Low Serum Paraoxonase-1 Activity Associates with Incident Cardiovascular Disease Risk in Subjects with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein
Abstract
1. Introduction
2. Experimental Section
2.1. Study Population
2.2. Clinical Parameters and Biomarkers
2.3. Outcomes
2.4. Data Analysis
3. Results
3.1. Study Population
3.2. Correlation of PON1 Activity with Blood Biomarker Levels
3.3. PON1 Activity as a Marker of CVD Risk in LR, HR1, and HR2 Populations
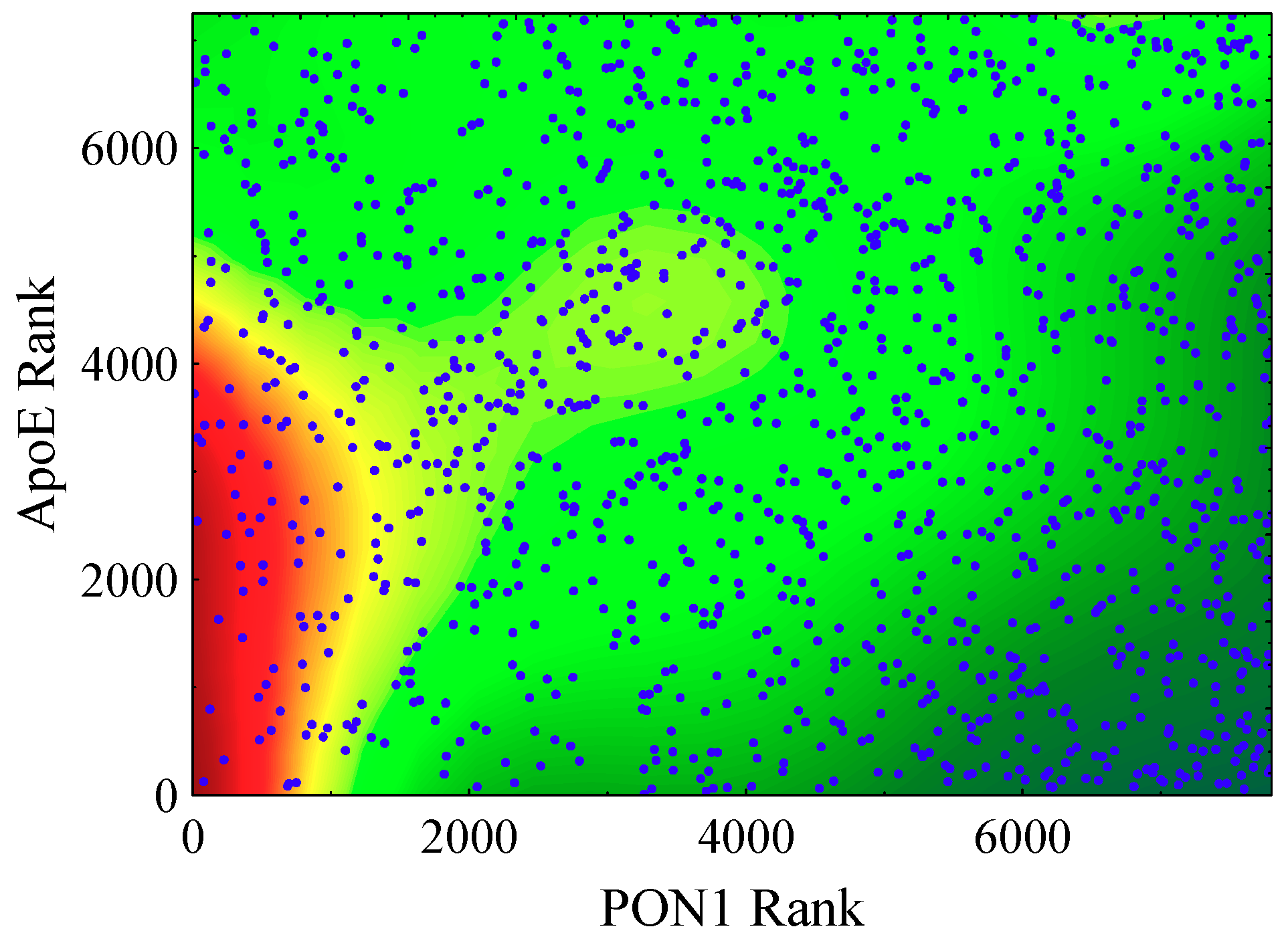
3.4. HDL Particle Constituents in Addition to PON1 as Markers of CVD Risk in HR2
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reddy, S.T.; Devarajan, A.; Bourquard, N.; Shih, D.; Fogelman, A.M. Is it just paraoxonase 1 or are other members of the paraoxonase gene family implicated in atherosclerosis? Curr. Opin. Lipidol. 2008, 19, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Orekhov, A.N.; Bobryshev, Y.V. Paraoxonase and atherosclerosis-related cardiovascular diseases. Biochimie 2017, 132, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1); Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Manolescu, B.N.; Busu, C.; Badita, D.; Stanculescu, R.; Berteanu, M. Paraoxoase 1–an update of the antioxidant properties of high-density lipoproteins. Medica J. Clin. Med. 2015, 10, 173–177. [Google Scholar]
- Kowalska, K.; Socha, E.; Milerowicz, H. The role of paraoxonase in cardiovascular disease. Ann. Clin. Lab. Sci. 2015, 45, 226–233. [Google Scholar] [PubMed]
- Shunmoogam, N.; Naidoo, P.; Chilton, R. Paraoxonase (PON1): A brief overview on genetics, structure, polymorphisms and clinical relevance. Vasc. Health Risk Manag. 2018, 14, 137–143. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Bisgaier, C.L.; Newton, R.S.; Primo-Parmo, S.L.; La Du, B.N. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. J. Clin. Investig. 1998, 101, 1581–1590. [Google Scholar] [CrossRef]
- Ahmed, Z.; Ravandi, A.; Maguire, G.F.; Emili, A.; Draganow, D.; La Du, B.N.; Kuksis, A.; Connelly, P.W. Apolipoprotein A-I promotes the formation of phosphatidylcholine core aldehydes that are hydrolyzed by paraoxonase (PON-1) during high density lipoprotein oxidation with a peroxynitrite donor. J. Biol. Chem. 2001, 276, 24473–24481. [Google Scholar] [CrossRef]
- Mackness, M.; Durrington, P.; Mackness, B. Paraoxonase 1 activity, concentration and genotype in cardiovascular disease. Curr. Opin. Lipidol. 2004, 15, 399–404. [Google Scholar] [CrossRef]
- Mastorikou, M.; Mackness, M.; Mackness, B. Defective metabolism of oxidized phospholipid by HDL from people with type 2 diabetes. Diabetes 2006, 55, 3099–3103. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Ma, Y.; Fang, Y.; Liu, L.; Wu, S.; Fu, D.; Wang, X. Association between PON1 activity and coronary heart disease risk: A meta-analysis based on 43 studies. Mol. Genet. Metab. 2012, 105, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lang, X.; Cui, S.; Zou, L.; Cao, J.; Wang, S.; Wu, X. Quantitative assessment of the influence of paraoxonase activity and coronary heart disease risk. DNA Cell Biol. 2012, 31, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: The PREVEND study and meta-analysis of prospective population studies. Atherosclerosis 2016, 245, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Goswami, B.; Tayal, D.; Gupta, N.; Mallika, V. Paraoxonase: A multifaceted biomolecule. Clin. Chem. Acta 2009, 410, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Annema, W.; von Eckardstein, A. Dysfunctional high-density lipoproteins in coronary heart disease: Implications for diagnostics and therapy. Transl. Res. 2016, 173, 30–57. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell Biochem. 2018, 440, 167–187. [Google Scholar] [CrossRef]
- Chang, T.I.; Streja, E.; Moradi, H. Could high-density lipoprotein cholesterol predict increased cardiovascular risk? Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 140–147. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Ryan, D.; Rainwater, D.L.; Moss, A.J.; Zareba, W.; Sparks, C.E. Cholesteryl Ester Transfer Protein Polymorphism (TaqIB) Associates with Risk in Postinfarction Patients with High C-Reactive Protein and High-Density Lipoprotein Cholesterol Levels. Atheroscler. Thromb. Vasc. Biol. 2010, 30, 1657–1664. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Gansevoort, R.T.; Sparks, C.E.; Dullaart, R.P.F. Inflammation reduces HDL protection against primary cardiac risk. Eur. J. Clin. Investig. 2010, 40, 483–489. [Google Scholar] [CrossRef]
- Corsetti, J.P.; Gansevoort, R.T.; Bakker, S.J.L.; Navis, G.J.; Sparks, C.E.; Dullaart, R.P.F. Apolipoprotein E Predicts Incident Cardiovascular Disease Risk in Women but not in Men with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein. Metab. Clin. Exp. 2012, 61, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, J.P.; Bakker, S.J.L.; Sparks, C.E.; Dullaart, R.P.F. Apolipoprotein A-II Influences Apolipoprotein E-Linked Cardiovascular Disease Risk in Women with High Levels of HDL Cholesterol and C-Reactive Protein. PLoS ONE 2012, 7, e39110. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, J.P.; Gansevoort, R.T.; Navis, G.J.; Sparks, C.E.; Dullaart, R.P.F. LPL Polymorphism (D9N) Predicts Cardiovascular Disease Risk Directly and Through Interaction with CETP Polymorphism (TaqIB) in Women with High HDL Cholesterol and CRP. Atherosclerosis 2011, 214, 373–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corsetti, J.P.; Ryan, D.; Moss, A.J.; McCarthy, J.J.; Goldenberg, I.; Zareba, W.; Sparks, C.E. Thrombospondin-4 Polymorphism (A387P) Predicts Cardiovascular Risk in Postinfarction Patients with High HDL Cholesterol and C-Reactive Protein Levels. Thromb. Haemost. 2011, 106, 1170–1178. [Google Scholar] [PubMed]
- Le, N.T.; Corsetti, J.P.; Dehoff-Sparks, J.L.; Sparks, C.E.; Fujiwara, K.; Abe, J.I. Reactive Oxygen Species (ROS), SUMOylation, and Endothelial Inflammation. Int. J. Inflamm. 2012, 2012, 678190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corsetti, J.P.; Salzman, P.; Ryan, D.; Moss, A.J.; Zareba, W.; Sparks, C.E. Plasminogen Activator Inhibitor-2 Polymorphism Associates with Recurrent Coronary Event Risk in Patients with High HDL and C-Reactive Protein Levels. PLoS ONE 2013, 8, e68920. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pinto-Sietsma, S.J.; Janssen, W.M.; Hillege, H.L.; Navis, G.; De Zeeuw, D.; De Jong, P.E. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J. Am. Soc. Nephrol. 2000, 11, 1882–1888. [Google Scholar]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F. Apolipoprotein B/A-I and total cholesterol/high-density lipoprotein cholesterol ratios both predict cardiovascular events in the general population independently of nonlipid risk factors, albuminuria and C-reactive protein. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef]
- De Greeff, A.; Reggiori, F.; Shennan, A.H. Clinical assessment of the DINAMAP ProCare monitor in an adult population according to the British Hypertension Society Protocol. Blood Press. Monit. 2007, 12, 51–55. [Google Scholar] [CrossRef]
- Borggreve, S.E.; Hillege, H.L.; Wolffenbuttel, B.H.R.; de Jong, P.E.; Zuurman, M.W.; van der Steege, G.; van Tol, A.; Dullaart, R.P.F.; PREVEND Study Group. An increased coronary risk is paradoxically associated with common cholesteryl ester transfer protein gene variations that relate to higher high-density lipoprotein cholesterol: A population-based study. J. Clin. Endocrinol. Metab. 2006, 91, 3382–3388. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, E.H.; Gruppen, E.G.; James, R.W.; Bakker, S.J.L.; Dullaart, R.P.F. Serum paraoxonase 1 activity is paradoxically maintained in nonalcoholic fatty liver disease despite low HDL cholesterol. J. Lipid Res. 2019, 60, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gaidukov, L.; Viji, R.I.; Yacobson, S.; Rosenblat, M.; Aviram, M.; Tawfik, D.S. ApoE induces serum paraoxonase PON1 activity and stability similar to apoA-I. Biochemistry 2010, 49, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.F.; Kwakernaak, A.J.; Dallinga-Thie, G.M. The positive relationship of serum paraoxonase-1 activity with apolipoprotein E is abrogated in metabolic syndrome. Atherosclerosis 2013, 230, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Dullaart, R.P.F.; Otvos, J.D.; James, R.W. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in Type 2 diabetic and non-diabetic subjects. Clin. Biochem. 2014, 47, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
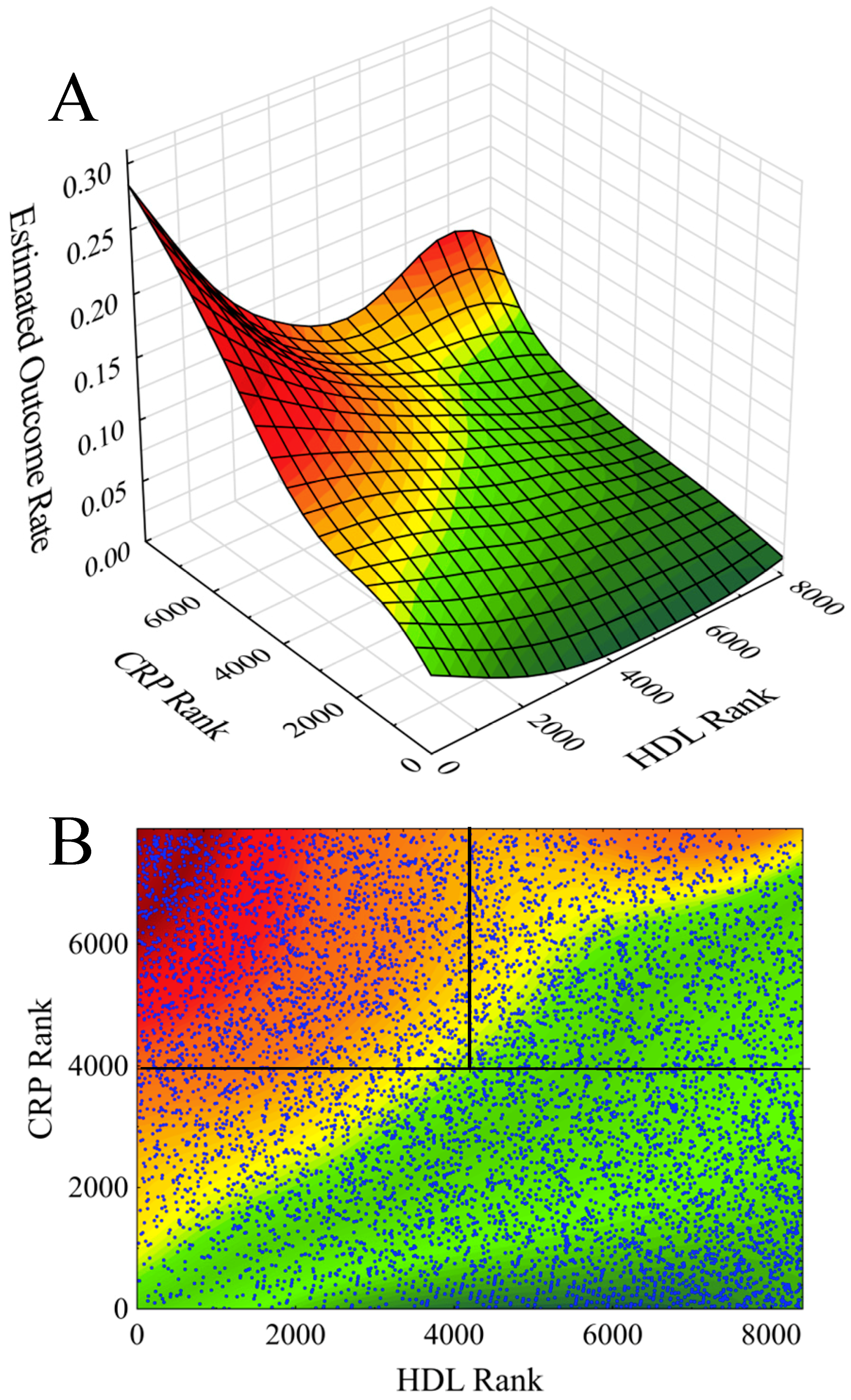
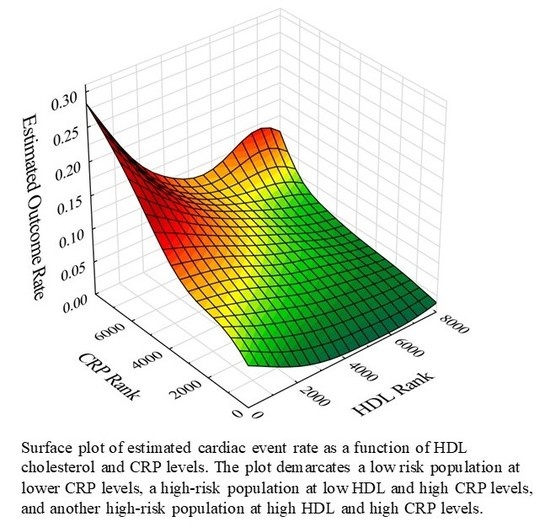
| Parameter | Total Population | Low Risk (86.1% of Subjects) | High-Risk 1 | High-Risk 2 | pa | pb | |||
|---|---|---|---|---|---|---|---|---|---|
| Subjects N (%) | 7766 | 3889 (50.1) | 2294 (29.5) | 1583 (20.4) | |||||
| Outcome events N (%) | 643 (8.3) | 192 (4.9) | 331 (14.4) | 120 (7.6) | <0.001 | ||||
| Age (years) | 49.1 ± 12.6 | 46.2 ± 11.8 | 52.8 ± 12.5 | 50.9 ± 13.2 | <0.001 | ||||
| Females (%) | 50.1 | 49.0 | 36.5 | 72.5 | <0.001 | ||||
| Cardiac history (%) | 4.4 | 2.5 | 8.1 | 3.9 | <0.001 | ||||
| Diabetes (%) | 3.2 | 1.4 | 7.0 | 2.1 | <0.001 | ||||
| Metabolic Syndrome | 24.4 | 13.4 | 51.3 | 9.7 | <0.001 | ||||
| Statins (%) | 4.1 | 3.1 | 6.3 | 3.3 | <0.001 | ||||
| Anti-hypertensives (%) | 13.6 | 8.4 | 20.8 | 15.7 | <0.001 | ||||
| Current Smoker (%) | 33.3 | 27.7 | 42.7 | 33.6 | <0.001 | ||||
| Ethanol Use (>1 drink/day %) | 25.3 | 25.9 | 24.1 | 25.7 | 0.28 | ||||
| Pulse Rate (per minute) | 69.0 | 68.7 | 70.4 | 70.3 | <0.001 | <0.001 | |||
| Systolic BP (mmHg) | 129 ± 20 | 124 ± 18 | 135 ± 21 | 131 ± 22 | <0.001 | <0.001 | |||
| Diastolic BP (mmHg) | 74 ± 10 | 72 ± 9 | 77 ± 10 | 74 ± 10 | <0.001 | <0.001 | |||
| BMI (kg/m2) | 26.1 ± 4.2 | 24.6 ± 3.4 | 28.0 ± 4.3 | 26.2 ± 4.2 | <0.001 | <0.001 | |||
| HDL-C (mM) | 1.32 ± 0.40 | 1.40 ± 0.41 | 1.01 ± 0.18 | 1.62 ± 0.29 | <0.001 | <0.001 | |||
| CRP (mg/L) | 1.28 (0.56–2.98) | 0.54 (0.30–0.82) | 2.76 (1.79–4.62) | 2.52 (1.71–4.16) | <0.001 | <0.001 | |||
| PON1 (U/L) | 53.2 (43.2–65.1) | 53.7 (43.7–65.6) | 50.8 (40.9–62.0) | 56.1 (46.6–68.3) | <0.001 | <0.001 | |||
| ApoA-I (μM) | 47.8 ± 9.9 | 48.0 ± 10.0 | 42.8 ± 6.8 | 54.7 ± 9.3 | <0.001 | <0.001 | |||
| ApoA-II (μM) | 19.6 ± 3.8 | 19.5 ± 3.8 | 18.7 ± 3.2 | 21.4 ± 4.0 | <0.001 | <0.001 | |||
| ApoA-I/HDL-C (μM/mM) | 37.8 ± 8.2 | 35.7 ± 7.7 | 43.2 ± 7.7 | 34.1 ± 5.1 | <0.001 | <0.001 | |||
| ApoA-II/HDL-C (μM/mM) (μmol/mmol) | 15.9 ± 4.6 | 14.8 ± 4.4 | 19.0 ± 4.1 | 13.4 ± 2.7 | <0.001 | <0.001 | |||
| Cholesterol (mM) | 5.65 ± 1.13 | 5.46 ± 1.08 | 5.88 ± 1.18 | 5.75 ± 1.11 | <0.001 | <0.001 | |||
| NonHDL-C (mM) | 4.33 ± 1.21 | 4.05 ± 1.14 | 4.87 ± 1.19 | 4.13 ± 1.13 | <0.001 | <0.001 | |||
| LDL-C (mM) | 3.69 ± 1.05 | 3.51 ± 0.99 | 4.04 ± 1.04 | 3.58 ± 1.05 | <0.001 | <0.001 | |||
| Triglycerides (mM) | 1.16 (0.85–1.68) | 1.00 (0.74–1.40) | 1.59 (1.14–2.27) | 1.09 (0.83–1.42) | <0.001 | <0.001 | |||
| ApoB (g/L) | 1.04 ± 0.30 | 0.96 ± 0.28 | 1.17 ± 0.32 | 1.01 ± 0.27 | <0.001 | <0.001 | |||
| Glucose (mM) | 4.89 ± 1.19 | 4.67 ± 0.79 | 5.24 ± 1.58 | 4.76 ± 0.96 | <0.001 | <0.001 | |||
| Creatinine (μM) | 83.9 ± 19.5 | 83.1 ± 14.3 | 87.8 ± 26.3 | 80.7 ± 14.5 | <0.001 | <0.001 | |||
| UAE (mg/24 h) | 9.5 (6.3–17.8) | 8.4 (6.0–13.6) | 11.8 (7.2–27.1) | 9.4 (6.1–17.4) | <0.001 | <0.001 | |||
| eGFR (mL/min/1.73 m2) | 84.0 ± 15.6 | 86.5 ± 14.8 | 81.3 ± 16.1 | 81.5 ± 15.5 | <0.001 | <0.001 | |||
| ApoE (μM) | 1.15 ± 0.47 | 1.09 ± 0.42 | 1.26 ± 0.57 | 1.14 ± 0.39 | <0.001 | <0.001 | |||
| Paraoxonase/HDL-C (U/mM) | 41.7 (32.3–53.8) | 39.8 (30.7–50.7) | 50.0 (40.5–63.8) | 35.0 (28.6–43.5) | <0.001 | <0.001 | |||
| 9 | 9 | 9 | 9 | ||||||
| Parameter | Total Population | Low Risk | High-Risk 1 | High-Risk 2 |
|---|---|---|---|---|
| HDL-C | 0.20 *** | 0.19 *** | 0.11 *** | 0.15 *** |
| CRP | −0.05 *** | −0.02 | −0.06 ** | 0.02 |
| ApoA-I | 0.19 *** | 0.17 *** | 0.10 *** | 0.15 *** |
| ApoA-II | 0.28 *** | 0.26 *** | 0.23 *** | 0.26 *** |
| ApoA-I/HDL-C | −0.12 *** | −0.11 *** | −0.04 | 0.01 |
| ApoA-II/HDL-C (μmol/mmol) | −0.04 *** | −0.03 | 0.08 *** | 0.13 *** |
| Cholesterol | 0.08 *** | 0.13 *** | 0.09 *** | 0.01 |
| NonHDL-C | 0.01 | 0.05 ** | 0.07 ** | −0.03 |
| LDL-C | 0.00 | 0.04 * | 0.04 | −0.06 * |
| Triglycerides | 0.03 * | 0.03 * | 0.09 *** | 0.13 *** |
| ApoB | 0.00 | 0.03 | 0.05 * | −0.02 |
| Glucose | −0.09 *** | −0.09 *** | −0.05 * | −0.07 ** |
| Creatinine | −0.03 ** | 0.01 | −0.04 | −0.02 |
| UAE | −0.02 | 0.02 | −0.01 | 0.01 |
| eGFR | 0.02 * | −0.01 | 0.05 * | 0.03 |
| ApoE | 0.00 | 0.04 * | 0.04 | −0.07 ** |
| Population | Model Adjustments | HR | 95% CI (86.1% of Subjects) | p-Value |
|---|---|---|---|---|
| LR | unadjusted | 0.86 | 0.75–0.98 | 0.024 |
| LR | gender, age | 0.95 | 0.82–1.09 | 0.46 |
| HR1 | unadjusted | 0.93 | 0.83–1.04 | 0.22 |
| HR1 | gender, age | 1.02 | 0.91–1.14 | 0.72 |
| HR2 | unadjusted | 0.62 | 0.000001 | 0.000001 |
| HR2 | gender, age | 0.72 | 0.59–0.87 | 0.0007 |
| HR2 | gender, age, UAE | 0.70 | 0.58–0.86 | 0.0004 |
| HR2 | gender, age, UAE, apoB | 0.70 | 0.57–0.85 | 0.0003 |
| HR2 | gender, age, UAE, past CVD | 0.71 | 0.58–0.86 | 0.0005 |
| HR2 | gender, age, UAE, DM | 0.71 | 0.58–0.86 | 0.0006 |
| HR2 | gender, age, UAE, statins | 0.69 | 0.57–0.83 | 0.0002 |
| HR2 | gender, age, UAE, anti-hypertensives | 0.68 | 0.56–0.83 | 0.0001 |
| HR2 | gender, age, UAE, SBP | 0.70 | 0.58–0.85 | 0.0004 |
| HR2 | gender, age, UAE, DBP | 0.70 | 0.58–0.85 | 0.0004 |
| HR2 | gender, age, UAE, smoking | 0.70 | 0.58–0.85 | 0.0004 |
| HR2 | gender, age, UAE, ethanol use | 0.69 | 0.57–0.84 | 0.0003 |
| HR2 | gender, age, UAE, eGFR | 0.72 | 0.59–0.87 | 0.0009 |
| HR2 | gender, age, UAE, past CVD, DM, | 0.68 | 0.55–0.83 | 0.0003 |
| apoB, statins, anti-hypertensives | ||||
| smoking, ethanol use, eGFR, SBP, DBP |
| Models without Interaction | Models with Interaction | |||||
|---|---|---|---|---|---|---|
| Parameters | HR | 95% CI | p | HR | 95% CI | p |
| PON1 | 0.67 | 0.54−0.83 | 0.0003 | 0.76 | 0.57−1.01 | 0.059 |
| HDL-C | 1.04 | 0.75−1.45 | 0.82 | 1.01 | 0.72−1.40 | 0.98 |
| interaction | 0.81 | 0.057−1.16 | 0.25 | |||
| PON1 | 0.69 | 0.56−0.86 | 0.0007 | 0.69 | 0.54−0.89 | 0.0036 |
| ApoA-I | 0.76 | 0.58−1.00 | 0.051 | 0.76 | 0.58−1.00 | 0.054 |
| interaction | 0.99 | 0.77−1.29 | 0.96 | |||
| PON1 | 0.70 | 0.57−0.88 | 0.002 | 0.70 | 0.57−0.87 | 0.001 |
| ApoA-II | 0.78 | 0.60−1.02 | 0.070 | 0.79 | 0.61−1.02 | 0.070 |
| interaction | 1.09 | 0.90−1.31 | 0.39 | |||
| PON1 | 0.66 | 0.54−0.82 | 0.0001 | 0.71 | 0.55−0.92 | 0.008 |
| ApoA-I/HDL-C | 0.56 | 0.37−0.85 | 0.0057 | 0.59 | 0.39−0.89 | 0.013 |
| interaction | 1.17 | 0.87−1.57 | 0.30 | |||
| PON1 | 0.68 | 0.55–0.84 | 0.0004 | 0.81 | 0.60−1.08 | 0.15 |
| ApoA-II/HDL-C | 0.63 | 0.40−1.00 | 0.051 | 0.65 | 0.41−1.03 | 0.065 |
| interaction | 1.30 | 0.94−1.80 | 0.12 | |||
| PON1 | 0.66 | 0.53−0.82 | 0.0002 | 0.64 | 0.51−0.80 | 0.0001 |
| ApoE | 0.82 | 0.60−1.12 | 0.21 | 0.85 | 0.62−1.16 | 0.30 |
| interaction | 1.77 | 1.29−2.41 | 0.0003 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsetti, J.P.; Sparks, C.E.; James, R.W.; Bakker, S.J.L.; Dullaart, R.P.F. Low Serum Paraoxonase-1 Activity Associates with Incident Cardiovascular Disease Risk in Subjects with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein. J. Clin. Med. 2019, 8, 1357. https://doi.org/10.3390/jcm8091357
Corsetti JP, Sparks CE, James RW, Bakker SJL, Dullaart RPF. Low Serum Paraoxonase-1 Activity Associates with Incident Cardiovascular Disease Risk in Subjects with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein. Journal of Clinical Medicine. 2019; 8(9):1357. https://doi.org/10.3390/jcm8091357
Chicago/Turabian StyleCorsetti, James P., Charles E. Sparks, Richard W. James, Stephan J. L. Bakker, and Robin P. F. Dullaart. 2019. "Low Serum Paraoxonase-1 Activity Associates with Incident Cardiovascular Disease Risk in Subjects with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein" Journal of Clinical Medicine 8, no. 9: 1357. https://doi.org/10.3390/jcm8091357
APA StyleCorsetti, J. P., Sparks, C. E., James, R. W., Bakker, S. J. L., & Dullaart, R. P. F. (2019). Low Serum Paraoxonase-1 Activity Associates with Incident Cardiovascular Disease Risk in Subjects with Concurrently High Levels of High-Density Lipoprotein Cholesterol and C-Reactive Protein. Journal of Clinical Medicine, 8(9), 1357. https://doi.org/10.3390/jcm8091357