Alpha-1-Antitrypsin Ameliorates Pristane Induced Diffuse Alveolar Hemorrhage in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Splenocyte Stimulation
2.3. Flow Cytometry
2.4. Cytokine Assays
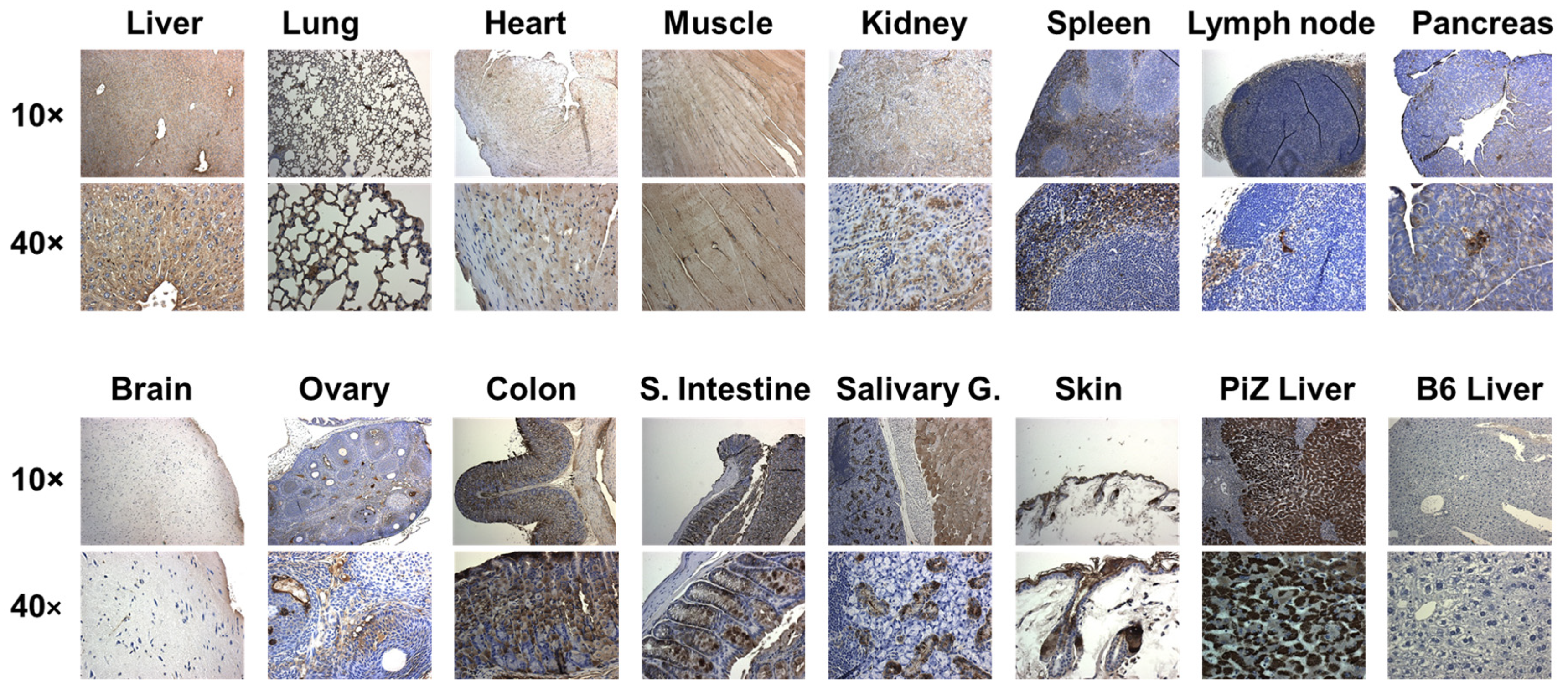
2.5. Detection of hAAT
2.6. DAH and Lung Pathological Evaluation
2.7. Statistical Analysis
3. Results
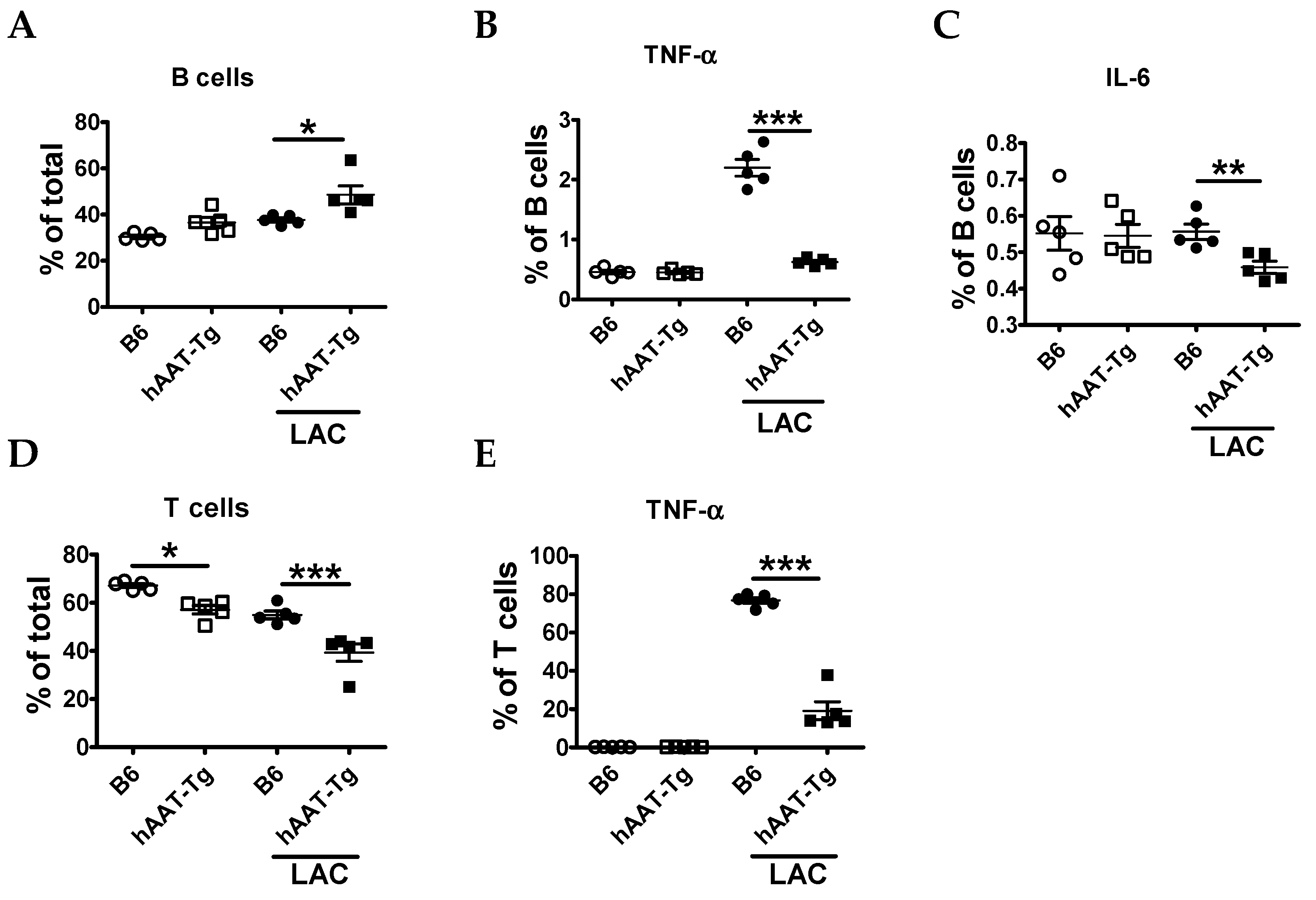
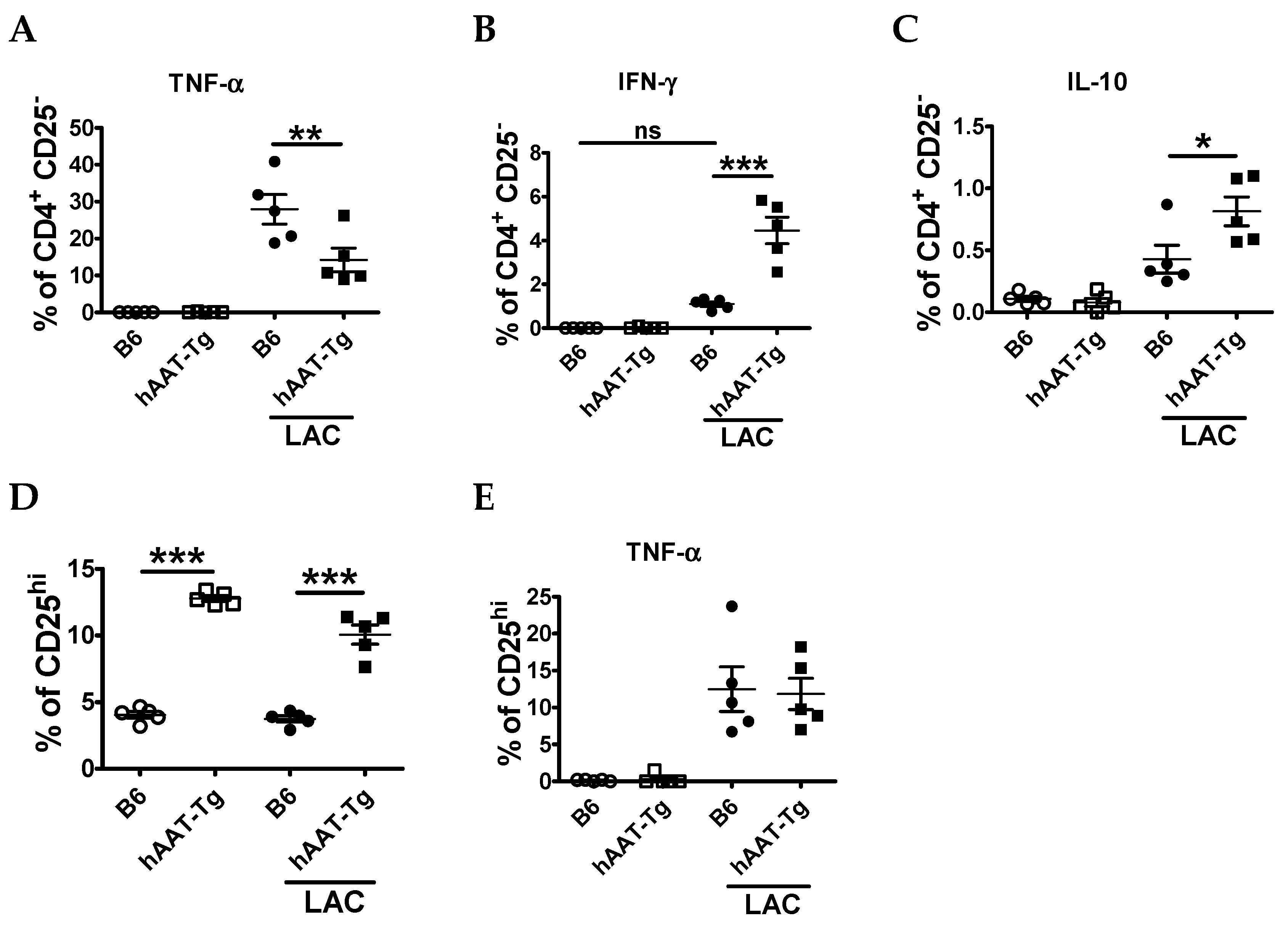
3.1. Splenocytes from hAAT-Tg Mice Are Less Susceptible to Activation
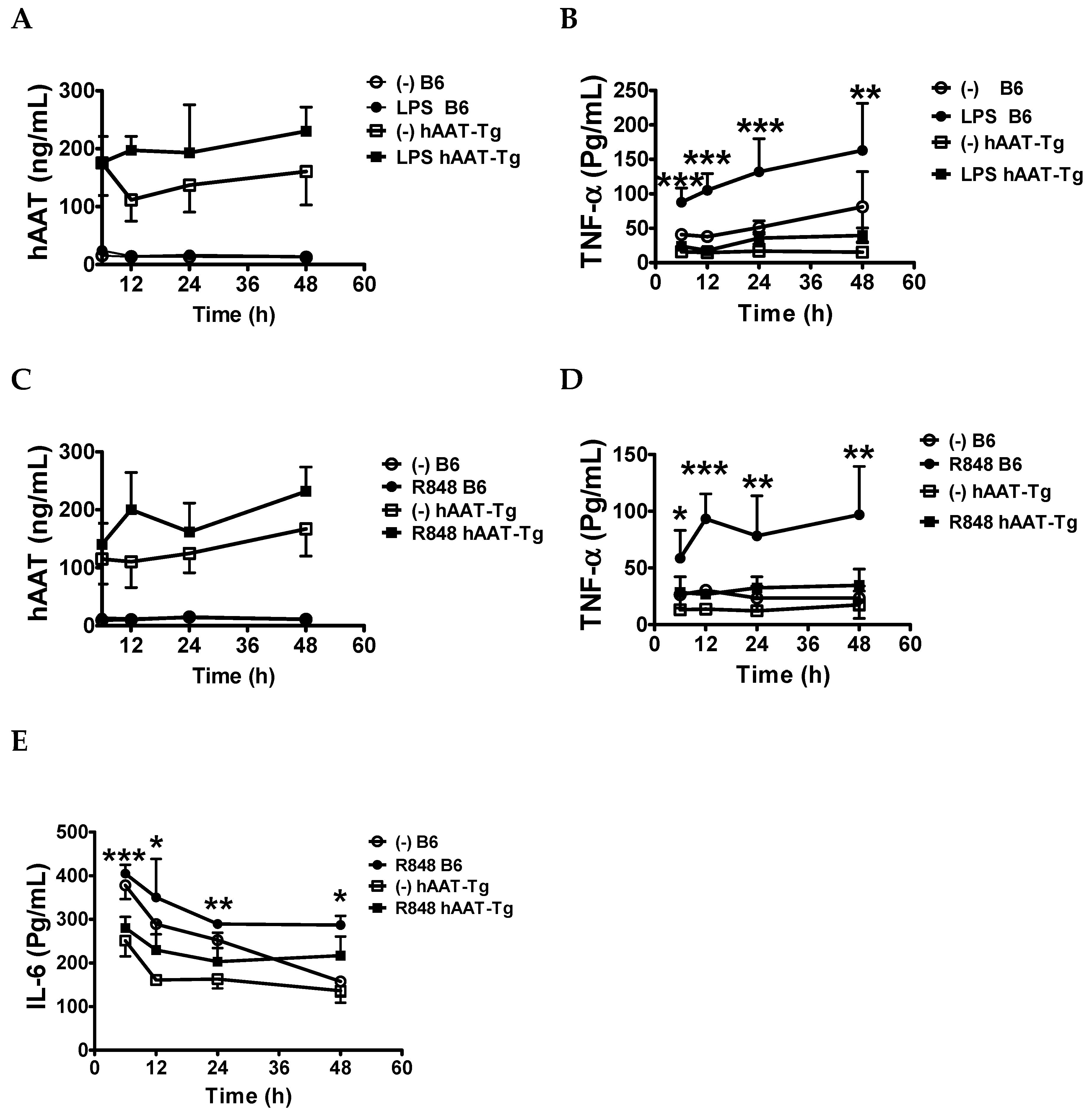
3.2. Transgenic Expression of hAAT Inhibited Splenocyte Activation by TLR4 and TLR7/8agonists
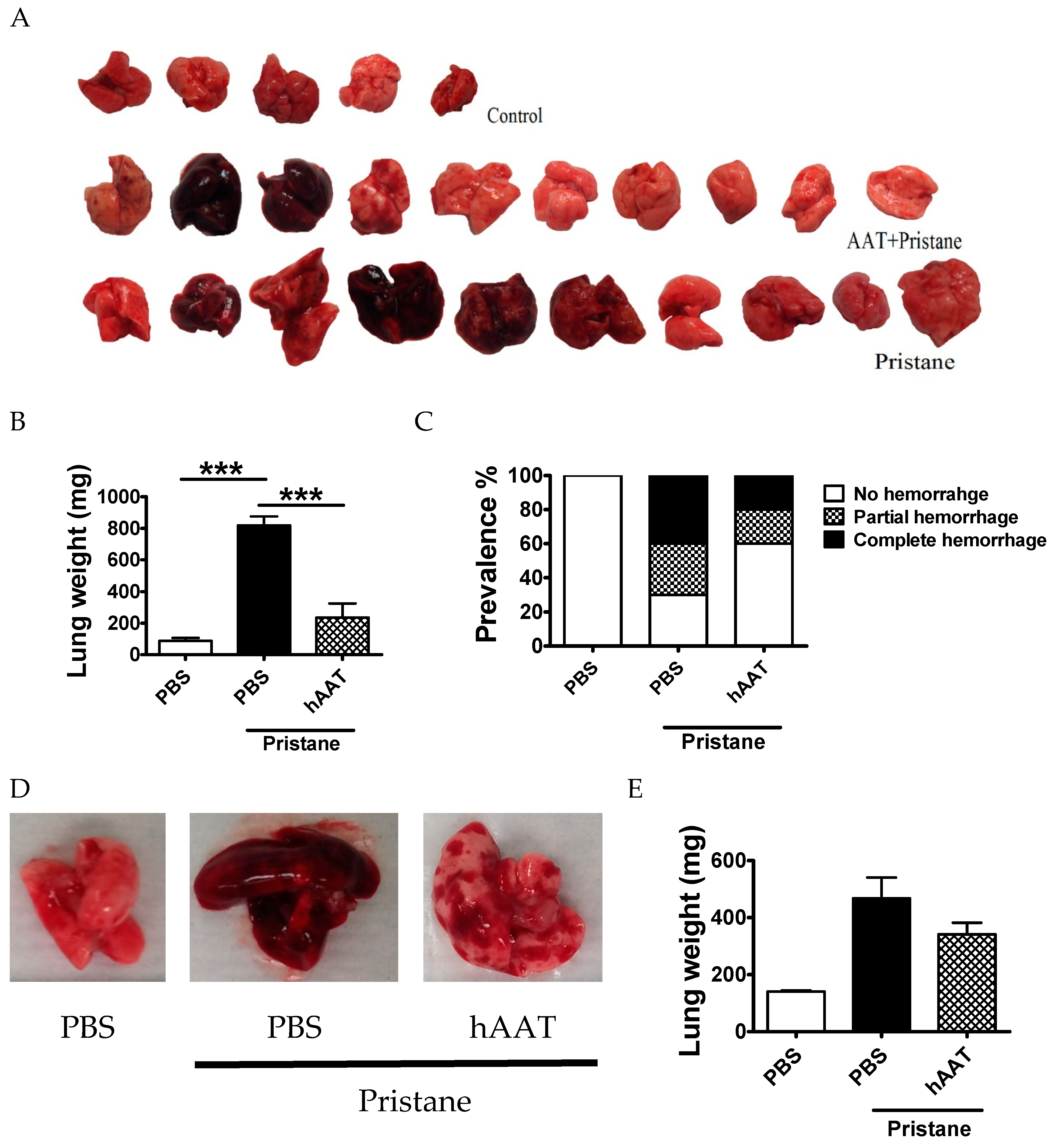
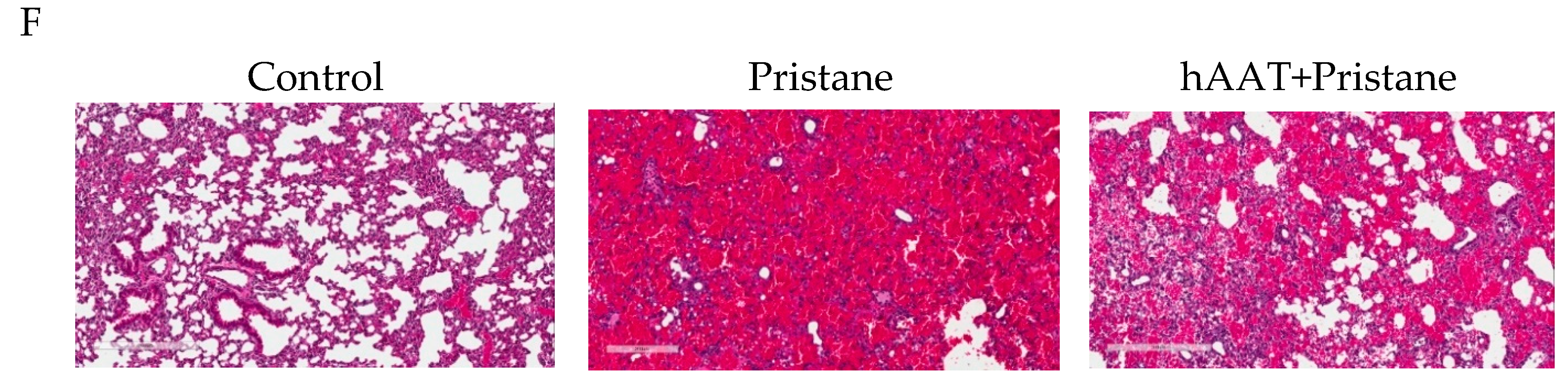
3.3. Transgenic Expression of hAAT Prevents Pristane Induced DAH
3.4. Protective Effect of hAAT Protein Therapy against DAH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, W.; Wu, J.; Yang, H.; Xiong, Y.; Jiang, R.; Cui, T.; Ye, D. Milk fat globule-EGF factor 8 suppresses the aberrant immune response of systemic lupus erythematosus-derived neutrophils and associated tissue damage. Cell Death Differ. 2017, 24, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Han, S.; Lee, P.Y.; Khaybullin, R.; Shumyak, S.; Lu, L.; Chatha, A.; Afaneh, A.; Zhang, Y.; Xie, C.; et al. Pathogenesis of diffuse alveolar hemorrhage in murine lupus. Arthritis Rheumatol. 2017, 69, 1280–1293. [Google Scholar] [CrossRef] [PubMed]
- Barker, T.T.; Lee, P.Y.; Kelly-Scumpia, K.M.; Weinstein, J.S.; Nacionales, D.C.; Kumagai, Y.; Akira, S.; Croker, B.P.; Sobel, E.S.; Reeves, W.H.; et al. Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Lab. Investig. J. Tech. Methods Pathol. 2011, 91, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Kamen, D.L.; Strange, C. Pulmonary manifestations of systemic lupus erythematosus. Clin. Chest Med. 2010, 31, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ocampo, A.S.; Mandell, B.F.; Fessler, B.J. Alveolar hemorrhage in systemic lupus erythematosus: Presentation and management. Chest 2000, 118, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Zamora, M.R.; Warner, M.L.; Tuder, R.; Schwarz, M.I. Diffuse alveolar hemorrhage and systemic lupus erythematosus. Clinical presentation, histology, survival, and outcome. Medicine (Baltimore) 1997, 76, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tsuboi, N.; Furuhashi, K.; Du, Q.; Horinouchi, A.; Maeda, K.; Kosugi, T.; Matsuo, S.; Maruyama, S. Pristane-induced granulocyte recruitment promotes phenotypic conversion of macrophages and protects against diffuse pulmonary hemorrhage in Mac-1 deficiency. J. Immunol. 2014, 193, 5129–5139. [Google Scholar] [CrossRef]
- Jarrot, P.A.; Tellier, E.; Plantureux, L.; Crescence, L.; Robert, S.; Chareyre, C.; Daniel, L.; Secq, V.; Garcia, S.; Dignat-George, F.; et al. Neutrophil extracellular traps are associated with the pathogenesis of diffuse alveolar hemorrhage in murine lupus. J. Autoimmun. 2019, 100, 120–130. [Google Scholar] [CrossRef]
- Myers, J.L.; Katzenstein, A.A. Microangiitis in lupus-induced pulmonary hemorrhage. Am. J. Clin. Pathol. 1986, 85, 552–556. [Google Scholar] [CrossRef]
- Chowdhary, V.R.; Grande, J.P.; Luthra, H.S.; David, C.S. Characterization of haemorrhagic pulmonary capillaritis: Another manifestation of Pristane-induced lupus. Rheumatology (Oxford) 2007, 46, 1405–1410. [Google Scholar] [CrossRef]
- Badsha, H.; Teh, C.L.; Kong, K.O.; Lian, T.Y.; Chng, H.H. Pulmonary hemorrhage in systemic lupus erythematosus. Semin. Arthritis Rheum. 2004, 33, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.J.; Costenbader, K.H. Dyspnoea in a young woman with active systemic lupus erythematosus. Lupus 2009, 18, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Kumagai, Y.; Li, Y.; Takeuchi, O.; Yoshida, H.; Weinstein, J.; Kellner, E.S.; Nacionales, D.; Barker, T.; Kelly-Scumpia, K.; et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J. Exp. Med. 2008, 205, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, S.; Rosal-Vela, A.; Botta, D.; Cumba Garcia, L.M.; Zumaquero, E.; Prados-Maniviesa, V.; Cerezo-Wallis, D.; Lo Buono, N.; Robles-Guirado, J.A.; Guerrero, S.; et al. CD38 promotes pristane-induced chronic inflammation and increases susceptibility to experimental lupus by an apoptosis-driven and TRPM2-dependent mechanism. Sci. Rep. 2018, 8, 3357. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhuang, H.; Shumyak, S.; Wu, J.; Xie, C.; Li, H.; Yang, L.J.; Reeves, W.H. Liver X receptor agonist therapy prevents diffuse alveolar hemorrhage in murine lupus by repolarizing macrophages. Front. Immunol. 2018, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Li, Y.; Kumagai, Y.; Xu, Y.; Weinstein, J.S.; Kellner, E.S.; Nacionales, D.C.; Butfiloski, E.J.; van Rooijen, N.; Akira, S.; et al. Type I interferon modulates monocyte recruitment and maturation in chronic inflammation. Am. J. Pathol. 2009, 175, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Nacionales, D.C.; Kelly, K.M.; Lee, P.Y.; Zhuang, H.; Li, Y.; Weinstein, J.S.; Sobel, E.; Kuroda, Y.; Akaogi, J.; Satoh, M.; et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2,6,10,14-tetramethyl-pentadecane (pristane). Am. J. Pathol. 2006, 168, 1227–1240. [Google Scholar] [CrossRef]
- Lee, P.Y.; Weinstein, J.S.; Nacionales, D.C.; Scumpia, P.O.; Li, Y.; Butfiloski, E.; van Rooijen, N.; Moldawer, L.; Satoh, M.; Reeves, W.H. A novel Type I IFN-producing cell subset in murine lupus. J. Immunol. 2008, 180, 5101–5108. [Google Scholar] [CrossRef]
- Lomas-Neira, J.; Perl, M.; Venet, F.; Chung, C.S.; Ayala, A. The role and source of tumor necrosis factor-alpha in hemorrhage-induced priming for septic lung injury. Shock 2012, 37, 611–620. [Google Scholar] [CrossRef]
- Keatings, V.M.; O’Connor, B.J.; Wright, L.G.; Huston, D.P.; Corrigan, C.J.; Barnes, P.J. Late response to allergen is associated with increased concentrations of tumor necrosis factor-alpha and IL-5 in induced sputum. J. Allergy Clin. Immunol. 1997, 99, 693–698. [Google Scholar] [CrossRef]
- Keatings, V.M.; Collins, P.D.; Scott, D.M.; Barnes, P.J. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am. J. Respir. Crit. Care Med. 1996, 153, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Vuillemenot, B.R.; Rodriguez, J.F.; Hoyle, G.W. Lymphoid tissue and emphysema in the lungs of transgenic mice inducibly expressing tumor necrosis factor-alpha. Am. J. Respir. Cell Mol. Biol. 2004, 30, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Libura, J.; Bettens, F.; Radkowski, A.; Tiercy, J.M.; Piguet, P.F. Risk of chemotherapy-induced pulmonary fibrosis is associated with polymorphic tumour necrosis factor-a2 gene. Eur. Respir. J. 2002, 19, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Han, S.; Xu, Y.; Li, Y.; Wang, H.; Yang, L.J.; Reeves, W.H. Toll-like receptor 7-stimulated tumor necrosis factor alpha causes bone marrow damage in systemic lupus erythematosus. Arthritis Rheumatol. 2014, 66, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Beech, J.T.; Thompson, S.J. Anti-tumour necrosis factor therapy ameliorates joint disease in a chronic model of inflammatory arthritis. Br. J. Rheumatol. 1997, 36, 1129. [Google Scholar] [CrossRef]
- Tsuda, Y.; Takahashi, H.; Kobayashi, M.; Hanafusa, T.; Herndon, D.N.; Suzuki, F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 2004, 21, 215–226. [Google Scholar] [CrossRef]
- Bao, J.; Pan, G.; Poncz, M.; Wei, J.; Ran, M.; Zhou, Z. Serpin functions in host-pathogen interactions. PeerJ 2018, 6, e4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.J.; Kim, S.; Choi, Y.; Nielsen, T.B.; Yan, J.; Lu, A.; Ruan, J.; Lee, H.R.; Wu, H.; Spellberg, B.; et al. SERPINB1-mediated checkpoint of inflammatory caspase activation. Nat. Immunol. 2019, 20, 276–287. [Google Scholar] [CrossRef]
- Cameron, C.; Hota-Mitchell, S.; Chen, L.; Barrett, J.; Cao, J.X.; Macaulay, C.; Willer, D.; Evans, D.; McFadden, G. The complete DNA sequence of myxoma virus. Virology 1999, 264, 298–318. [Google Scholar] [CrossRef]
- Macen, J.L.; Garner, R.S.; Musy, P.Y.; Brooks, M.A.; Turner, P.C.; Moyer, R.W.; McFadden, G.; Bleackley, R.C. Differential inhibition of the Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc. Natl. Acad. Sci. USA 1996, 93, 9108–9113. [Google Scholar] [CrossRef]
- Lucas, A.; Liu, L.; Dai, E.; Bot, I.; Viswanathan, K.; Munuswamy-Ramunujam, G.; Davids, J.A.; Bartee, M.Y.; Richardson, J.; Christov, A.; et al. The serpin saga; development of a new class of virus derived anti-inflammatory protein immunotherapeutics. Adv. Exp. Med. Biol. 2009, 666, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Yaron, J.R.; Zhang, L.; Ambadapadi, S. Overview of serpins and their roles in biological systems. Methods Mol. Biol. 2018, 1826, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Yaron, J.R.; Zhang, L.; Macaulay, C.; McFadden, G. Serpins: Development for therapeutic applications. Methods Mol. Biol. 2018, 1826, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.; Richardson, J.; Togonu-Bickersteth, B.; Dai, E.; Liu, L.; Vatsya, P.; Sun, Y.M.; Yu, J.; Munuswamy-Ramanujam, G.; Baker, H.; et al. Myxoma viral serpin, Serp-1, inhibits human monocyte adhesion through regulation of actin-binding protein filamin B. J. Leukoc. Biol. 2009, 85, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Brahn, E.; Lee, S.; Lucas, A.; McFadden, G.; Macaulay, C. Suppression of collagen-induced arthritis with a serine proteinase inhibitor (serpin) derived from myxoma virus. Clin. Immunol. 2014, 153, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Chen, H.; Bartee, M.Y.; Williams, J.; Davids, J.A.; Lomas, D.A.; McFadden, G.; Lucas, A.R. Myxomaviral anti-inflammatory serpin reduces myeloid-derived suppressor cells and human pancreatic cancer cell growth in mice. J. Cancer Sci. Ther. 2013, 5, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, D.; Abbott, J.; Liu, L.; Bartee, M.Y.; Long, M.; Davids, J.; Williams, J.; Feldmann, H.; Strong, J.; et al. Myxomavirus-derived serpin prolongs survival and reduces inflammation and hemorrhage in an unrelated lethal mouse viral infection. Antimicrob. Agents Chemother. 2013, 57, 4114–4127. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; L’Allier, P.L.; Gregoire, J.; Ibrahim, R.; McFadden, G.; Kostuk, W.; Knudtson, M.; Labinaz, M.; Waksman, R.; Pepine, C.J.; et al. A randomized controlled, phase 2 trial of the viral serpin Serp-1 in patients with acute coronary syndromes undergoing percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2010, 3, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Lu, Y.; Elshikha, A.S. In vivo analysis of alpha-1-antitrypsin functions in autoimmune disease models. Methods Mol. Biol. 2018, 1826, 143–155. [Google Scholar] [CrossRef]
- Baraldo, S.; Turato, G.; Lunardi, F.; Bazzan, E.; Schiavon, M.; Ferrarotti, I.; Molena, B.; Cazzuffi, R.; Damin, M.; Balestro, E.; et al. Immune activation in alpha1-antitrypsin-deficiency emphysema. Beyond the protease-antiprotease paradigm. Am. J. Respir. Crit. Care Med. 2015, 191, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; DiCiaccio, B.; Li, Y.; Elshikha, A.S.; Titov, D.; Brenner, B.; Seifer, L.; Pan, H.; Karic, N.; Akbar, M.A.; et al. Anti-inflammaging effects of human alpha-1 antitrypsin. Aging Cell 2018, 17, e12694. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Al-Omari, M.; Maegel, L.; Muller, M.; Izykowski, N.; Hong, J.; Hong, K.; Kim, S.H.; Dorsch, M.; Mahadeva, R.; et al. Anti-inflammatory and immunomodulatory properties of alpha1-antitrypsin without inhibition of elastase. Proc. Natl. Acad. Sci. USA 2013, 110, 15007–15012. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.A.; Nardo, D.; Chen, M.J.; Elshikha, A.S.; Ahamed, R.; Elsayed, E.M.; Bigot, C.; Holliday, L.S.; Song, S. Alpha-1 antitrypsin inhibits RANKL-induced osteoclast formation and functions. Mol. Med. 2017, 23, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Janciauskiene, S.M.; Bals, R.; Koczulla, R.; Vogelmeier, C.; Kohnlein, T.; Welte, T. The discovery of alpha1-antitrypsin and its role in health and disease. Respir. Med. 2011, 105, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cai, Q.; Oh, Y. Therapeutic potential of alpha-1 antitrypsin in human disease. Ann. Pediatr. Endocrinol. Metab. 2018, 23, 131–135. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Reeves, E.P.; McElvaney, N.G. The role of neutrophils in alpha-1 antitrypsin deficiency. Ann. Am. Thorac. Soc. 2016, 13, S297–S304. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.A.; Reeves, E.P.; Hurley, K.; Wolfe, R.; Jameel, R.; Fitzgerald, S.; McElvaney, N.G. The circulating proteinase inhibitor alpha-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci. Transl. Med. 2014, 6, 217ra1. [Google Scholar] [CrossRef]
- Elshikha, A.S.; Lu, Y.; Chen, M.J.; Akbar, M.; Zeumer, L.; Ritter, A.; Elghamry, H.; Mahdi, M.A.; Morel, L.; Song, S. Alpha 1 antitrypsin inhibits dendritic cell activation and attenuates nephritis in a mouse model of lupus. PLoS ONE 2016, 11, e0156583. [Google Scholar] [CrossRef] [PubMed]
- Elshikha, A.S.; Yuan, Y.; Lu, Y.; Chen, M.J.; Abboud, G.; Akbar, M.A.; Plate, H.; Wolney, H.; Hoffmann, T.; Tagari, E.; et al. Alpha 1 antitrypsin gene therapy extends the lifespan of lupus-prone mice. Mol. Ther. Methods Clin. Dev. 2018, 11, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Borel, F.; Sun, H.; Zieger, M.; Cox, A.; Cardozo, B.; Li, W.; Oliveira, G.; Davis, A.; Gruntman, A.; Flotte, T.R.; et al. Editing out five Serpina1 paralogs to create a mouse model of genetic emphysema. Proc. Natl. Acad. Sci. USA 2018, 115, 2788–2793. [Google Scholar] [CrossRef]
- Lu, Y.; Song, S. Distinct immune responses to transgene products from rAAV1 and rAAV8 vectors. Proc. Natl. Acad. Sci. USA 2009, 106, 17158–17162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimstein, C.; Choi, Y.K.; Wasserfall, C.H.; Satoh, M.; Atkinson, M.A.; Brantly, M.L.; Campbell-Thompson, M.; Song, S. Alpha-1 antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model. J. Transl. Med. 2011, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Goudy, K.; Campbell-Thompson, M.; Wasserfall, C.; Scott-Jorgensen, M.; Wang, J.; Tang, Q.; Crawford, J.M.; Ellis, T.M.; Atkinson, M.A.; et al. Recombinant adeno-associated virus-mediated alpha-1 antitrypsin gene therapy prevents type I diabetes in NOD mice. Gene Ther. 2004, 11, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, B.; Lu, Y.; Jorgensen, M.; Petersen, B.; Song, S. Adipose tissue-derived mesenchymal stem cell-based liver gene delivery. J. Hepatol. 2011, 54, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucchini, N.; Bessou, G.; Robbins, S.H.; Chasson, L.; Raper, A.; Crocker, P.R.; Dalod, M. Individual plasmacytoid dendritic cells are major contributors to the production of multiple innate cytokines in an organ-specific manner during viral infection. Int. Immunol. 2008, 20, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Petrache, I.; Hajjar, J.; Campos, M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biol. Targets Ther. 2009, 3, 193–204. [Google Scholar] [CrossRef]
- Lu, Y.; Tang, M.; Wasserfall, C.; Kou, Z.; Campbell-Thompson, M.; Gardemann, T.; Crawford, J.; Atkinson, M.; Song, S. Alpha1-antitrypsin gene therapy modulates cellular immunity and efficiently prevents type 1 diabetes in nonobese diabetic mice. Hum. Gene Ther. 2006, 17, 625–634. [Google Scholar] [CrossRef]
- Ma, H.; Lu, Y.; Li, H.; Campbell-Thompson, M.; Parker, M.; Wasserfall, C.; Haller, M.; Brantly, M.; Schatz, D.; Atkinson, M.; et al. Intradermal alpha1-antitrypsin therapy avoids fatal anaphylaxis, prevents type 1 diabetes and reverses hyperglycaemia in the NOD mouse model of the disease. Diabetologia 2010, 53, 2198–2204. [Google Scholar] [CrossRef]
- Grimstein, C.; Choi, Y.K.; Satoh, M.; Lu, Y.; Wang, X.; Campbell-Thompson, M.; Song, S. Combination of alpha-1 antitrypsin and doxycycline suppresses collagen-induced arthritis. J. Gene Med. 2010, 12, 35–44. [Google Scholar] [CrossRef]
- Moldthan, H.L.; Hirko, A.C.; Thinschmidt, J.S.; Grant, M.B.; Li, Z.; Peris, J.; Lu, Y.; Elshikha, A.S.; King, M.A.; Hughes, J.A.; et al. Alpha 1-antitrypsin therapy mitigated ischemic stroke damage in rats. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, e355–e363. [Google Scholar] [CrossRef]
- Cao, J.J.; Gregoire, B.R.; Sun, L.; Song, S. Alpha-1 antitrypsin reduces ovariectomy-induced bone loss in mice. Ann. N. Y. Acad. Sci. 2011, 1240, E31–E35. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.A.; Cao, J.J.; Lu, Y.; Nardo, D.; Chen, M.J.; Elshikha, A.S.; Ahamed, R.; Brantly, M.; Holliday, L.S.; Song, S. Alpha-1 antitrypsin gene therapy ameliorates bone loss in ovariectomy-induced osteoporosis mouse model. Hum. Gene Ther. 2016, 27, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.A.; Lu, Y.; Elshikha, A.S.; Chen, M.J.; Yuan, Y.; Whitley, E.M.; Holliday, L.S.; Chang, L.J.; Song, S. Transplantation of Adipose Tissue-Derived Mesenchymal Stem Cell (ATMSC) Expressing alpha-1 antitrypsin reduces bone loss in ovariectomized osteoporosis mice. Hum. Gene Ther. 2017, 28, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, A.M.; Karoopongse, E.; Lesnikova, M.; Margineantu, D.; Welte, T.; Dinarello, C.A.; Hockenbery, D.; Janciauskiene, S.; Deeg, H.J. alpha-1-antitrypsin (AAT)-modified donor cells suppress GVHD but enhance the GVL effect: A role for mitochondrial bioenergetics. Blood 2014, 124, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Magenau, J.M.; Goldstein, S.C.; Peltier, D.; Soiffer, R.J.; Braun, T.; Pawarode, A.; Riwes, M.M.; Kennel, M.; Antin, J.H.; Cutler, C.S.; et al. alpha1-Antitrypsin infusion for treatment of steroid-resistant acute graft-versus-host disease. Blood 2018, 131, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.A.; Reeves, E.P.; Meleady, P.; Henry, M.; McElvaney, O.J.; Carroll, T.P.; Condron, C.; Chotirmall, S.H.; Clynes, M.; O’Neill, S.J.; et al. alpha-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J. Clin. Investig. 2010, 120, 4236–4250. [Google Scholar] [CrossRef] [PubMed]
- Hadeiba, H.; Sato, T.; Habtezion, A.; Oderup, C.; Pan, J.; Butcher, E.C. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 2008, 9, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C.; Mizrahi, M.; Toledano, M.; Defelice, N.; Wright, J.L.; Churg, A.; Shapiro, L.; Dinarello, C.A. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 16236–16241. [Google Scholar] [CrossRef]
- Song, S. Alpha-1 Antitrypsin Therapy for Autoimmune Disorders. Chronic. Obstr. Pulm. Dis. 2018, 5, 289–301. [Google Scholar] [CrossRef] [Green Version]
- O’Dwyer, C.A.; O’Brien, M.E.; Wormald, M.R.; White, M.M.; Banville, N.; Hurley, K.; McCarthy, C.; McElvaney, N.G.; Reeves, E.P. The BLT1 Inhibitory Function of alpha-1 Antitrypsin Augmentation Therapy Disrupts Leukotriene B4 Neutrophil Signaling. J. Immunol. 2015, 195, 3628–3641. [Google Scholar] [CrossRef]
- Silverman, G.A.; Bird, P.I.; Carrell, R.W.; Church, F.C.; Coughlin, P.B.; Gettins, P.G.; Irving, J.A.; Lomas, D.A.; Luke, C.J.; Moyer, R.W.; et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001, 276, 33293–33296. [Google Scholar] [CrossRef] [PubMed]
- Bergin, D.A.; Hurley, K.; McElvaney, N.G.; Reeves, E.P. Alpha-1 antitrypsin: A potent anti-inflammatory and potential novel therapeutic agent. Arch. Immunol. Ther. Exp. 2012, 60, 81–97. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elshikha, A.S.; Abboud, G.; van der Meijden-Erkelens, L.; Lu, Y.; Chen, M.-J.; Yuan, Y.; Ponjee, G.; Zeumer, L.; Satoh, M.; Morel, L.; et al. Alpha-1-Antitrypsin Ameliorates Pristane Induced Diffuse Alveolar Hemorrhage in Mice. J. Clin. Med. 2019, 8, 1341. https://doi.org/10.3390/jcm8091341
Elshikha AS, Abboud G, van der Meijden-Erkelens L, Lu Y, Chen M-J, Yuan Y, Ponjee G, Zeumer L, Satoh M, Morel L, et al. Alpha-1-Antitrypsin Ameliorates Pristane Induced Diffuse Alveolar Hemorrhage in Mice. Journal of Clinical Medicine. 2019; 8(9):1341. https://doi.org/10.3390/jcm8091341
Chicago/Turabian StyleElshikha, Ahmed S., Georges Abboud, Lonneke van der Meijden-Erkelens, Yuanqing Lu, Mong-Jen Chen, Ye Yuan, Godelieva Ponjee, Leilani Zeumer, Minoru Satoh, Laurence Morel, and et al. 2019. "Alpha-1-Antitrypsin Ameliorates Pristane Induced Diffuse Alveolar Hemorrhage in Mice" Journal of Clinical Medicine 8, no. 9: 1341. https://doi.org/10.3390/jcm8091341



