Physiological and Pathological Role of Circadian Hormones in Osteoarthritis: Dose-Dependent or Time-Dependent?
Abstract
1. Introduction
2. Source and Synthesis of Circadian Hormones
3. Secretion Pattern of Melatonin
3.1. Concentration-Based In Vitro Studies of Melatonin in Osteoarthritis
3.2. Concentration-Based In Vivo Studies of Melatonin in Osteoarthritis
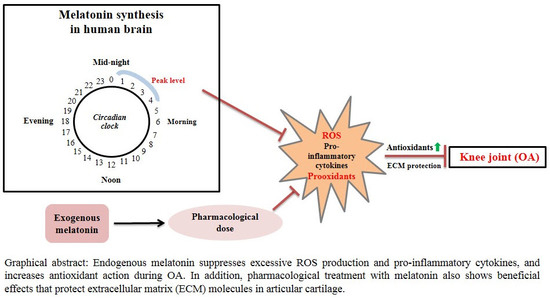
4. Regulatory Effects of Melatonin on Osteoarthritis
5. Anabolic and Catabolic Effects of Thyroid-Stimulating Hormone on Articular Cartilage and Bone
6. Inflammatory Effects of Cortisol in Osteoarthritis
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuettner, K.E.; Cole, A.A. Cartilage degeneration in different human joints. Osteoarthr. Cartil. 2005, 13, 93–103. [Google Scholar] [CrossRef] [PubMed]
- March, L.; Smith, E.U.; Hoy, D.G.; Cross, M.J.; Sanchez-Riera, L.; Blyth, F.; Buchbinder, R.; Vos, T.; Woolf, A.D. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract. Res. Clin. Rheumatol. 2014, 28, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; Amann, M.; Anderson, H.R.; Andrews, K.G.; Aryee, M.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef]
- Arden, N.; Nevitt, M. Osteoarthritis: Epidemiology. Best Pract. Res. Clin. Rheumatol. 2006, 20, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Ono, K.; Miyazaki, J.; Olsen, B.R.; Muragaki, Y.; Adachi, E.; Yamamura, K.; Kimura, T. Osteoarthritis associated with mild chondrodysplasia in transgenic mice expressing alpha 1(IX) collagen chains with a central deletion. Proc. Natl. Acad. Sci. USA 1993, 90, 2870–2874. [Google Scholar] [CrossRef] [PubMed]
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef]
- Yuan, X.L.; Meng, H.Y.; Wang, Y.C.; Peng, J.; Guo, Q.Y.; Wang, A.Y.; Lu, S.B. Bone–cartilage interface crosstalk in osteoarthritis: Potential pathways and future therapeutic strategies. Osteoarthr. Cartil. 2014, 22, 1077–1089. [Google Scholar] [CrossRef]
- Almonte-Becerril, M.; Navarro-Garcia, F.; Gonzalez-Robles, A.; Vega-Lopez, M.A.; Lavalle, C.; Kouri, J.B. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis 2010, 15, 631–638. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E.; Brandt, K.; Hawker, G.; Peeva, E.; Schreyer, E.; Tsuji, W.; Hochberg, M.C. OARSI-FDA initiative: Defining the disease state of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 478–482. [Google Scholar] [CrossRef]
- Tellegen, A.R.; Rudnik-Jansen, I.; Pouran, B.; de Visser, H.M.; Weinans, H.H.; Thomas, R.E.; Kik, M.J.L.; Grinwis, G.C.M.; Thies, J.C.; Woike, N.; et al. Controlled release of celecoxib inhibits inflammation, bone cysts and osteophyte formation in a preclinical model of osteoarthritis. Drug Deliv. 2018, 25, 1438–1447. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Halawa, F.; Mostafa, T.; Atta, H. Melatonin hormone profile in infertile males. Int. J. Androl. 2006, 29, 409–413. [Google Scholar] [CrossRef]
- Rodríguez, M.I.; Escames, G.; López, L.C.; López, A.; García, J.A.; Ortiz, F.; Acuña-Castroviejo, D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J. Pineal Res. 2007, 42, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Sugden, D. Psychopharmacological effects of melatonin in mouse and rat. J. Pharmacol. Exp. Ther. 1983, 227, 587–591. [Google Scholar]
- Bilici, D.; Akpinar, E.; Kiziltunç, A. Protective effect of melatonin in carrageenan-induced acute local inflammation. Pharmacol. Res. 2002, 46, 133–139. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef]
- Suzuki, N.; Somei, M.; Kitamura, K.; Reiter, R.J.; Hattori, A. Novel bromomelatonin derivatives suppress osteoclastic activity and increase osteoblastic activity: Implications for the treatment of bone diseases. J. Pineal Res. 2008, 44, 326–334. [Google Scholar] [CrossRef]
- Suzuki, N.; Hattori, A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J. Pineal Res. 2002, 33, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Amstrup, A.K.; Sikjaer, T.; Heickendorff, L.; Mosekilde, L.; Rejnmark, L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: A randomized controlled trial. J. Pineal Res. 2015, 59, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Witt-Enderby, P.A. Melatonin effects on bone: Potential use for the prevention and treatment for osteopenia, osteoporosis, and periodontal disease and for use in bone-grafting procedures. J. Pineal Res. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Spence, D.W.; Moscovitch, A.; Trakht, I.; Brown, G.M.; Cardinali, D.P. Potential use of melatonergic drugs in analgesia: Mechanisms of action. Brain Res. Bull. 2010, 81, 362–371. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Sanchez-Barcelo, E.; Mediavilla, M.D.; Reiter, R.J. Significance of high levels of endogenous melatonin in Mammalian cerebrospinal fluid and in the central nervous system. Curr. Neuropharmacol. 2010, 8, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Nakao, N.; Ono, H.; Yamamura, T.; Anraku, T.; Takagi, T.; Higashi, K.; Yasuo, S.; Katou, Y.; Kageyama, S.; Uno, Y.; et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 2008, 452, 317–322. [Google Scholar] [CrossRef]
- Askari, A.; Ehrampoush, E.; Homayounfar, R.; Bahramali, E.; Farjam, M. Serum insulin in pathogenesis and treatment of osteoarthritis. Med. Hypotheses 2017, 99, 45–46. [Google Scholar] [CrossRef]
- Maeda, K.; Kato, Y.; Ohgo, S.; Chihara, K.; Yoshimoto, Y.; Yamaguchi, N.; Kuromaru, S.; Imura, H. Growth Hormone and Prolactin Release After Injection of Thyrotropin-Releasing Hormone in Patients with Depression. J. Clin. Endocrinol. Metab. 1975, 40, 501–505. [Google Scholar] [CrossRef]
- Tashjian, A.H., Jr.; Barowsky, N.J.; Jensen, D.K. Thyrotropin releasing hormone: Direct evidence for stimulation of prolactin production by pituitary cells in culture. Biochem. Biophys. Res. Commun. 1971, 43, 516–523. [Google Scholar] [CrossRef]
- Abe, E.; Marians, R.C.; Yu, W.; Wu, X.B.; Ando, T.; Li, Y.; Iqbal, J.; Eldeiry, L.; Rajendren, G.; Blair, H.C.; et al. TSH Is a Negative Regulator of Skeletal Remodeling. Cell 2003, 115, 151–162. [Google Scholar] [CrossRef]
- Sampath, T.K.; Simic, P.; Sendak, R.; Draca, N.; Bowe, A.E.; O’Brien, S.; Schiavi, S.C.; McPherson, J.M.; Vukicevic, S. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J. Bone Miner. Res. 2007, 22, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Kalev-Zylinska, M.L.; Hearn, J.I.; Rong, J.; Zhu, M.; Munro, J.; Cornish, J.; Dalbeth, N.; Poulsen, R.C. Altered N-methyl D-aspartate receptor subunit expression causes changes to the circadian clock and cell phenotype in osteoarthritic chondrocytes. Osteoarthr. Cartil. 2018, 26, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Debono, M. Replication of cortisol circadian rhythm: New advances in hydrocortisone replacement therapy. Ther. Adv. Endocrinol. Metab. 2010, 1, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, J.R.; Veldhuis, J.D.; Leyo, S.A.; Iranmanesh, A.; Rogol, A.D. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J. Clin. Endocrinol. Metab. 1993, 76, 1505–1510. [Google Scholar] [PubMed]
- Linder, B.L.; Esteban, N.V.; Yergey, A.L.; Winterer, J.C.; Loriaux, D.L.; Cassorla, F. Cortisol production rate in childhood and adolescence. J. Pediatr. 1990, 117, 892–896. [Google Scholar] [CrossRef]
- Bierhaus, A.; Wolf, J.; Andrassy, M.; Rohleder, N.; Humpert, P.M.; Petrov, D.; Ferstl, R.; von Eynatten, M.; Wendt, T.; Rudofsky, G.; et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Parvez, S. Role of melatonin in traumatic brain injury and spinal cord injury. Sci. World J. 2014, 2014, 586270. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin and human reproduction. Ann. Med. 1998, 30, 103–108. [Google Scholar] [CrossRef]
- Stehle, J.H.; Saade, A.; Rawashdeh, O.; Ackermann, K.; Jilg, A.; Sebestény, T.; Maronde, E. A survey of molecular details in the human pineal gland in the light of phylogeny, structure, function and chronobiological diseases. J. Pineal Res. 2011, 51, 17–43. [Google Scholar] [CrossRef]
- Yonei, Y.; Hattori, A.; Tsutsui, K.; Okawa, M.; Ishizuka, B. Effects of Melatonin: Basics Studies and Clinical Applications. Anti Aging Med. 2010, 7, 85–91. [Google Scholar]
- Magner, J. Historical note: Many steps led to the “discovery” of thyroid-stimulating hormone. Eur. Thyroid J. 2014, 3, 95–100. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lechan, R.M.; Fekete, C. The TRH neuron: A hypothalamic integrator of energy metabolism. Prog. Brain Res. 2006, 153, 209–235. [Google Scholar] [PubMed]
- Bianco, A.C.; Kim, B.W. Deiodinases: Implications of the local control of thyroid hormone action. J. Clin. Investig. 2006, 116, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.; Harrison, R.F.; Smith, N.; Darzy, K.; Shalet, S.; Weetman, A.P.; Ross, R.J. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J. Clin. Endocrinol. Metab. 2008, 93, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C.; Alford, F.P.; Burger, H.G. The 24-hour plasma thyrotrophin profile. Clin. Sci. 1972, 43, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lucke, C.; Hehrmann, R.; von Mayersbach, K.; von zur Mühlen, A. Studies on circadian variations of plasma TSH, thyroxine and triiodothyronine in man. Acta Endocrinol. 1977, 86, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.M.; Dieguez, C.; Lewis, M.D.; Scanlon, M.F. Dopamine stimulates release of thyrotrophin-releasing hormone from perfused intact rat hypothalamus via hypothalamic D2-receptors. J. Endocrinol. 1987, 115, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Damerow, S.; Kiessling, S.; Jakubcakova, V.; Abraham, D.; Tian, J.; Hoffmann, M.W.; Eichele, G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006, 4, 163–173. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef]
- Sae-Teaw, M.; Johns, J.; Johns, N.P.; Subongkot, S. Serum melatonin levels and antioxidant capacities after consumption of pineapple, orange, or banana by healthy male volunteers. J. Pineal Res. 2013, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Barsacchi, R.; Kusmic, C.; Damiani, E.; Carloni, P.; Greci, L.; Donato, L. Vitamin E consumption induced by oxidative stress in red blood cells is enhanced by melatonin and reduced by N-acetylserotonin. Free Radic. Biol. Med. 1998, 24, 1187–1192. [Google Scholar] [CrossRef]
- Banki, K.; Hutter, E.; Gonchoroff, N.J.; Perl, A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J. Immunol. 1999, 162, 1466–1479. [Google Scholar] [PubMed]
- Wölfler, A.; Caluba, H.C.; Abuja, P.M.; Dohr, G.; Schauenstein, K.; Liebmann, P.M. Prooxidant activity of melatonin promotes fas-induced cell death in human leukemic Jurkat cells. FEBS Lett. 2001, 502, 127–131. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, D.; Reiter, R.J.; Sewerynek, E.; Hara, M.; Chen, L.; Nisticò, G. Paraquat toxicity and oxidative damage. Reduction by melatonin. Biochem. Pharmacol. 1996, 51, 1095–1099. [Google Scholar] [CrossRef]
- Tan, D.X.; Pöeggeler, B.; Reiter, R.J.; Chen, L.D.; Chen, S.; Manchester, L.C.; Barlow-Walden, L.R. The pineal hormone melatonin inhibits DNA-adduct formation induced by the chemical carcinogen safrole in vivo. Cancer Lett. 1993, 70, 65–71. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Poeggeler, B.; Menendez-Pelaez, A.; Chen, L.D.; Saarela, S. Melatonin as a free radical scavenger: Implications for aging and age-related diseases. Ann. N. Y. Acad. Sci. 1994, 719, 1–12. [Google Scholar] [CrossRef]
- Reiter, R.J.; Melchiorri, D.; Sewerynek, E.; Poeggeler, B.; Barlow-Walden, L.; Chuang, J.; Ortiz, G.G.; Acuña-Castroviejo, D. A review of the evidence supporting melatonin’s role as an antioxidant. J. Pineal Res. 1995, 18, 1–11. [Google Scholar] [CrossRef]
- Osseni, R.A.; Rat, P.; Bogdan, A.; Warnet, J.M.; Touitou, Y. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci. 2000, 68, 387–399. [Google Scholar] [CrossRef]
- Harms, C.; Lautenschlager, M.; Bergk, A.; Freyer, D.; Weih, M.; Dirnagl, U.; Weber, J.R.; Hörtnagl, H. Melatonin is protective in necrotic but not in caspase-dependent, free radical-independent apoptotic neuronal cell death in primary neuronal cultures. FASEB J. 2000, 14, 1814–1824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Clapp-Lilly, K.L.; Smith, M.A.; Perry, G.; Harris, P.L.; Zhu, X.; Duffy, L.K. Melatonin acts as antioxidant and pro-oxidant in an organotypic slice culture model of Alzheimer’s disease. Neuroreport 2001, 12, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, H.; Lee, Y.; Lee, S.; Kim, K.; Jin, Y.; Lee, S.R.; Chang, K.T.; Hong, Y. Salutary effects of melatonin combined with treadmill exercise on cartilage damage. J. Pineal Res. 2014, 57, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Buyukavci, M.; Ozdemir, O.; Buck, S.; Stout, M.; Ravindranath, Y.; Savasan, S. Melatonin cytotoxicity in human leukemia cells: Relation with its pro-oxidant effect. Fundam. Clin. Pharmacol. 2006, 20, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Ghaffari Novin, M.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin modifies histone acetylation during in vitro maturation of mouse oocytes. Cell J. 2018, 20, 244–249. [Google Scholar] [PubMed]
- Albertini, M.C.; Radogna, F.; Accorsi, A.; Uguccioni, F.; Paternoster, L.; Cerella, C.; De Nicola, M.; D’Alessio, M.; Bergamaschi, A.; Magrini, A.; et al. Intracellular pro-oxidant activity of melatonin deprives U937 cells of reduced glutathione without affecting glutathione peroxidase activity. Ann. N. Y. Acad. Sci. 2006, 1091, 10–16. [Google Scholar] [CrossRef]
- Cristofanon, S.; Uguccioni, F.; Cerella, C.; Radogna, F.; Dicato, M.; Ghibelli, L.; Diederich, M. Intracellular prooxidant activity of melatonin induces a survival pathway involving NF-κB activation. Ann. N. Y. Acad. Sci. 2009, 1171, 472–478. [Google Scholar] [CrossRef]
- Gao, C.; Han, H.B.; Tian, X.Z.; Tan, D.X.; Wang, L.; Zhou, G.B.; Zhu, S.E.; Liu, G.S. Melatonin promotes embryonic development and reduces reactive oxygen species in vitrified mouse 2-cell embryos. J. Pineal Res. 2012, 52, 305–311. [Google Scholar] [CrossRef]
- Ali, T.; Rehman, S.U.; Shah, F.A.; Kim, M.O. Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain. J. Neuroinflamm. 2018, 15, 119. [Google Scholar] [CrossRef]
- Kocyigit, A.; Guler, E.M.; Karatas, E.; Caglar, H.; Bulut, H. Dose-dependent proliferative and cytotoxic effects of melatonin on human epidermoid carcinoma and normal skin fibroblast cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 829, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, D.S.; Anoopkumar-Dukie, S.; Glass, B.D.; Antunes, E.M.; Lack, B.; Walker, R.B.; Daya, S. The identification of the UV degradants of melatonin and their ability to scavenge free radicals. J. Pineal Res. 2002, 32, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, G.; Coşkun, S.; Erbaş, D.; Hasanoglu, E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. Jpn. J. Physiol. 2000, 50, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Antolín, I.; Rodríguez, C.; Saínz, R.M.; Mayo, J.C.; Uría, H.; Kotler, M.L.; Rodríguez-Colunga, M.J.; Tolivia, D.; Menéndez-Peláez, A. Neurohormone melatonin prevents cell damage: Effect on gene expression for antioxidant enzymes. FASEB J. 1996, 10, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ng, T.B. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem. Cell Biol. 2000, 78, 447–453. [Google Scholar] [CrossRef]
- Shen, Y.X.; Xu, S.Y.; Wei, W.; Sun, X.X.; Liu, L.H.; Yang, J.; Dong, C. The protective effects of melatonin from oxidative damage induced by amyloid beta-peptide 25-35 in middle-aged rats. J. Pineal Res. 2002, 32, 85–89. [Google Scholar] [CrossRef]
- Shen, Y.-X.; Xu, S.Y.; Wei, W.; Sun, X.X.; Yang, J.; Liu, L.H.; Dong, C. Melatonin reduces memory changes and neural oxidative damage in mice treated with D-galactose. J. Pineal Res. 2002, 32, 173–178. [Google Scholar] [CrossRef]
- Wakatsuki, A.; Okatani, Y.; Shinohara, K.; Ikenoue, N.; Kaneda, C.; Fukaya, T. Melatonin protects fetal rat brain against oxidative mitochondrial damage. J. Pineal Res. 2001, 30, 22–28. [Google Scholar] [CrossRef]
- Benot, S.; Molinero, P.; Soutto, M.; Goberna, R.; Guerrero, J.M. Circadian variations in the rat serum total antioxidant status: Correlation with melatonin levels. J. Pineal Res. 1998, 25, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Favero, G.; Rodella, L.F.; Moghadasian, M.H.; Rezzani, R. Melatonin modulation of Sirtuin-1 attenuates liver injury in a hypercholesterolemic mouse model. BioMed Res. Int. 2018, 2018, 7968452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Li, Y.Y.; Cui, W.; Li, L.B.; Zhang, Z.C.; Tian, B.P.; Zhang, G.S. Melatonin attenuates pain hypersensitivity and decreases astrocyte-mediated spinal neuroinflammation in a rat model of oxaliplatin-induced pain. Inflammation 2017, 40, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.; Tian, Y.; Wang, H.; Liu, F.; Xie, G. Protective effects of melatonin on lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2015, 29, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Abe, K.; Mekada, K.; Yoshiki, A.; Kato, T. Genetic variation of melatonin productivity in laboratory mice under domestication. Proc. Natl. Acad. Sci. USA 2010, 107, 6412–6417. [Google Scholar] [CrossRef] [PubMed]
- Semenova, N.V.; Madaeva, I.M.; Bairova, T.A.; Zhambalova, R.M.; Sholokhov, L.F.; Kolesnikova, L.I. Association of the melatonin circadian rhythms with clock 3111T/C gene polymorphism in Caucasian and Asian menopausal women with insomnia. Chronobiol. Int. 2018, 35, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Pablos, M.I.; Reiter, R.J.; Ortiz, G.G.; Guerrero, J.M.; Agapito, M.T.; Chuang, J.I.; Sewerynek, E. Rhythms of glutathione peroxidase and glutathione reductase in brain of chick and their inhibition by light. Neurochem. Int. 1998, 32, 69–75. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Zingarelli, B.; Gilad, E.; Hake, P.; Salzman, A.L.; Szabó, C. Protective effect of melatonin in carrageenan-induced models of local inflammation: Relationship to its inhibitory effect on nitric oxide production and its peroxynitrite scavenging activity. J. Pineal Res. 1997, 23, 106–116. [Google Scholar] [CrossRef]
- Takaishi, H.; Kimura, T.; Dalal, S.; Okada, Y.; D’Armiento, J. Joint diseases and matrix metalloproteinases: A role for MMP-13. Curr. Pharm. Biotechnol. 2008, 9, 47–54. [Google Scholar] [CrossRef]
- Pei, M.; He, F.; Wei, L.; Rawson, A. Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes. J. Pineal Res. 2009, 46, 181–187. [Google Scholar] [CrossRef]
- Lim, H.D.; Kim, Y.S.; Ko, S.H.; Yoon, I.J.; Cho, S.G.; Chun, Y.H.; Choi, B.J.; Kim, E.C. Cytoprotective and anti-inflammatory effects of melatonin in hydrogen peroxide-stimulated CHON-001 human chondrocyte cell line and rabbit model of osteoarthritis via the SIRT1 pathway. J. Pineal Res. 2012, 53, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Meng, Q.J. The brain–joint axis in osteoarthritis: Nerves, circadian clocks and beyond. Nat. Rev. Rheumatol. 2016, 12, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Yang, N.; Borysiewicz, E.; Dudek, M.; Williams, J.L.; Li, J.; Maywood, E.S.; Adamson, A.; Hastings, M.H.; Bateman, J.F.; et al. Catabolic cytokines disrupt the circadian clock and the expression of clock-controlled genes in cartilage via an NFкB-dependent pathway. Osteoarthr. Cartil. 2015, 23, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, Y.; Chen, S.; Tan, Z.; Xiong, K.; Li, Y.; Ye, Y.; Luo, Z.P.; He, F.; Gong, Y. Rescue of proinflammatory cytokine-inhibited chondrogenesis by the antiarthritic effect of melatonin in synovium mesenchymal stem cells via suppression of reactive oxygen species and matrix metalloproteinases. Free Radic. Biol. Med. 2014, 68, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Rong, J.; Zhu, M.; Munro, J.; Cornish, J.; McCarthy, G.M.; Dalbeth, N.; Poulsen, R.C. Altered expression of the core circadian clock component PERIOD2 contributes to osteoarthritis-like changes in chondrocyte activity. Chronobiol. Int. 2019, 36, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Snelling, S.J.B.; Forster, A.; Mukherjee, S.; Price, A.J.; Poulsen, R.C. The chondrocyte-intrinsic circadian clock is disrupted in human osteoarthritis. Chronobiol. Int. 2016, 33, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kang, X.; Liu, J.; Li, H.; Ma, Z.; Jin, X.; Qian, Z.; Xie, T.; Qin, N.; Feng, D.; et al. Clock Gene Bmal1 Modulates Human Cartilage Gene Expression by Crosstalk with Sirt1. Endocrinology 2016, 157, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kim, H.; Lee, S.; Jin, Y.; Choi, J.; Lee, S.-R.; Chang, K.-T.; Hong, Y. Role of melatonin combined with exercise as a switch-like regulator for circadian behavior in advanced osteoarthritic knee. Oncotarget 2017, 8, 97633–97647. [Google Scholar] [CrossRef]
- Lassová, L.; Niu, Z.; Golden, E.B.; Cohen, A.J.; Adams, S.L. Thyroid hormone treatment of cultured chondrocytes mimics in vivo stimulation of collagen X mRNA by increasing BMP 4 expression. J. Cell. Physiol. 2009, 219, 595–605. [Google Scholar] [CrossRef]
- Robson, H.; Siebler, T.; Stevens, D.A.; Shalet, S.M.; Williams, G.R. Thyroid hormone acts directly on growth plate chondrocytes to promote hypertrophic differentiation and inhibit clonal expansion and cell proliferation. Endocrinology 2000, 141, 3887–3897. [Google Scholar] [CrossRef]
- Miura, M.; Tanaka, K.; Komatsu, Y.; Suda, M.; Yasoda, A.; Sakuma, Y.; Ozasa, A.; Nakao, K. Thyroid hormones promote chondrocyte differentiation in mouse ATDC5 cells and stimulate endochondral ossification in fetal mouse tibias through iodothyronine deiodinases in the growth plate. J. Bone Miner. Res. 2002, 17, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Himeno, M.; Enomoto, H.; Liu, W.; Ishizeki, K.; Nomura, S.; Kitamura, Y.; Komori, T. Impaired vascular invasion of Cbfa1-deficient cartilage engrafted in the spleen. J. Bone Miner. Res. 2002, 17, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Makihira, S.; Yan, W.; Murakami, H.; Furukawa, M.; Kawai, T.; Nikawa, H.; Yoshida, E.; Hamada, T.; Okada, Y.; Kato, Y. Thyroid hormone enhances aggrecanase-2/ADAM-TS5 expression and proteoglycan degradation in growth plate cartilage. Endocrinology 2003, 144, 2480–2488. [Google Scholar] [CrossRef]
- Burch, W.M.; Lebovitz, H.E. Triiodothyronine stimulates maturation of porcine growth-plate cartilage in vitro. J. Clin. Investig. 1982, 70, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Murata, Y.; Horwitz, A.L.; Philipson, L.; Refetoff, S. Regulation of glycosaminoglycan synthesis by thyroid hormone in vitro. J. Clin. Investig. 1982, 70, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Otsuka, T.; Adachi, S.; Matsushima-Nishiwaki, R.; Natsume, H.; Kozawa, O.; Tokuda, H. (-)-Epigallocatechin gallate inhibits thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Mol. Med. Rep. 2011, 4, 297–300. [Google Scholar]
- Kondo, A.; Otsuka, T.; Kato, K.; Matsushima-Nishiwaki, R.; Kuroyanagi, G.; Mizutani, J.; Tokuda, H.; Kozawa, O. AMP-activated protein kinase regulates thyroid hormone-stimulated osteocalcin synthesis in osteoblasts. Int. J. Mol. Med. 2013, 31, 1457–1462. [Google Scholar] [CrossRef]
- Endo, T.; Kobayashi, T. Excess TSH causes abnormal skeletal development in young mice with hypothyroidism via suppressive effects on the growth plate. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E660–E666. [Google Scholar] [CrossRef]
- Boutin, A.; Eliseeva, E.; Gershengorn, M.C.; Neumann, S. β-Arrestin-1 mediates thyrotropin-enhanced osteoblast differentiation. FASEB J. 2014, 28, 3446–3455. [Google Scholar] [CrossRef]
- Hase, H.; Ando, T.; Eldeiry, L.; Brebene, A.; Peng, Y.; Liu, L.; Amano, H.; Davies, T.F.; Sun, L.; Zaidi, M.; et al. TNFalpha mediates the skeletal effects of thyroid-stimulating hormone. Proc. Natl. Acad. Sci. USA 2006, 103, 12849–12854. [Google Scholar] [CrossRef]
- Kaneki, H.; Guo, R.; Chen, D.; Yao, Z.; Schwarz, E.M.; Zhang, Y.E.; Boyce, B.F.; Xing, L. Tumor Necrosis Factor Promotes Runx2 Degradation through Up-regulation of Smurf1 and Smurf2 in Osteoblasts. J. Biol. Chem. 2006, 281, 4326–4333. [Google Scholar] [CrossRef] [PubMed]
- Steeve, K.T.; Marc, P.; Sandrine, T.; Dominique, H.; Yannick, F. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [CrossRef]
- Sun, L.; Vukicevic, S.; Baliram, R.; Yang, G.; Sendak, R.; McPherson, J.; Zhu, L.-L.; Iqbal, J.; Latif, R.; Natrajan, A.; et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc. Natl. Acd. Sci. USA 2008, 105, 4289–4294. [Google Scholar] [CrossRef] [PubMed]
- Hugues, J.N.; Reinberg, A.; Lagoguey, M.; Modigliani, E.; Sebaoun, J. Biological rhythms of thyrotropin secretion. Ann. Med. Interne 1983, 134, 84–94. [Google Scholar]
- Cooper, M.S.; Rabbitt, E.H.; Goddard, P.E.; Bartlett, W.A.; Hewison, M.; Stewart, P.M. Osteoblastic 11β-Hydroxysteroid Dehydrogenase Type 1 Activity Increases with Age and Glucocorticoid Exposure. J. Bone. Miner. Res. 2002, 17, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.S.; Wan, C.; Liu, Q.; Wang, Y.; Almeida, M.; O’Brien, C.A.; Thostenson, J.; Roberson, P.K.; Boskey, A.L.; Clemens, T.L.; et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 2010, 9, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Zhang, P.; Ji, Z.; Henneicke, H.; Li, J.; Kim, S.; Swarbrick, M.M.; Wu, Y.; Little, C.B.; Seibel, M.J.; et al. Disruption of glucocorticoid signalling in osteoblasts attenuates age-related surgically induced osteoarthritis. Osteoarthr. Cartil. 2019. [Google Scholar] [CrossRef]
- Debono, M.; Ghobadi, C.; Rostami-Hodjegan, A.; Huatan, H.; Campbell, M.J.; Newell-Price, J.; Darzy, K.; Merke, D.P.; Arlt, W.; Ross, R.J. Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. 2009, 94, 1548–1554. [Google Scholar] [CrossRef]
- Weitzman, E.D.; Fukushima, D.; Nogeire, C.; Roffwarg, H.; Gallagher, T.F.; Hellman, L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J. Clin. Endocrinol. Metab. 1971, 33, 14–22. [Google Scholar] [CrossRef]
- Pace, T.W.; Mletzko, T.C.; Alagbe, O.; Musselman, D.L.; Nemeroff, C.B.; Miller, A.H.; Heim, C.M. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry 2006, 163, 1630–1633. [Google Scholar] [CrossRef]




| Cell Line and Species | Melatonin Concentration | Effect of Melatonin with Dose Variation | Ref. |
|---|---|---|---|
| Primary cultured chondrocyte (rat) | 10−3 M, 10−6 M and 10−9 M | 10−3 M: Increased cytotoxic effect; high concentration failed to recover Col2a1 10−6–10−9 M: Inhibition of cell death, recovered cell surface area, and increased Col2a1 expression via MMP-13 inhibition. | [64] |
| HepG2 (human) | 10−3–10−4 M 10−6–10−8 M | 10−3–10−4 M: Increased pro-oxidant activity, increased ROS level after 96 h 10−6–10−8 M: Decreased cell viability, showed antioxidant action at 24 h | [61] |
| Jurkat T cell, (human) | (0.1–1) × 10−3 M | Increased ROS, fas-induced apoptosis occurred by decreasing antioxidant activity | [55] |
| MOLT-4, CMK, (human) | 10−3 M | Increased cytotoxicity and ROS production | [65] |
| B6D2F1 (mouse) | 10−9 and 10−6 M | 10-6 M: increased ROS level and GSH level decreased compared with 10−9 M in oocytes. | [66] |
| U937 (human) | 10−3 M | Increased ROS production and ameliorated GSH level | [67] |
| U937 (human) | 10−3 M | NF-κB activation, ROS generation and apoptosis | [68] |
| Mouse 2-cell embryo (mouse) | 10−9 M, 10−3 M | 10−3 M: Possibility of cell injury and lower rate of blastocyst 10−9 M: Improved at maximum blastocyst rate and hatching blastocyst rate | [69] |
| HT22 and BV2 (mouse) | 100 × 10−6 M | Reduced the elevated ROS and oxidative stress, reduced p38 MAPK Prevent apoptosis through the suppression of activated caspase-3 | [70] |
| A-431, CCD- 1079Sk (human) | (0.03–0.125) × 10−3 M (0.125–5) × 10−3 M | (0.03–0.125) × 10−3 M: Increased cell proliferation, decreased ROS production (0.125–5) × 10−3 M: Leads to increase ROS, DNA damage, apoptosis, and decreased cell viability | [71] |
| Route of Administration and Animals | Dose of Melatonin | Effects | Ref. |
|---|---|---|---|
| Subcutaneous injection (rat) | 10 mg/kg | Increased Col2a1 level through MMP-13 inhibition, suppressed pro-inflammatory cytokines, and catalytic transcription factors were found in OA knee. | [64] |
| Oral administration (mouse) | 10 mg/kg | Prevented cytotoxicity, and increased serum SOD and glutathione (GSH) levels. | [82] |
| Intraperitoneal injection (rat) | 20 mg/kg | Decreased apoptosis, repressed IL-1β and TNF-α in the spinal dorsal horn; anti-nociceptive effect. | [83] |
| Intraperitoneal injection (rat) | 20 mg/kg | Reduced ROS and oxidative stress, activated antioxidant mechanism, and inhibited neuroinflammation by reducing NF-κB in mouse embryos. | [69] |
| Intravenous injection (mouse) | 5, 10, or 20 mg/kg | Anti-inflammatory action through activating PPAR-γ; inhibited TNF-α, IL-1, and IL-6 production, and 20 mg/kg was more effective for reduction. | [84] |
| Subcutaneous injection (rat) | 10 mg/kg | Increased SOD activity, decreased nitrite levels in the liver. | [75] |
| Subcutaneous injection (Syrian hamster) | 500 µg/kg | Decreased percent of damaged cells, increased CuZn-SOD and Mn-SOD in the Harderian gland. | [76] |
| Intraperitoneal injection (rat) | 5 mg/kg | Increased SOD activity and glutathione reductase in kidney, liver, and brain tissue. | [77] |
| Intragastric administration (mouse) | 0.1, 1, or 10 mg/kg | Ameliorated SOD and CuZn-SOD in brain tissue. | [79] |
| Intraperitoneal injection (rat) | 10 mg/kg | Prevented oxidative mitochondrial damage by the activation of glutathione peroxidase (GSH-Px) in brain tissue. | [80] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, F.M.; Hong, Y.; Jin, Y.; Choi, J.; Hong, Y. Physiological and Pathological Role of Circadian Hormones in Osteoarthritis: Dose-Dependent or Time-Dependent? J. Clin. Med. 2019, 8, 1415. https://doi.org/10.3390/jcm8091415
Hossain FM, Hong Y, Jin Y, Choi J, Hong Y. Physiological and Pathological Role of Circadian Hormones in Osteoarthritis: Dose-Dependent or Time-Dependent? Journal of Clinical Medicine. 2019; 8(9):1415. https://doi.org/10.3390/jcm8091415
Chicago/Turabian StyleHossain, Farhad Md., Yunkyung Hong, Yunho Jin, Jeonghyun Choi, and Yonggeun Hong. 2019. "Physiological and Pathological Role of Circadian Hormones in Osteoarthritis: Dose-Dependent or Time-Dependent?" Journal of Clinical Medicine 8, no. 9: 1415. https://doi.org/10.3390/jcm8091415
APA StyleHossain, F. M., Hong, Y., Jin, Y., Choi, J., & Hong, Y. (2019). Physiological and Pathological Role of Circadian Hormones in Osteoarthritis: Dose-Dependent or Time-Dependent? Journal of Clinical Medicine, 8(9), 1415. https://doi.org/10.3390/jcm8091415