Periprosthetic Joint Infection Caused by Gram-Positive Versus Gram-Negative Bacteria: Lipopolysaccharide, but not Lipoteichoic Acid, Exerts Adverse Osteoclast-Mediated Effects on the Bone
Abstract
:1. Introduction
2. Patients and Methods
2.1. Experimental Animal Studies
2.2. Ibudilast Treatment
2.3. Serum Osteocalcin Assay
2.4. Micro-Computed Tomography Bone Imaging
2.5. Histochemistry and Immunofluorescence Staining
2.6. Cell Culture and Osteoclast Differentiation
2.7. Statistical Analysis
3. Results
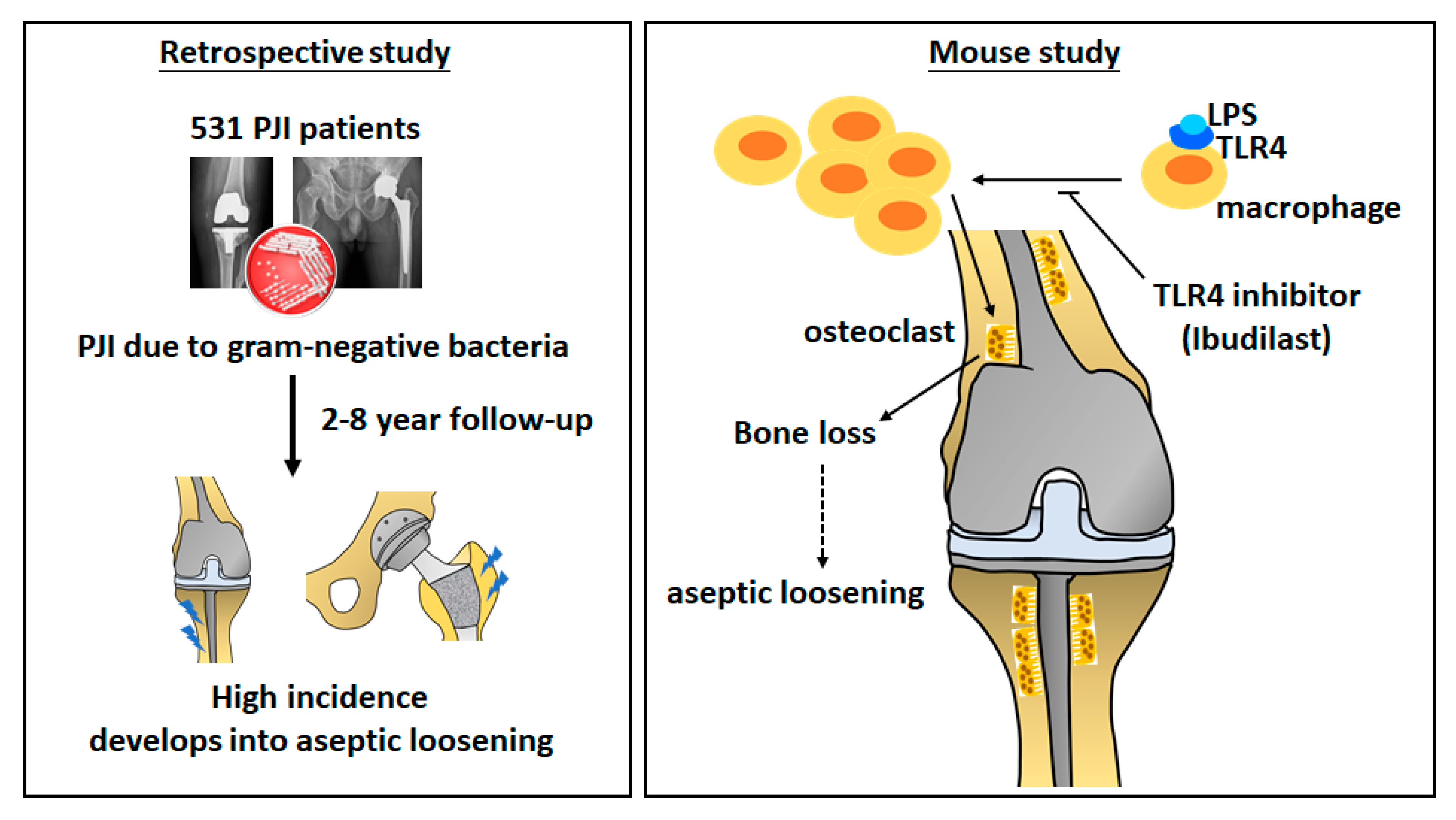
3.1. PJI Caused by Gram-Negative Bacteria Increases the Risk of Aseptic Loosening
3.2. Intrafemoral Injection of LPS, but not LTA, Results in a Decreased Number of Trabeculae and a Lower Bone Density
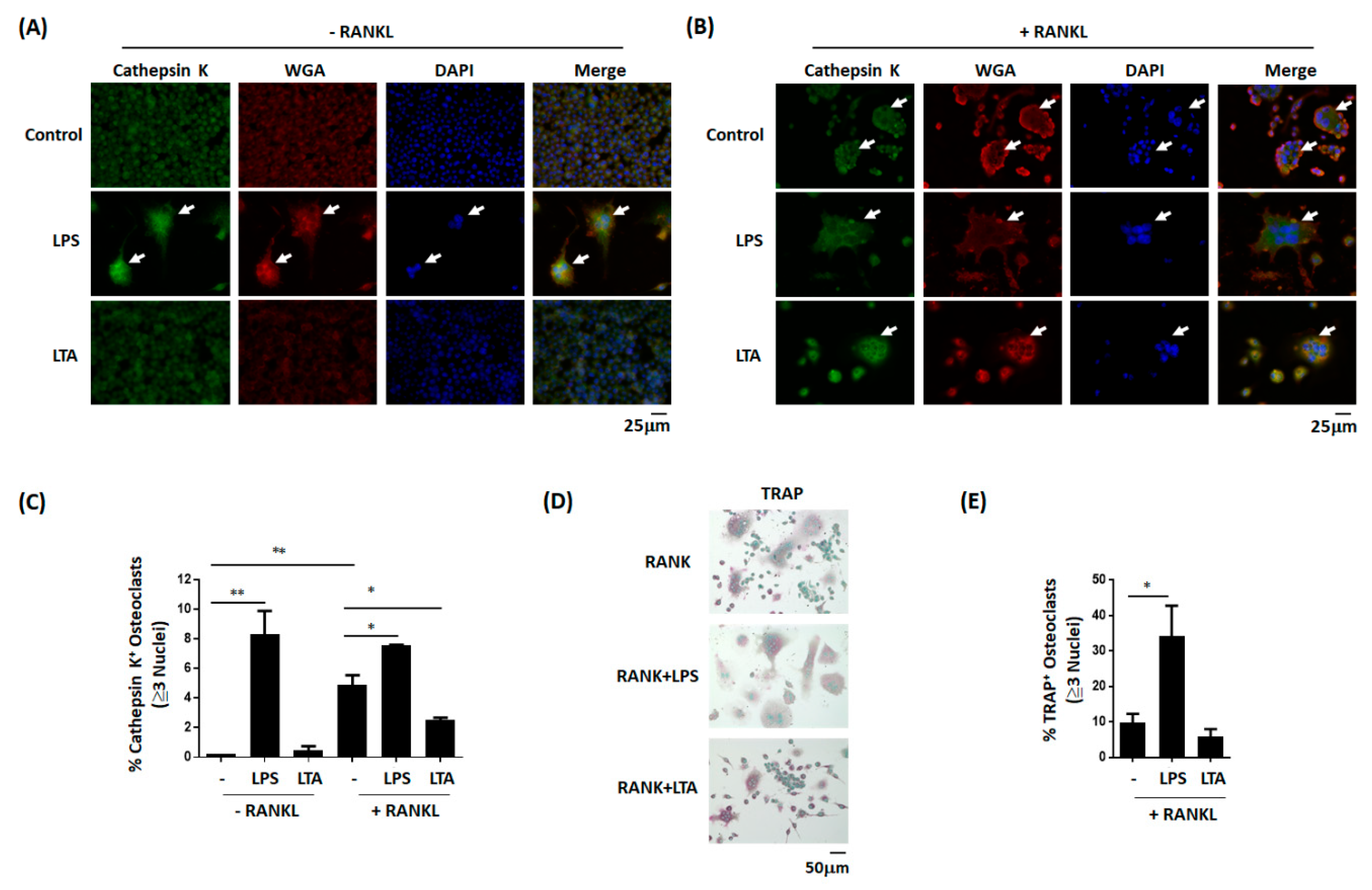
3.3. LPS, But Not LTA, Increases the Number of Osteoclasts
3.4. Intrafemoral Injection of LPS in Mice Decreases the Body Weight and Increases Serum Osteocalcin Concentrations
3.5. LPS, but not LTA, Promotes the Differentiation of Monocytes into Osteoclast-Like Cells
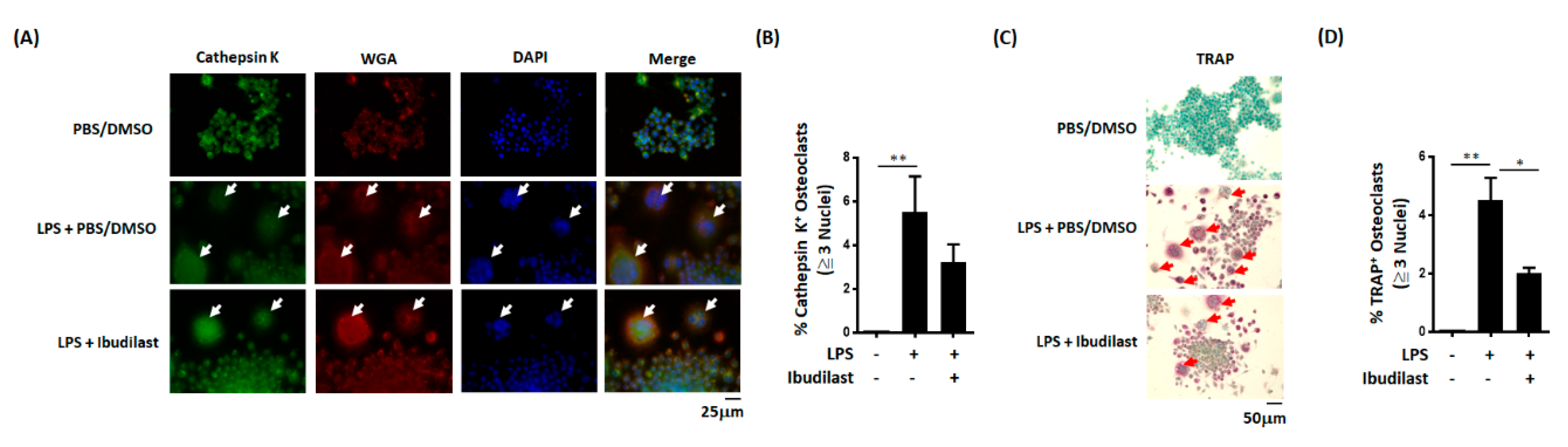
3.6. In Vitro Effects of Ibudilast on LPS-Induced Osteoclast Differentiation
3.7. Ibudilast Attenuates LPS-Induced Femoral Bone Loss in Mice
3.8. Ibudilast Attenuates LPS-Induced Bone Loosening and Reduces the Number of Osteoclasts In Vivo
4. Discussion
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| PJI | periprosthetic joint infection |
| GP | Gram-positive |
| GN | Gram-negative |
| LPS | lipopolysaccharide |
| LTA | lipoteichoic acid |
| TLR | toll-like receptor |
| BMD | bone mineral density |
| TRAP | tartrate-resistant acid phosphatase |
| FBS | fetal bovine serum |
| RANKL | receptor activator of nuclear factor kappa-Β ligand |
| WGA | wheat germ agglutinin |
| BS | bone surface |
| BV | bone volume |
| TV | tissue volume |
| Tb.N | trabecular number |
| Tb.Th | trabecular thickness |
| Tb.Sp | trabecular spacing |
References
- Hooper, G. The challenge of the increasing demand for joint replacement. N. Z. Med. J. 2016, 129, 8–9. [Google Scholar] [PubMed]
- Lombardi, A.V.; Berend, K.R.; Adams, J.B. Why knee replacements fail in 2013: Patient, surgeon, or implant? Bone Joint J. 2014, 96-B (11 Supple A), 101–104. [Google Scholar] [CrossRef] [PubMed]
- Weston, J.T.; Watts, C.D.; Mabry, T.M.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Irrigation and debridement with chronic antibiotic suppression for the management of infected total knee arthroplasty: A Contemporary Analysis. Bone Joint J. 2018, 100-B, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Gwam, C.U.; Mistry, J.B.; Mohamed, N.S.; Thomas, M.; Bigart, K.C.; Mont, M.A.; Delanois, R.E. Current Epidemiology of Revision Total Hip Arthroplasty in the United States: National Inpatient Sample 2009 to 2013. J. Arthroplast. 2017, 32, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.K.; Elbuluk, A.; Dundon, J.; Herrero, C.; Hernandez, C.; Vigdorchik, J.M.; Schwarzkopf, R.; Iorio, R.; Long, W.J. Surgical approach significantly affects the complication rates associated with total hip arthroplasty. Bone Joint J. 2019, 101-B, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Parmar, D.; Ibrahim, M.S.; Kayani, B.; Haddad, F.S. Outcomes of repeat two-stage exchange hip arthroplasty for prosthetic joint infection. Bone Joint J. 2019, 101-B (6 Supple B), 110–115. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.J.; Ting, J.; Simpson, A.; Gaston, P. Outcomes following debridement, antibiotics and implant retention in the management of periprosthetic infections of the hip: A review of cohort studies. Bone Joint J. 2017, 99-B, 1458–1466. [Google Scholar] [CrossRef]
- Hexter, A.T.; Hislop, S.M.; Blunn, G.W.; Liddle, A.D. The effect of bearing surface on risk of periprosthetic joint infection in total hip arthroplasty: A systematic review and meta-analysis. Bone Joint J. 2018, 100-B, 134–142. [Google Scholar] [CrossRef]
- Suarez, J.; Griffin, W.; Springer, B.; Fehring, T.; Mason, J.B.; Odum, S. Why do revision knee arthroplasties fail? J. Arthroplast. 2008, 23 (6 Suppl. 1), 99–103. [Google Scholar] [CrossRef]
- Brown, T.S.; Fehring, K.A.; Ollivier, M.; Mabry, T.M.; Hanssen, A.D.; Abdel, M.P. Repeat two-stage exchange arthroplasty for prosthetic hip re-infection. Bone Joint J. 2018, 100-B, 1157–1161. [Google Scholar] [CrossRef]
- Chalmers, B.P.; Weston, J.T.; Osmon, D.R.; Hanssen, A.D.; Berry, D.J.; Abdel, M.P. Prior hip or knee prosthetic joint infection in another joint increases risk three-fold of prosthetic joint infection after primary total knee arthroplasty: A matched control study. Bone Joint J. 2019, 101-B (7 Supple C), 91–97. [Google Scholar] [CrossRef]
- Drago, L.; Clerici, P.; Morelli, I.; Ashok, J.; Benzakour, T.; Bozhkova, S.; Alizadeh, C.; Del Sel, H.; Sharma, H.K.; Peel, T.; et al. The World Association against Infection in Orthopaedics and Trauma (WAIOT) procedures for Microbiological Sampling and Processing for Periprosthetic Joint Infections (PJIs) and other Implant-Related Infections. J. Clin. Med. 2019, 8, 933. [Google Scholar] [CrossRef]
- Fei, Y.; Wang, W.; Kwiecinski, J.; Josefsson, E.; Pullerits, R.; Jonsson, I.M.; Magnusson, M.; Jin, T. The combination of a tumor necrosis factor inhibitor and antibiotic alleviates staphylococcal arthritis and sepsis in mice. J. Infect. Dis. 2011, 204, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zhu, X.; Kwiecinski, J.; Gjertsson, I.; Lindholm, C.; Iwakura, Y.; Wang, X.; Lycke, N.; Josefsson, E.; Pullerits, R.; et al. Antibiotic-killed Staphylococcus aureus induces destructive arthritis in mice. Arthritis Rheumatol. 2015, 67, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Rochford, E.T.; Sabate Bresco, M.; Zeiter, S.; Kluge, K.; Poulsson, A.; Ziegler, M.; Richards, R.G.; O’Mahony, L.; Moriarty, T.F. Monitoring immune responses in a mouse model of fracture fixation with and without Staphylococcus aureus osteomyelitis. Bone 2016, 83, 82–92. [Google Scholar] [CrossRef]
- Dapunt, U.; Radzuweit-Mihaljevic, S.; Lehner, B.; Haensch, G.M.; Ewerbeck, V. Bacterial Infection and Implant Loosening in Hip and Knee Arthroplasty: Evaluation of 209 Cases. Materials 2016, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, L.A.; Anderson, J.R.; Lee, Z.; Goldberg, V.M.; Greenfield, E.M. Adherent lipopolysaccharide inhibits the osseointegration of orthopedic implants by impairing osteoblast differentiation. Bone 2013, 52, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandow, K.; Maeda, A.; Kakimoto, K.; Kusuyama, J.; Shamoto, M.; Ohnishi, T.; Matsuguchi, T. Molecular mechanisms of the inhibitory effect of lipopolysaccharide (LPS) on osteoblast differentiation. Biochem. Biophys. Res. Commun. 2010, 402, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yuan, L.; Wang, J.G.; Wang, F.; Yang, X.K.; Zhang, F.H.; Song, J.L.; Ma, X.Y.; Cheng, Q.; Song, G.H. Lipopolysaccharide (LPS) induces the apoptosis and inhibits osteoblast differentiation through JNK pathway in MC3T3-E1 cells. Inflammation 2014, 37, 621–631. [Google Scholar] [CrossRef]
- Huang, R.L.; Yuan, Y.; Zou, G.M.; Liu, G.; Tu, J.; Li, Q. LPS-stimulated inflammatory environment inhibits BMP-2-induced osteoblastic differentiation through crosstalk between TLR4/MyD88/NF-kappaB and BMP/Smad signaling. Stem Cells Dev. 2014, 23, 277–289. [Google Scholar] [CrossRef]
- Xiao, L.; Zhou, Y.; Zhu, L.; Yang, S.; Huang, R.; Shi, W.; Peng, B.; Xiao, Y. SPHK1-S1PR1-RANKL Axis Regulates the Interactions Between Macrophages and BMSCs in Inflammatory Bone Loss. J. Bone Miner. Res. 2018, 33, 1090–1104. [Google Scholar] [CrossRef]
- Yang, J.; Park, O.J.; Kim, J.; Baik, J.E.; Yun, C.H.; Han, S.H. Lipoteichoic Acid of Enterococcus faecalis Inhibits the Differentiation of Macrophages into Osteoclasts. J. Endod. 2016, 42, 570–574. [Google Scholar] [CrossRef]
- Tomomatsu, N.; Aoki, K.; Alles, N.; Soysa, N.S.; Hussain, A.; Nakachi, H.; Kita, S.; Shimokawa, H.; Ohya, K.; Amagasa, T. LPS-induced inhibition of osteogenesis is TNF-alpha dependent in a murine tooth extraction model. J. Bone Miner. Res. 2009, 24, 1770–1781. [Google Scholar] [CrossRef]
- Metzger, C.E.; Gong, S.; Aceves, M.; Bloomfield, S.A.; Hook, M.A. Osteocytes reflect a pro-inflammatory state following spinal cord injury in a rodent model. Bone 2019, 120, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Raynaud-Messina, B.; Verollet, C.; Maridonneau-Parini, I. The osteoclast, a target cell for microorganisms. Bone 2019, 127, 315–323. [Google Scholar] [CrossRef]
- Chen, M.F.; Chang, C.H.; Yang, L.Y.; Hsieh, P.H.; Shih, H.N.; Ueng, S.W.N.; Chang, Y. Synovial fluid interleukin-16, interleukin-18, and CRELD2 as novel biomarkers of prosthetic joint infections. Bone Joint Res. 2019, 8, 179–188. [Google Scholar] [CrossRef]
- Yu, J.; Adapala, N.S.; Doherty, L.; Sanjay, A. Cbl-PI3K interaction regulates Cathepsin K secretion in osteoclasts. Bone 2019, 127, 376–385. [Google Scholar] [CrossRef]
- Hohman, E.E.; Hodges, J.K.; Wastney, M.E.; Lachcik, P.J.; Han, C.Y.; Dwyer, D.; Peacock, M.; Kostenuik, P.J.; Weaver, C.M. Serum calcium concentration is maintained when bone resorption is suppressed by osteoprotegerin in young growing male rats. Bone 2018, 116, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Coffey, C.S.; Conwit, R.; Cudkowicz, M.E.; Gleason, T.; Goodman, A.; Klawiter, E.C.; Matsuda, K.; McGovern, M.; Naismith, R.T.; et al. Phase 2 Trial of Ibudilast in Progressive Multiple Sclerosis. N. Engl. J. Med. 2018, 379, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J. Impact of the economic downturn on total joint replacement demand in the United States: Updated projections to 2021. J. Bone Joint Surg. Am. 2014, 96, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Vail, T.P.; Berry, D.J. The epidemiology of revision total hip arthroplasty in the United States. J. Bone Joint Surg. Am. 2009, 91, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.M.; Molligan, J.; Austin, M.S.; Purtill, J.J.; Hozack, W.J.; Parvizi, J. Failure following revision total knee arthroplasty: Infection is the major cause. Int. Orthop. 2011, 35, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.H.; Sierra, R.J.; Trousdale, R.T.; Lewallen, D.G.; Berry, D.J.; Abdel, M.P. The Lawrence D. Dorr Surgical Techniques & Technologies Award: Why Are Contemporary Revision Total Hip Arthroplasties Failing? An Analysis of 2500 Cases. J. Arthroplast. 2019. [Google Scholar] [CrossRef]
- Akgun, D.; Muller, M.; Perka, C.; Winkler, T. A positive bacterial culture during re-implantation is associated with a poor outcome in two-stage exchange arthroplasty for deep infection. Bone Joint J. 2017, 99-B, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.S.; Twaij, H.; Haddad, F.S. Two-stage revision for the culture-negative infected total hip arthroplasty: A comparative study. Bone Joint J. 2018, 100-B (1 Supple A), 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hipfl, C.; Janz, V.; Lochel, J.; Perka, C.; Wassilew, G.I. Cup-cage reconstruction for severe acetabular bone loss and pelvic discontinuity: Mid-term Results of a Consecutive Series of 35 Cases. Bone Joint J. 2018, 100-B, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Munro, J.T.; Millar, J.S.; Fernandez, J.W.; Walker, C.G.; Howie, D.W.; Shim, V.B. Risk analysis of patients with an osteolytic acetabular defect after total hip arthroplasty using subject-specific finite-element modelling. Bone Joint J. 2018, 100-B, 1455–1462. [Google Scholar] [CrossRef]
- Sabry, F.Y.; Buller, L.; Ahmed, S.; Klika, A.K.; Barsoum, W.K. Preoperative prediction of failure following two-stage revision for knee prosthetic joint infections. J. Arthroplast. 2014, 29, 115–121. [Google Scholar] [CrossRef]
- Ragab, A.A.; Van De Motter, R.; Lavish, S.A.; Goldberg, V.M.; Ninomiya, J.T.; Carlin, C.R.; Greenfield, E.M. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J. Orthop. Res. 1999, 17, 803–809. [Google Scholar] [CrossRef]
- Bi, Y.; Seabold, J.M.; Kaar, S.G.; Ragab, A.A.; Goldberg, V.M.; Anderson, J.M.; Greenfield, E.M. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J. Bone Miner. Res. 2001, 16, 2082–2091. [Google Scholar] [CrossRef]
- Bi, Y.; Collier, T.O.; Goldberg, V.M.; Anderson, J.M.; Greenfield, E.M. Adherent endotoxin mediates biological responses of titanium particles without stimulating their phagocytosis. J. Orthop. Res. 2002, 20, 696–703. [Google Scholar] [CrossRef]
- Tatro, J.M.; Taki, N.; Islam, A.S.; Goldberg, V.M.; Rimnac, C.M.; Doerschuk, C.M.; Stewart, M.C.; Greenfield, E.M. The balance between endotoxin accumulation and clearance during particle-induced osteolysis in murine calvaria. J. Orthop. Res. 2007, 25, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Virdi, A.S.; Sena, K.; Hughes, W.F.; Sumner, D.R. Bone turnover markers correlate with implant fixation in a rat model using LPS-doped particles to induced implant loosening. J. Biomed. Mater. Res. A 2012, 100, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Abu-Amer, Y.; Ross, F.P.; Edwards, J.; Teitelbaum, S.L. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J. Clin. Investig. 1997, 100, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, J.; Park, O.J.; Kang, S.S.; Kim, W.S.; Kurokawa, K.; Yun, C.H.; Kim, H.H.; Lee, B.L.; Han, S.H. Lipoproteins are an important bacterial component responsible for bone destruction through the induction of osteoclast differentiation and activation. J. Bone Miner. Res. 2013, 28, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Muthukuru, M.; Darveau, R.P. TLR signaling that induces weak inflammatory response and SHIP1 enhances osteogenic functions. Bone Res. 2014, 2, 14031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uster, S.; Coelho, F.M.; Aeberli, D.; Stein, J.V.; Hofstetter, W.; Engelhardt, B.; Seitz, M. TNFalpha blockade mediates bone protection in antigen-induced arthritis by reducing osteoclast precursor supply. Bone 2018, 107, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, Y.; Hwang, H.G.; Sung, S.H.; Lee, M.; Son, Y.J. Betulin Suppresses Osteoclast Formation via Down-Regulating NFATc1. J. Clin. Med. 2018, 7, 154. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, W.S.; Byun, J.E.; Choi, J.H.; Yoon, S.R.; Choi, I.; Jung, H. Inhibition of Osteoclastogenesis by Thioredoxin-Interacting Protein-Derived Peptide (TN13). J. Clin. Med. 2019, 8, 431. [Google Scholar] [CrossRef]
- Lin, S.H.; Ho, J.C.; Li, S.C.; Chen, J.F.; Hsiao, C.C.; Lee, C.H. MiR-146a-5p Expression in Peripheral CD14(+) Monocytes from Patients with Psoriatic Arthritis Induces Osteoclast Activation, Bone Resorption, and Correlates with Clinical Response. J. Clin. Med. 2019, 8, 110. [Google Scholar] [CrossRef]



| Bacterial PJI | Reoperation Rate % | Reoperation Rate Due to Aseptic Loosening % |
|---|---|---|
| GP | 25.1% (63/251) | 6.3% (4/63) |
| GN | 23.2% (16/69) | 31.3% (5/16) * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.-F.; Chang, C.-H.; Hu, C.-C.; Wu, Y.-Y.; Chang, Y.; Ueng, S.W.N. Periprosthetic Joint Infection Caused by Gram-Positive Versus Gram-Negative Bacteria: Lipopolysaccharide, but not Lipoteichoic Acid, Exerts Adverse Osteoclast-Mediated Effects on the Bone. J. Clin. Med. 2019, 8, 1289. https://doi.org/10.3390/jcm8091289
Chen M-F, Chang C-H, Hu C-C, Wu Y-Y, Chang Y, Ueng SWN. Periprosthetic Joint Infection Caused by Gram-Positive Versus Gram-Negative Bacteria: Lipopolysaccharide, but not Lipoteichoic Acid, Exerts Adverse Osteoclast-Mediated Effects on the Bone. Journal of Clinical Medicine. 2019; 8(9):1289. https://doi.org/10.3390/jcm8091289
Chicago/Turabian StyleChen, Mei-Feng, Chih-Hsiang Chang, Chih-Chien Hu, Ying-Yu Wu, Yuhan Chang, and Steve W. N. Ueng. 2019. "Periprosthetic Joint Infection Caused by Gram-Positive Versus Gram-Negative Bacteria: Lipopolysaccharide, but not Lipoteichoic Acid, Exerts Adverse Osteoclast-Mediated Effects on the Bone" Journal of Clinical Medicine 8, no. 9: 1289. https://doi.org/10.3390/jcm8091289
APA StyleChen, M.-F., Chang, C.-H., Hu, C.-C., Wu, Y.-Y., Chang, Y., & Ueng, S. W. N. (2019). Periprosthetic Joint Infection Caused by Gram-Positive Versus Gram-Negative Bacteria: Lipopolysaccharide, but not Lipoteichoic Acid, Exerts Adverse Osteoclast-Mediated Effects on the Bone. Journal of Clinical Medicine, 8(9), 1289. https://doi.org/10.3390/jcm8091289




