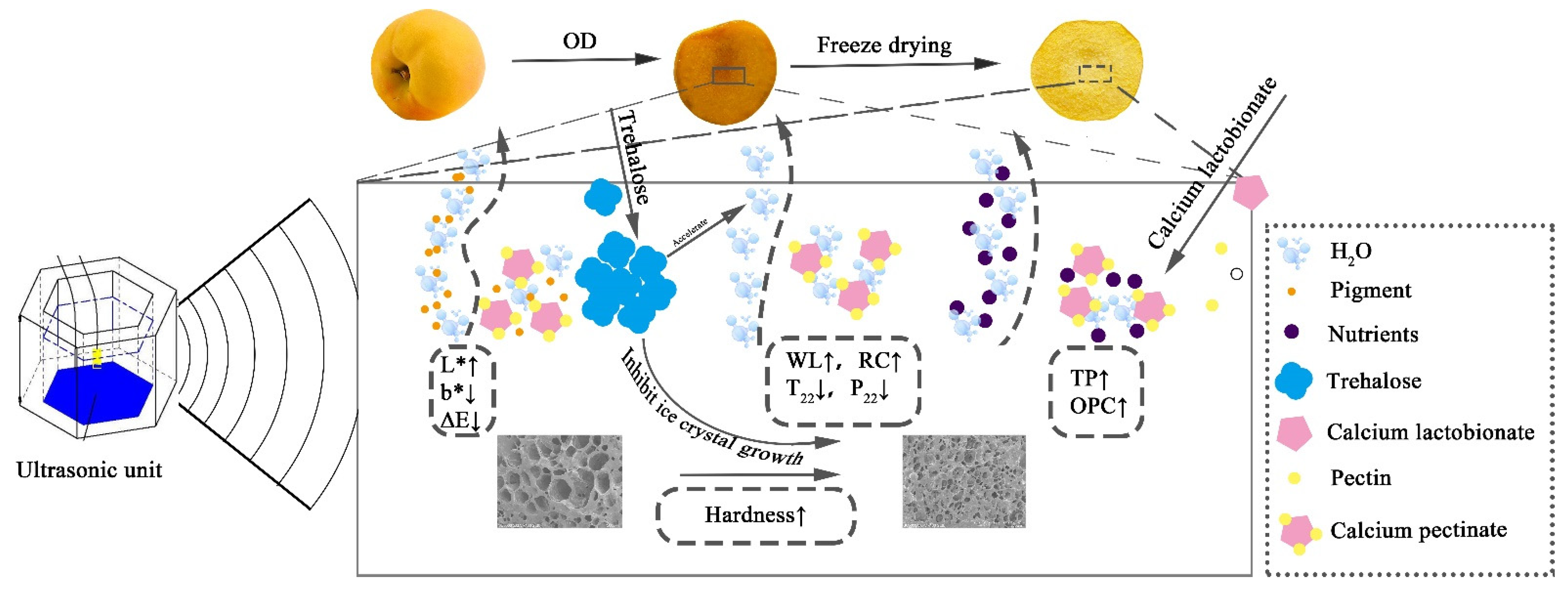
3.5. Schematic Illustration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cano-Salazar, J.; Echeverría, G.; Crisosto, C.H.; Lopez, L. Cold-storage potential of four yellow-fleshed peach cultivars defined by their volatile compounds emissions, standard quality parameters, and consumer acceptance. J. Agric. Food Chem. 2012, 60, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gonzalles, G.; Liu, J.; Dai, Z.; Li, D.; Liu, C.; Zhang, M. Optimization of explosion puffing drying for high-value yellow-fleshed peach crisps using response surface methodology. Dry. Technol. 2019, 37, 929–940. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, Y.; Liao, L.; Shi, D.; An, K.; Jun, W.; Liu, S. Effects of ultrasound and ultra-high pressure pretreatments on volatile and taste compounds of vacuum-freeze dried strawberry slice. LWT 2021, 112012. [Google Scholar] [CrossRef]
- Jia, Y.; Khalifa, I.; Hu, L.; Zhu, W.; Li, J.; Li, K.; Li, C. Influence of three different drying techniques on persimmon chips’ characteristics: A comparison study among hot-air, combined hot-air-microwave, and vacuum-freeze drying techniques. Food Bioprod. Process. 2019, 118, 67–76. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. Nutr. 2017, 57, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, C.; Andrzej, L. Rehydration and sorption properties of osmotically pretreated freeze-dried strawberries. J. Food Eng. 2010, 97, 267–274. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, M.; Devahastin, S.; Yu, D. Effect of ultrasound-assisted osmotic dehydration pretreatments on drying and quality characteristics of pulsed fluidized bed microwave freeze-dried strawberries. LWT 2021, 145, 111300. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Hasanzadeh, H.; Mokhtari-Dizaji, M.; Zahra Bathaie, S.; Hassan, Z.M.; Nilchiani, V.; Goudarzi, H. Enhancement and control of acoustic cavitation yield by low-level dual frequency sonication: A subharmonic analysis. Ultrason. Sonochem. 2011, 18, 394–400. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, P.; Sun, D.-W. Effects of multi-frequency ultrasound on freezing rates and quality attributes of potatoes. Ultrason. Sonochem. 2020, 60, 104733. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Simultaneous application of ultrasounds and firming agents to improve the quality properties of osmotic + freeze-dried foods. LWT 2018, 96, 402–410. [Google Scholar] [CrossRef]
- Alonso, S.; Rendueles, M.; Díaz, M. Bio-production of lactobionic acid: Current status, applications and future prospects. Biotechnol. Adv. 2013, 31, 1275–1291. [Google Scholar] [CrossRef]
- Sáez-Orviz, S.; Camilleri, P.; Marcet, I.; Rendueles, M.; Díaz, M. Microencapsulation of calcium lactobionate for protection from microorganisms in a solid phase food. Biochem. Eng. J. 2019, 150, 107281. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, L.; Qiao, Y.; Wang, C.; Shi, D.; An, K.; Hu, J. Effects of ultrahigh pressure and ultrasound pretreatments on properties of strawberry chips prepared by vacuum-freeze drying. Food Chem. 2020, 303, 125386. [Google Scholar] [CrossRef]
- Wei, S.; Mei, J.; Xie, J. Effects of different carbon dioxide-modified atmosphere packaging and low-temperature storage at 13 °C on the quality and metabolism in mango (Mangifera indica L.). Agriculture 2021, 11, 636. [Google Scholar] [CrossRef]
- Yoon, Y.-S.; Ameer, K.; Song, B.-S.; Kim, J.-K.; Park, H.-Y.; Lee, K.-C.; Eun, J.-B.; Park, J.-H. Effects of X-ray irradiation on the postharvest quality characteristics of ‘Maehyang’ strawberry (Fragaria × ananassa). Food Chem. 2020, 325, 126817. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K.; Raghavarao, K.S.M.S. Water and solute diffusion coefficients of carrot as a function of temperature and concentration during osmotic dehydration. J. Food Eng. 1997, 34, 429–440. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; An, K.; Zou, B.; Yang, W. Effect of ultrasound-assisted osmotic dehydration pretreatment on the drying characteristics and quality properties of Sanhua plum (Prunus salicina L.). LWT 2021, 138, 110653. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Wang, W. Ultrasound-assisted osmotic dehydration pretreatment before pulsed fluidized bed microwave freeze-drying (PFBMFD) of Chinese yam. Food Biosci. 2020, 35, 100548. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Lenart, A.; Gręda, K.J. Effect of pre-treatment conditions on content and activity of water and colour of freeze-dried pumpkin. LWT Food Sci. Technol. 2014, 59, 1075–1081. [Google Scholar] [CrossRef]
- Albano, K.M.; Nicoletti, V.R. Ultrasound impact on whey protein concentrate-pectin complexes and in the O/W emulsions with low oil soybean content stabilization. Ultrason. Sonochem. 2018, 41, 562–571. [Google Scholar] [CrossRef] [Green Version]
- Bizzani, M.; William Menezes Flores, D.; Bueno Moraes, T.; Alberto Colnago, L.; David Ferreira, M.; Helena Fillet Spoto, M. Non-invasive detection of internal flesh breakdown in intact Palmer mangoes using time-domain nuclear magnetic resonance relaxometry. Microchem. J. 2020, 158, 105208. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H. Effect of vacuum impregnated fish gelatin and grape seed extract on moisture state, microbiota composition, and quality of chilled seabass fillets. Food Chem. 2021, 354, 129581. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Effect of trehalose and ultrasound-assisted osmotic dehydration on the state of water and glass transition temperature of broccoli (Brassica oleracea L. var. botrytis L.). J. Food Eng. 2013, 119, 640–647. [Google Scholar] [CrossRef]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Evaluation of the effects of different freezing and thawing methods on color, polyphenol and ascorbic acid retention in strawberries (Fragaria×ananassa Duch.). Food Res. Int. 2012, 48, 241–248. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, L.; Li, D.; Liu, C.; Ma, R.; Song, J.; Zhao, J. Low intensity ultrasound as a pretreatment to drying of daylilies: Impact on enzyme inactivation, color changes and nutrition quality parameters. Ultrason. Sonochem. 2017, 36, 50–58. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Pawlak, G. Effect of drying on microstructure of plant tissue. Dry. Technol. 2003, 21, 657–683. [Google Scholar] [CrossRef]
- Prosapio, V.; Norton, I. Influence of osmotic dehydration pre-treatment on oven drying and freeze drying performance. LWT 2017, 80, 401–408. [Google Scholar] [CrossRef]
- Petzold, G.; Aguilera, J.M. Ice Morphology: Fundamentals and technological applications in foods. Food Biophys. 2009, 4, 378–396. [Google Scholar] [CrossRef]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. (Amsterdam) 2020, 268, 109365. [Google Scholar] [CrossRef]
- Bozkir, H.; Rayman Ergün, A.; Serdar, E.; Metin, G.; Baysal, T. Influence of ultrasound and osmotic dehydration pretreatments on drying and quality properties of persimmon fruit. Ultrason. Sonochem. 2019, 54, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yi, J.; Bi, J.; Zhao, Y.; Li, X.; Wu, X.; Du, Q. Effect of ultrasound on mass transfer kinetics and phenolic compounds of apple cubes during osmotic dehydration. LWT 2021, 151, 112186. [Google Scholar] [CrossRef]
- Ferrando, M.; Spiess, W.E. Cellular response of plant tissue during the osmotic treatment with sucrose, maltose, and trehalose solutions. J. Food Eng. 2001, 49, 115–127. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. REACTIVE OXYGEN SPECIES: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Chen, T.; Qin, G.; Li, B.; Tian, S. Synergistic action of antioxidative systems contributes to the alleviation of senescence in kiwifruit. Postharvest Biol. Technol. 2016, 111, 15–24. [Google Scholar] [CrossRef]
- Masuzawa, N.; Ohdaira, E.; Ide, M. Effects of ultrasonic irradiation on phenolic compounds in wine. Jpn. J. Appl. Phys. 2014, 39, 2978–2979. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, W.; Mezrigui, S.; Badwaik, L.S.; Bejar, A.K.; Perez, C.T.; Kechaou, N. Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason. Sonochem. 2017, 36, 286–300. [Google Scholar] [CrossRef] [PubMed]
| Treatment | L* | a* | b* | ∆E |
|---|---|---|---|---|
| Fresh | 60.37 ± 3.25 | 18.23 ± 0.91 | 45.27 ± 1.97 | 0 |
| FD | 75.98 ± 3.21 | 16.43 ± 1.22 | 28.28 ± 1.22 | 23.39 ± 1.30 |
| USOD-0 W | 68.83 ± 1.35b | 18.65 ± 3.25a | 31.14 ± 1.04c | 16.84 ± 1.19c |
| USOD-180 W | 63.22 ± 1.02a | 17.66 ± 1.74a | 34.52 ± 0.65b | 11.31 ± 0.75b |
| USOD-240 W | 63.17 ± 0.74a | 17.89 ± 0.63a | 37.64 ± 1.67a | 8.18 ± 1.73a |
| USOD-300 W | 64.57 ± 0.62a | 18.21 ± 2.64a | 32.66 ± 1.71bc | 13.58 ± 1.58b |
| USOD (CaLa)-0 W | 67.28 ± 0.99b | 17.25 ± 1.23a | 30.05 ± 1.71c | 16.81 ± 1.82c |
| USOD (CaLa)-180 W | 63.11 ± 0.65a | 17.99 ± 1.11a | 36.22 ± 1.37b | 9.54 ± 1.43a |
| USOD (CaLa)-240 W | 63.01 ± 1.21a | 18.24 ± 0.47a | 38.05 ± 1.04a | 7.81 ± 0.91a |
| USOD (CaLa)-300 W | 63.15 ± 0.47a | 18.10 ± 1.66a | 32.17 ± 0.25c | 13.50 ± 0.33b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Y.; Wei, S.; Ding, Z.; Mei, J.; Xie, J. Application of Ultrasound and Curing Agent during Osmotic Dehydration to Improve the Quality Properties of Freeze-Dried Yellow Peach (Amygdalus persica) Slices. Agriculture 2021, 11, 1069. https://doi.org/10.3390/agriculture11111069
Chu Y, Wei S, Ding Z, Mei J, Xie J. Application of Ultrasound and Curing Agent during Osmotic Dehydration to Improve the Quality Properties of Freeze-Dried Yellow Peach (Amygdalus persica) Slices. Agriculture. 2021; 11(11):1069. https://doi.org/10.3390/agriculture11111069
Chicago/Turabian StyleChu, Yuanming, Saichao Wei, Zhaoyang Ding, Jun Mei, and Jing Xie. 2021. "Application of Ultrasound and Curing Agent during Osmotic Dehydration to Improve the Quality Properties of Freeze-Dried Yellow Peach (Amygdalus persica) Slices" Agriculture 11, no. 11: 1069. https://doi.org/10.3390/agriculture11111069






