Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of Carotenoids
2.2. ABTS· Assay
2.3. Determination of Phenolics
2.4. Free Phenolic Acids (FPAs)
2.5. Statistical Analysis
3. Results
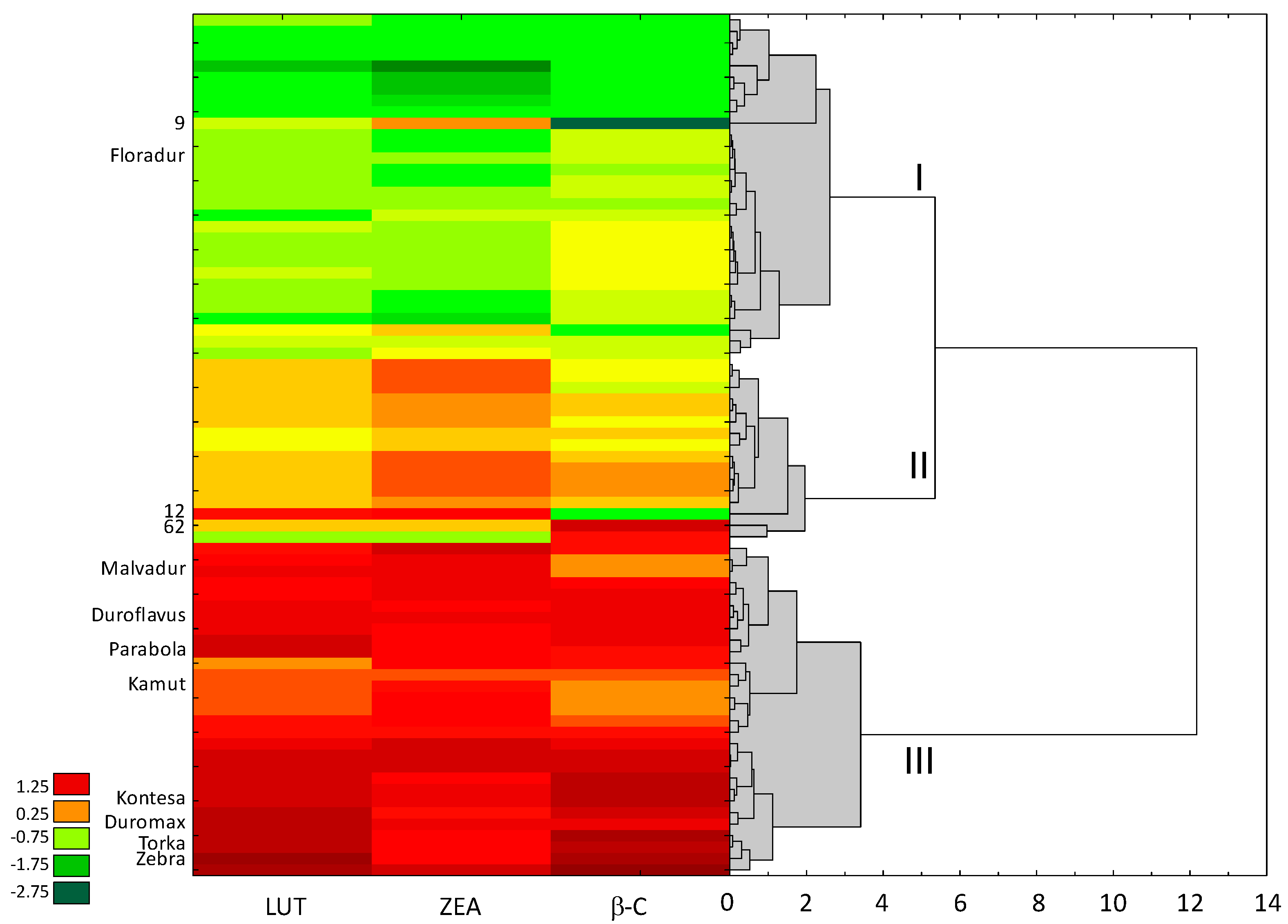
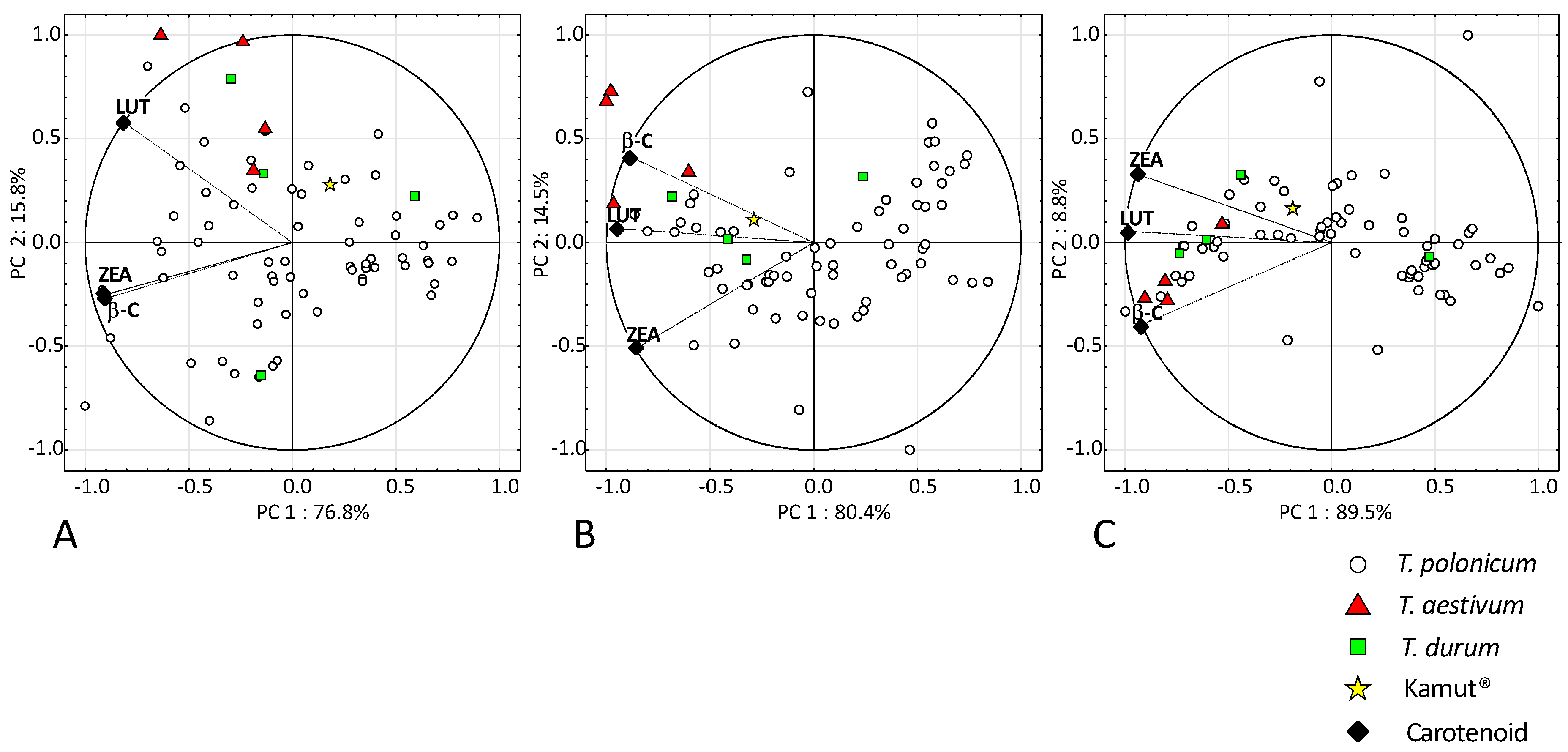
3.1. Antioxidant Activity (ABTS+) and Carotenoid Content
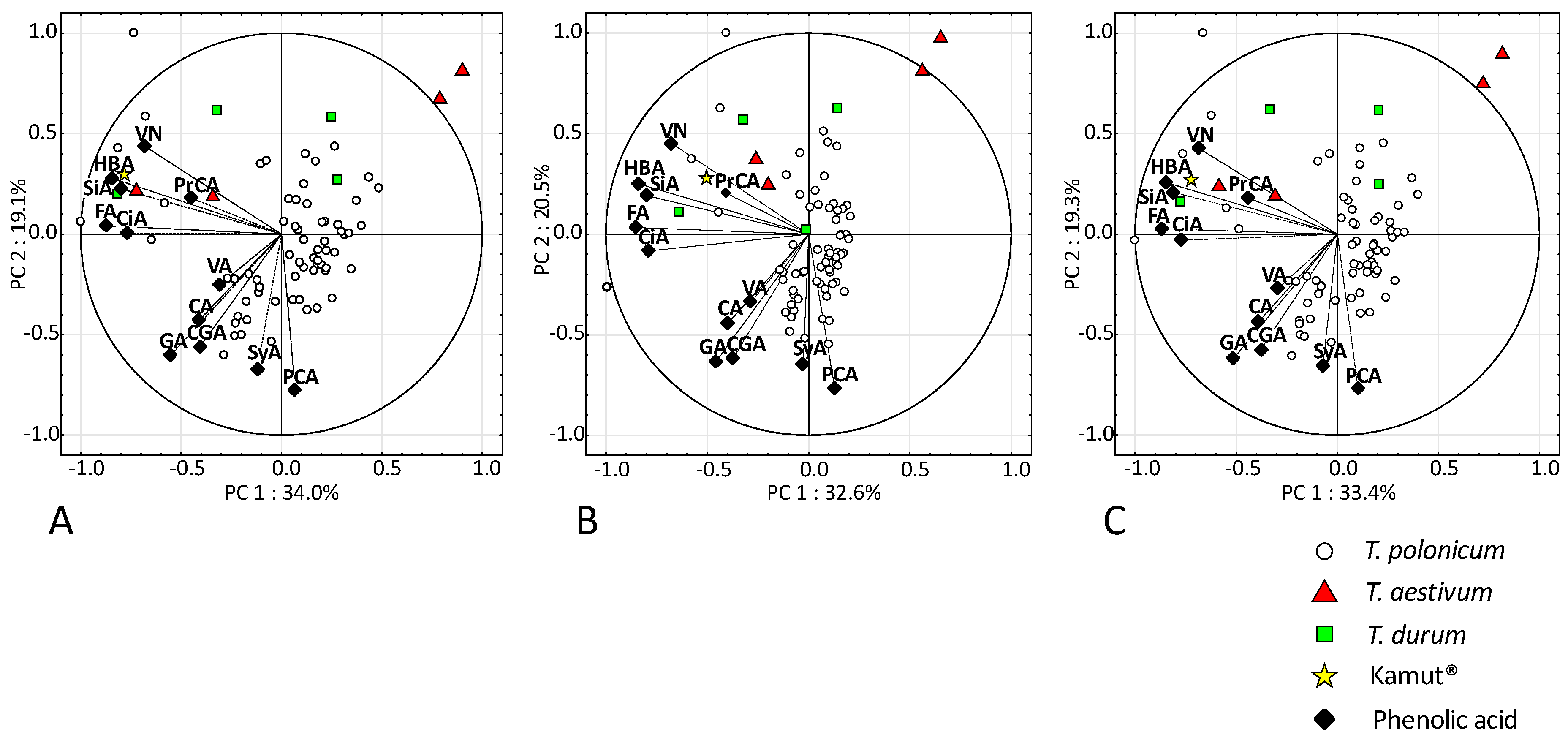
3.2. Content of Bound and Free Phenolic Acids (FPAs)
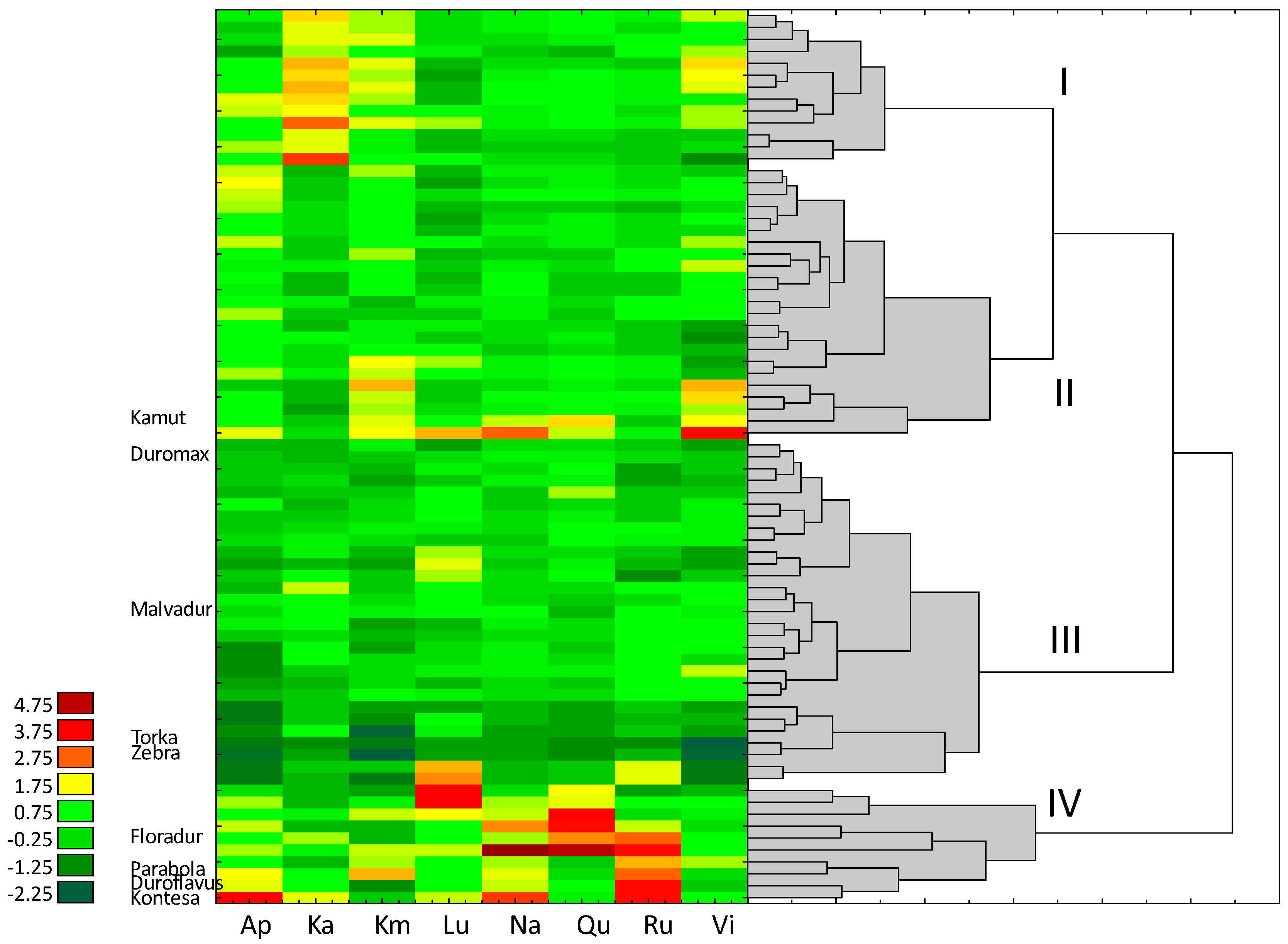
3.3. Content of Flavonoids
4. Discussion
4.1. Antioxidant Activity
4.2. Phenolic Acids
4.3. Flavonoids
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Đor₫ević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Brankovic, S.I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Food World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A. Nutritional properties of einkorn wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Leoncini, E.; Prata, C.; Malaguti, M.; Marotti, I.; Segura-Carretero, A.; Catizone, P.; Dinelli, G.; Hrelia, S. Phytochemical Profile and Nutraceutical Value of Old and Modern Common Wheat Cultivars. PLoS ONE 2012, 7, e45997. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.L.; Poutanen, K.; Gebruers, K.; Piironen, V.; Lampi, A.-M.; Nyström, L.; Andersson, A.A.; Boros, D.; Rakszegi, M.; Bedo, Z.; et al. The HEALTHGRAIN cereal diversity screen: Concept, results, and prospects. J. Agric. Food Chem. 2008, 56, 9699–9709. [Google Scholar] [CrossRef]
- Giambanelli, E.; Ferioli, F.; Kocaoglu, B.; Jorjadze, M.; Alexieva, I.; Darbinyan, N.; Antuono, F. A comparative study of bioactive compounds in primitive wheat populations from Italy, Turkey, Georgia, Bulgaria and Armenia. J. Sci. Food Agric. 2013, 93, 3490–3501. [Google Scholar] [CrossRef]
- Arzani, A.; Ashraf, M. Cultivated ancient wheats (Triticum spp.): A potential source of health-beneficial food products. Compr. Rev. Food Sci. F 2017, 16, 477–488. [Google Scholar] [CrossRef] [Green Version]
- Atanasova-Penichon, V.; Barreau, C.; Richard-Forget, F. Antioxidant Secondary Metabolites in Cereals: Potential Involvement in Resistance to Fusarium and Mycotoxin Accumulation. Front. Microbiol. 2016, 7, 566. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Aal, E.S.; Hucl, P.; Sosulski, F.W.; Graf, R.; Gillott, C.; Pietrzak, L. Screening Spring Wheat for Midge Resistance in Relation to Ferulic Acid Content. J. Agric. Food Chem. 2001, 49, 3559–3566. [Google Scholar] [CrossRef]
- Shahidi, F.; Liana-Pathirana, C. Antioxidant properties of wheat grain and its fractions. In Wheat Antioxidants; Yu, L., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008; pp. 7–23. [Google Scholar]
- Gorinstein, S.; Lojek, A.; Ciz, M.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Salas, I.A.; Goshev, I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int. J. Food Sci. Technol. 2008, 43, 629–637. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Ndolo, V.U.; Beta, T. Comparative studies on composition and distribution of phenolic acids in cereal grain botanical fractions. Cereal Chem. 2014, 91, 522–530. [Google Scholar] [CrossRef]
- Irakli, M.N.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Development and validation of an HPLC-method for determination of free and bound phenolic acids in cereals after solid-phase extraction. Food Chem. 2012, 134, 1624–1632. [Google Scholar] [CrossRef]
- Boz, H. Ferulic acid in cereals-a review. Czech J. Food Sci. 2015, 33, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Das, A.K.; Singh, V. Antioxidative free and bound phenolic constituents in pericarp, germ and endosperm of Indian dent (Zea mays var. indentata) and flint (Zea mays var. indurata) maize. J. Funct. Food 2015, 13, 363–374. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef]
- Shewry, P.R.; Piironen, V.; Lampi, A.-M.; Edelmann, M.; Kariluoto, S.; Nurmi, T.; Fernandez-Orozco, R.; Andersson, A.A.M.; Åman, P.; Fraś, A.; et al. Effects of Genotype and Environment on the Content and Composition of Phytochemicals and Dietary Fiber Components in Rye in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2010, 58, 9372–9383. [Google Scholar] [CrossRef]
- Falk, J.; Krahnstover, A.; van der Kooij, T.A.W.; Schlensog, M.; Krupinska, K. Tocopherol and tocotrienol accumulation during development of caryopses from barley (Hordeum vulgare L.). Phytochemistry 2004, 65, 2977–2985. [Google Scholar] [CrossRef]
- Onyeneho, S.N.; Hettiarachchy, N.S. Antioxidant activity of durum wheat bran. J. Agric. Food Chem. 1992, 40, 1496–1500. [Google Scholar] [CrossRef]
- Miller, H.E.; Rigelhof, F.; Marquart, L.; Prakash, A.; Kanter, M. Whole-grain products and antioxidants. Cereal Food World 2000, 45, 59–63. [Google Scholar]
- Quaglia, G. Other Durum Wheat Products. In Durum Wheat: Chemistry and Technology; Fabriani, G., Lintas, C., Eds.; American Association of Cereal Chemists: St. Paul, MN, USA, 1988; p. 263. [Google Scholar]
- Eticha, F.; Belay, G.; Bekele, E. Species diversity in wheat landrace populations from two regions of Ethiopia. Genet. Resour. Crop Evol. 2006, 53, 387–393. [Google Scholar] [CrossRef]
- Bieńkowska, T.; Suchowilska, E.; Kandler, W.; Krska, R.; Wiwart, M. Triticum polonicum L. as potential source material for the biofortification of wheat with essential micronutrients. Plant Genet. Resour. C 2019, 17, 213–220. [Google Scholar] [CrossRef]
- Suchowilska, E.; Szafrańska, A.; Słowik, E.; Wiwart, M. Flour from Triticum polonicum L. as a potential ingredient in bread production. Cereal Chem. 2019, 96, 554–563. [Google Scholar] [CrossRef]
- Wiwart, M.; Suchowilska, E.; Kandler, W.; Sulyok, M.; Groenwald, P.; Krska, R. Can Polish wheat (Triticum polonicum L.) be an interesting gene source for breeding wheat cultivars with increased resistance to Fusarium head blight? Genet. Resour. Crop Evol. 2013, 60, 2359–2373. [Google Scholar] [CrossRef]
- Witzenberger, A.; Van den Hack, H.; Boom, T. Erläuterungen zum BBCH-Dezimalcode für die Entwicklungsstadien des Getreides—Mit Abbildungen. Gesunde Pflanz. 1989, 41, 384–388. [Google Scholar]
- Przybylska-Balcerek, A.; Frankowski, J.; Stuper-Szablewska, K. Bioactive compounds in sorghum. Eur. Food Res. Technol. 2019, 245, 1075–1080. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), version 13. 2017. Available online: http://statistica.io (accessed on 27 September 2020).
- World-Grain.com. Available online: https://www.world-grain.com/articles/8777-global-durum-wheat-use-trending-upward (accessed on 27 September 2020).
- Statista.com. Available online: https://www.statista.com/statistics/267268/production-of-wheat-worldwide-since-1990/) (accessed on 27 September 2020).
- Bencze, S.; Makádi, M.; Aranyos, T.J.; Földi, M.; Hertelendy, P.; Mikó, P.; Bosi, S.; Negri, L.; Drexler, D. Re-Introduction of Ancient Wheat Cultivars into Organic Agriculture-Emmer and Einkorn Cultivation Experiences under Marginal Conditions. Sustainability 2020, 12, 1584. [Google Scholar] [CrossRef] [Green Version]
- Kwiatek, M.; Majka, M.; Majka, J.; Belter, J.; Suchowilska, E.; Wachowska, U.; Wiwart, M.; Wiśniewska, H. Intraspecific Polymorphisms of Cytogenetic Markers Mapped on Chromosomes of Triticum polonicum L. PLoS ONE 2016, 11, e0158883. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, A.; Danesi, F.; Di Nunzio, M.; Taccari, A.; Valli, V. Ancient wheat and health: A legend or the reality? A review on KAMUT khorasan wheat. Int. J. Food Sci. Nutr. 2017, 68, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalcová, V.; Dušinský, R.; Sabo, M.; Al Beyroutiová, M.; Hauptvogel, P.; Ivaničová, Z.; Švec, M. Taxonomical classification and origin of Kamut® wheat. Plant Syst. Evol. 2014, 300, 1749–1757. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Röder, M.S.; Grausgruber, H.; Börner, A. A DNA fingerprinting-based taxonomic allocation of Kamut wheat. Plant Genet. Resour. C 2006, 4, 172–180. [Google Scholar] [CrossRef]
- Ciudad-Mulero, M.; Barros, L.; Fernandes, Â.; Ferreira, I.C.F.R.; Callejo, M.; Matallana-González, M.C.; Fernández-Ruiz, V.; Morales, P.; Carrillo, J.M. Potential Health Claims of Durum and Bread Wheat Flours as Functional Ingredients. Nutrients 2020, 12, 504. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Yu, L.; Lu, Y.; Niu, Y.; Liu, L.; Costa, J.; Yu, L. Phytochemical compositions, and antioxidant properties, and antiproliferative activities of wheat flour. Food Chem. 2012, 135, 325–331. [Google Scholar] [CrossRef]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Klepacka, J.; Fornal, Ł. Ferulic acid and its position among the phenolic compounds of wheat. Crit. Rev. Food Sci. Nutr. 2007, 46, 639–647. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Bioactive Compounds and their Antioxidant Capacity in Selected Primitive and Modern Wheat Species. Open Agric. J. 2008, 2, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Kamut.com. Available online: https://www.kamut.com/en/discover/the-story (accessed on 27 September 2020).
- Di Loreto, A.; Di Silvestro, R.; Dinelli, G.; Bregola, V.; Stenico, V.; Sferrazza, R.E.; Marotti, I.; Quinn, R.; Bosi, S. Nutritional and nutraceutical aspects of KAMUT® khorasan wheat grown during the last two decades. J. Agric. Sci. 2017, 155, 954–965. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh, K.C. Syringic acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Kumar, N.; Kumar, S.; Mwaurah, P.W.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K.; Singh, V. Bioactive compounds, nutritional benefits and food applications of colored wheat: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Martinek, P.; Kotíkova, Z.; Orsak, M.; Sulc, M. Genetics and chemistry of pigments in wheat grain: A review. J. Cereal Sci. 2017, 74, 145–154. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Veronesi, M.; Grandi, E.; Dinelli, G.; Hrelia, S.; Borghi, C. Short-Term Hemodynamic Effects of Modern Wheat Products Substitution in Diet with Ancient Wheat Products: A Cross-Over, Randomized Clinical Trial. Nutrients 2018, 10, 1666. [Google Scholar] [CrossRef] [Green Version]



| Line | Name | Accession | Source | Line | Name | Accession | Source |
|---|---|---|---|---|---|---|---|
| Triticum polonicum | 40 | NK160 | TRI 4344 | IPK | |||
| 1 | NK118 | Cltr 13919 | NPGS | 41 | NK161 | TRI 5358 | IPK |
| 2 | NK119 | PI 29447 | NPGS | 42 | NK162 | TRI 6961 | IPK |
| 3 | NK120 | PI 56261 | NPGS | 43 | NK163 | TRI 9650 | IPK |
| 4 | NK121 | PI 56262 | NPGS | 44 | NK164 | TRI 28853 | IPK |
| 5 | NK122 | PI 134945 | NPGS | 45 | NK167 | TRI 19192 | IPK |
| 6 | NK123 | PI 185309 | NPGS | 46 | NK168 | TRI 19053 | IPK |
| 7 | NK124 | PI 191620 | NPGS | 47 | POL-1 | PL21801 | NCPGR |
| 8 | NK125 | PI 191837 | NPGS | 48 | POL-2 | PL 21802 | NCPGR |
| 9 | NK126 | PI 192666 | NPGS | 49 | POL-3 | PL 22488 | NCPGR |
| 10 | NK127 | PI 210845 | NPGS | 50 | POL-4 | PL 22479 | NCPGR |
| 11 | NK128 | PI 245663 | NPGS | 51 | POL-5 | PL 23047 | NCPGR |
| 12 | NK129 | PI 266846 | NPGS | 52 | POL-6 | PL 20770 | NCPGR |
| 13 | NK131 | PI 272565 | NPGS | 53 | POL-7 | PL 22991 | NCPGR |
| 14 | NK132 | PI 272566 | NPGS | 54 | POL-8 | PL 22746 | NCPGR |
| 15 | NK133 | PI 278647 | NPGS | 55 | POL-9 | PL 22195 | NCPGR |
| 16 | NK134 | PI 290512 | NPGS | 56 | POL-11 | PI 56262 | NPGS |
| 17 | NK135 | PI 306548 | NPGS | 57 | POL12 | Cltr 13919 | NPGS |
| 18 | NK136 | PI 306549 | NPGS | 58 | POL-12’ | Cltr 13919 | NPGS |
| 19 | NK137 | PI 330554 | NPGS | 59 | POL-12” | Cltr 13919 | NPGS |
| 20 | NK138 | PI 349052 | NPGS | 60 | POL-14 | PI 167622 | NPGS |
| 21 | NK139 | PI 352489 | NPGS | 61 | POL-14’ | PI 167622 | NPGS |
| 22 | NK140 | PI 387457 | NPGS | 62 | POL-14” | PI 167622 | NPGS |
| 23 | NK141 | PI 566593 | NPGS | 63 | POL-16 | PI 191881 | NPGS |
| 24 | NK142 | TRI 889 | IPK | 64 | POL-19 | PI 225335 | NPGS |
| 25 | NK143 | TRI 891 | IPK | 65 | POL-22 | PI 272570 | NPGS |
| 26 | NK144 | TRI 893 | IPK | 66 | POL-25 | PI 384265 | NPGS |
| 27 | NK146 | TRI 1896 | IPK | Triticum durum | |||
| 28 | NK147 | TRI 1950 | IPK | 67 | Duroflavus | cultivar | Saatbau Linz, Austria |
| 29 | NK148 | TRI 1951 | IPK | 68 | Duromax | cultivar | Saatbau Linz, Austria |
| 30 | NK150 | TRI 3248 | IPK | 69 | Floradur | cultivar | Probstdorfer Saatzucht, Austria |
| 31 | NK151 | TRI 3311 | IPK | 70 | Malvadur | cultivar | Probstdorfer Saatzucht, Austria |
| 32 | NK152 | TRI 3410 | IPK | Triticum aestivum | |||
| 33 | NK153 | TRI 3412 | IPK | 71 | Torka | Elite (E) cultivar | Hodowla Roślin Strzelce, Poland |
| 34 | NK154 | TRI 3440 | IPK | 72 | Parabola | Quality (A) cultivar | Małopolska Hodowla Roślin, Poland |
| 35 | NK155 | TRI 3442 | IPK | 73 | Kontesa | Quality (A) cultivar | Hodowla Roślin Strzelce, Poland |
| 36 | NK156 | TRI 3478 | IPK | 74 | Zebra | Elite (E) cultivar | DANKO, Hodowla Roślin, Poland |
| 37 | NK157 | TRI 3550 | IPK | Triticum turanicum | |||
| 38 | NK158 | TRI 3597 | IPK | 75 | Kamut® | cultivar | Supermarket, Austria |
| 39 | NK159 | TRI 4342 | IPK | ||||
| Extraction Rate (%) | LUT | ZEA | β-C | Total Carotenoids (LUT + ZEA+ β-C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | B | G | F | B | G | F | B | G | F | B | G | F | B | |
| T. polonicum (n = 66) | 75.6 a,b | 19.3 a,b | 1.18 b | 1.60 b | 0.73 b | 0.63 | 0.63 | 0.22 | 1.36 b | 0.26 | 2.21 b | 3.17 b | 2.49 b | 3.16 b |
| T. durum (n = 4) | 76.7 a | 17.8 b | 1.92 a | 2.39 a | 0.78 a,b | 0.89 | 0.89 | 0.22 | 2.04 a | 0.28 | 3.30 a | 4.84 a | 3.56 a | 4.30 a |
| T. aestivum (n = 4) | 73.5 b | 21.7 a | 1.56 a,b | 1.77 a,b | 0.92 a | 0.79 | 0.79 | 0.21 | 1.59 a,b | 0.26 | 2.69 a,b | 3.94 a,b | 2.82 a,b | 3.82 a,b |
| T. turanicum (Kamut®) | 74.9 a,b | 20.4 a,b | 1.35 a,b | 1.68 a,b | 0.78 a,b | 0.81 | 0.81 | 0.19 | 1.45 a,b | 0.22 | 2.68 a,b | 3.61 a,b | 2.71 b | 3.65 a,b |
| Mean (weighted) | 75.5 | 19.4 | 1.24 A | 1.65 A | 0.74 B | 0.65 A | 0.65 A | 0.22 B | 1.41 B | 0.26 C | 2.30 A | 3.31 A | 2.57 B | 3.26 A |
| Phenolic Acids (mg kg−1) | VN | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HBA | CA | CGA | FA | GA | PCA | PrCA | SiA | SyA | CiA | VA | ∑FA | FPA | ||
| Flour | ||||||||||||||
| T. polonicum | 41.2 b | 61.7 | 101.5 b | 463.8 b | 7.6 | 5.9 a | 26.9 | 39.9 | 25.9 a | 60.4 b | 12.3 | 0.350 | 256 | 0.32 b |
| T. durum | 64.5 b | 81.8 | 88.2 b | 466.3 b | 5.8 | 2.5 b | 28.6 | 43.6 | 16.8 a,b | 58.8 b | 14.6 | −1.154 | 233 | 0.88 a |
| T. aestivum | 31.8 b | 56.4 | 57.2 b | 353.8 b | 6.1 | 4.9 a,b | 19.7 | 43.5 | 7.3 b | 35.3 b | 13.4 | −6.651 | 221 | 0.87 a |
| T. turanicum (Kamut®) | 88.4 a | 16.6 | 142.4 a | 728.0 a | 7.4 | 3.1 a,b | 19.0 | 36.0 | 10.3 a,b | 109.2 a | 13.1 | 8.117 | 312 | 0.78 a |
| Mean (weighted) | 42.6B | 61.9 B | 99.0 C | 461.6 C | 7.4 B | 5.6 B | 26.5 B | 40.2 B | 24.2 B | 59.6 B | 12.5 B | 254 C | 0.40 B | |
| Bran | ||||||||||||||
| T. polonicum | 159.4 b | 242.3 a,b | 400.7 a,b | 1800.7 b | 29.6 a,b | 23.4 a | 104.9 | 155.2 | 100.8 a | 236.8 b | 48.4 | 0.418 | 3205 | 1.24 b |
| T. durum | 276.4 a,b | 352.6 a | 376.5 a,b | 1949.9 b | 25.3 a,b | 10.8 b | 121.1 | 187.9 | 75.6 a,b | 246.0 b | 61.9 | 1.314 | 2633 | 3.70 a |
| T. aestivum | 109.1 b | 190.2 a,b | 193.5 b | 1195.2 b | 20.5 b | 17.5 a,b | 66.2 | 146.4 | 24.4 c | 116.9 b | 46.4 | −9.785 | 2530 | 3.00 a |
| T. turanicum (Kamut®) | 413.7 | 77.6 b | 666.8 a | 3408.0 a | 34.8 a | 14.4 a,b | 89.0 | 167.6 | 48.3 bc | 511.0 a | 61.2 | 6.311 | 3625 | 3.70 a |
| Mean (weighted) | 166.3 A | 243.2 A | 391.9 A | 1797.8 A | 29.0 A | 22.3 A | 103.5 A | 156.6 A | 94.7 A | 234.6 A | 49.2A | 3144 A | 1.50 A | |
| Grain | ||||||||||||||
| T. polonicum | 65.2 b | 97.8 a,b | 161.2 b | 734.0 b | 12.0 a,b | 9.4 a | 42.7 | 63.2 | 41.0 a | 95.8 b | 19.5 | 0.369 | 631 | 0.51 |
| T. durum | 104.2 a,b | 132.3 a | 142.4 b | 749.9 b | 9.5 b | 4.0 b | 46.1 | 70.5 | 27.4 a,b | 94.6 b | 23.6 | −0.703 | 467 | 1.41 |
| T. aestivum | 49.1 b | 86.7 a,b | 88.0 b | 544.2 b | 9.3 b | 7.7 a,b | 30.3 | 66.9 | 11.1 b | 54.0 b | 20.8 | −7.343 | 561 | 1.35 |
| T. turanicum (Kamut®) | 176.7 a | 33.1 b | 284.8 a | 1455.8 a | 14.9 a | 6.2 a,b | 38.0 | 71.6 | 20.6 b | 218.3 a | 26.1 | 7.858 | 726 | 1.57 |
| Mean (weighted) | 67.9 B | 98.2 A,B | 157.9 B | 734.3 B | 11.8 B | 9.0 B | 42.2 B | 63.9 B | 38.4 B | 95.1 B | 19.9 B | 619 B | 0.60 B | |
| Ap | Ka | Km | Lu | Na | Qu | Ru | Vi | Total FLVs | |
|---|---|---|---|---|---|---|---|---|---|
| Flour | |||||||||
| T. polonicum | 22.9 | 9.4 | 9.7 | 23.1 | 15.1 | 27.8 | 5.2 a,b | 10.0 | 123.2 |
| RSD% | 44 | 73 | 36 | 74 | 82 | 71 | 38 | 42 | |
| T. durum | 24.4 | 11.1 | 6.4 | 25.3 | 23.4 | 35.4 | 8.9 a | 8.4 | 143.3 |
| RSD% | 43 | 43 | 29 | 30 | 31 | 84 | 48 | 21 | |
| T. aestivum | 27.7 | 9.1 | 7.3 | 20.2 | 23.5 | 12.8 | 7.8 a,b | 5.8 | 114.2 |
| RSD% | 105 | 101 | 103 | 101 | 109 | 109 | 78 | 78 | |
| T. turanicum (Kamut®) | 27.4 | 5.3 | 14.5 | 23.4 | 29.5 | 66.8 | 4.0 b | 16.9 | 187.8 |
| Bran | |||||||||
| T. polonicum | 90.2 | 37.2 | 38.4 | 89.4 | 58.4 | 109.2 | 20.4 b | 38.8 a,b | 482 |
| RSD% | 44 | 76 | 37 | 71 | 78 | 72 | 35 | 37 | |
| T. durum | 105.0 | 48.3 | 28.2 | 110.8 | 101.8 | 146.6 | 38.3 a | 36.4 a,b | 615.4 |
| RSD% | 40 | 45 | 39 | 33 | 32 | 80 | 44 | 25 | |
| T. aestivum | 92.8 | 30.7 | 25.6 | 67.8 | 78.7 | 42.9 | 26.6 a,b | 19.4 b | 384.5 |
| RSD% | 103 | 98 | 111 | 97 | 105 | 106 | 77 | 102 | |
| T. turanicum (Kamut®) | 100.5 | 19.5 | 53.1 | 96.8 | 108.2 | 245.2 | 14.8 b | 62.3 a | 700.4 |
| Grain | |||||||||
| T. polonicum | 36.4 | 14.9 | 15.5 | 36.5 | 23.9 | 44.1 | 8.3 a,b | 15.8 a,b | 195.4 |
| RSD% | 44 | 74 | 36 | 73 | 81 | 72 | 37 | 40 | |
| T. durum | 39.5 | 17.9 | 10.4 | 41.1 | 37.9 | 56.9 | 14.5 a | 13.5 a,b | 231.7 |
| RSD% | 43 | 43 | 30 | 30 | 31 | 84 | 48 | 22 | |
| T. aestivum | 42.6 | 14.0 | 11.4 | 31.0 | 36.1 | 19.6 | 12.1 a,b | 8.9 b | 175.7 |
| RSD% | 105 | 101 | 105 | 100 | 108 | 108 | 78 | 105 | |
| T. turanicum (Kamut®) | 42.9 | 8.3 | 22.7 | 41.4 | 46.2 | 104.8 | 6.3 b | 26.6 a | 299.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suchowilska, E.; Bieńkowska, T.; Stuper-Szablewska, K.; Wiwart, M. Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species. Agriculture 2020, 10, 591. https://doi.org/10.3390/agriculture10120591
Suchowilska E, Bieńkowska T, Stuper-Szablewska K, Wiwart M. Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species. Agriculture. 2020; 10(12):591. https://doi.org/10.3390/agriculture10120591
Chicago/Turabian StyleSuchowilska, Elżbieta, Teresa Bieńkowska, Kinga Stuper-Szablewska, and Marian Wiwart. 2020. "Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species" Agriculture 10, no. 12: 591. https://doi.org/10.3390/agriculture10120591
APA StyleSuchowilska, E., Bieńkowska, T., Stuper-Szablewska, K., & Wiwart, M. (2020). Concentrations of Phenolic Acids, Flavonoids and Carotenoids and the Antioxidant Activity of the Grain, Flour and Bran of Triticum polonicum as Compared with Three Cultivated Wheat Species. Agriculture, 10(12), 591. https://doi.org/10.3390/agriculture10120591





