Abducens Nerve Palsy as Initial Presentation of Multiple Myeloma and Intracranial Plasmacytoma
Abstract
:1. Introduction
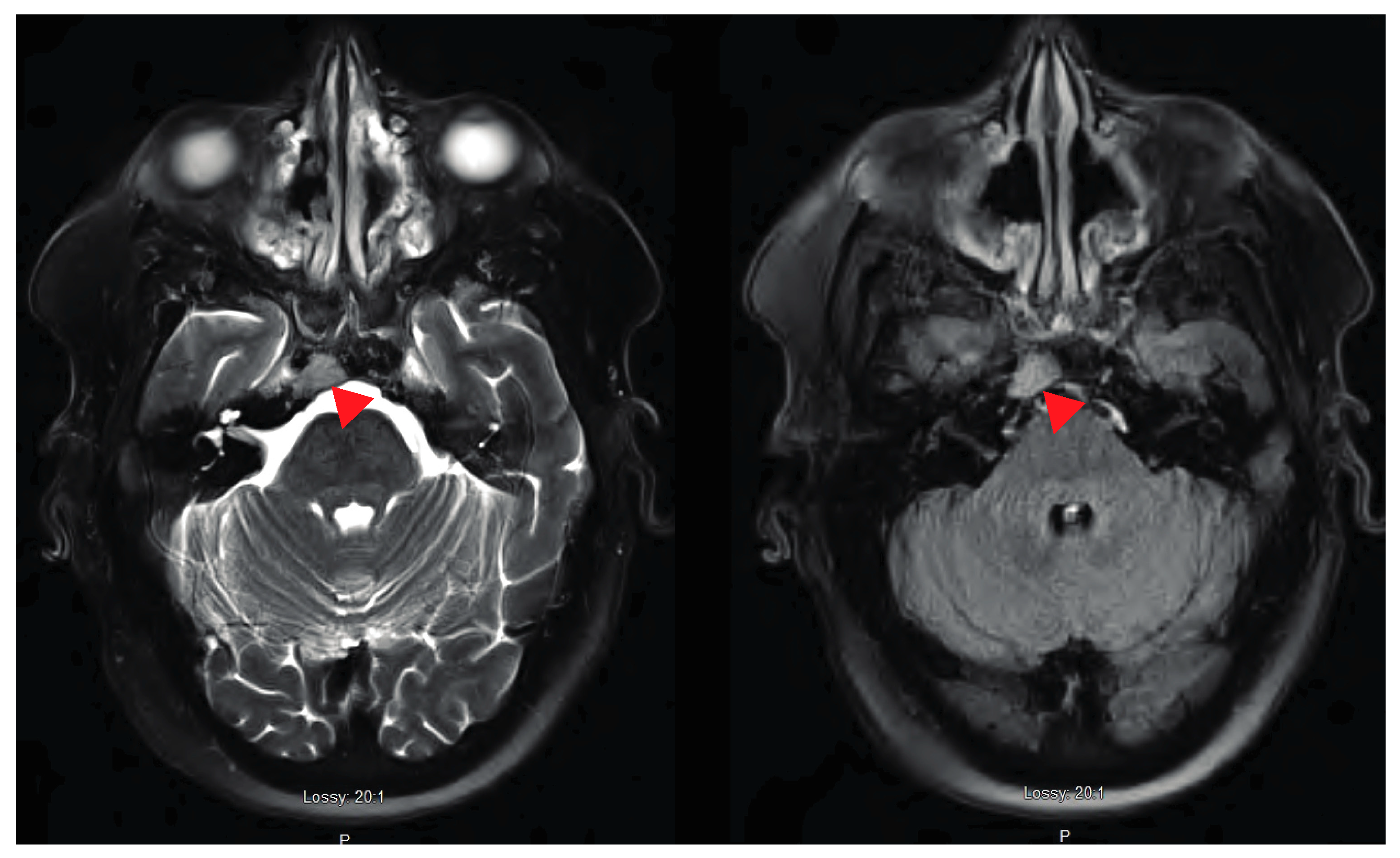
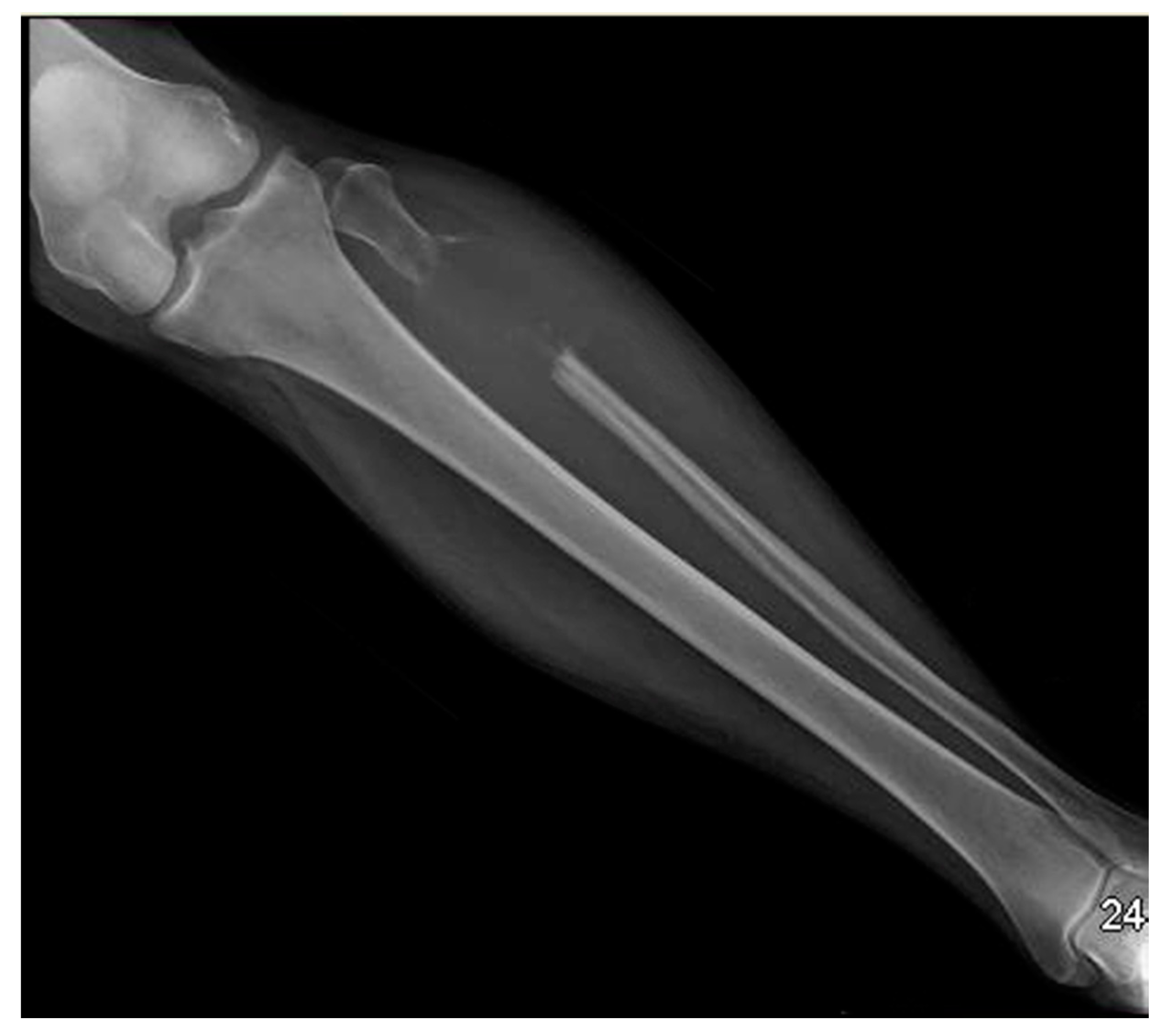
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Consent
References
- Sobol, U.; Stiff, P. Neurologic aspects of plasma cell disorders. Handb. Clin. Neurol. 2014, 120, 1083–1099. [Google Scholar]
- Fassas, A.B.; Ward, S.; Muwalla, F.; Van Hemert, R.; Schluterman, K.; Harik, S.; Harik, S.; Tricot, G. Myeloma of the central nervous system: Strong association with unfavorable chromosomal abnormalities and other high-risk disease features. Leuk. Lymphoma 2004, 45, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Na’ara, S.; Amit, M.; Gil, Z.; Billan, S. Plasmacytoma of the Skull Base: A Meta-Analysis. J. Neurol. Surg. B Skull Base 2016, 77, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef] [Green Version]
- Rajkumar, S.V. Evolving diagnostic criteria for multiple myeloma. Hematol. Am. Soc. Hematol. Educ. Program. 2015, 2015, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajkumar, S.V.; Dispenzieri, A.; Kyle, R.A. Monoclonal gammopathy of undetermined significance, Waldenstrom macroglobulinemia, AL amyloidosis, and related plasma cell disorders: Diagnosis and treatment. Mayo Clin. Proc. 2006, 81, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, E. Cranial and intracranial myelomas. Brain 1954, 77, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Farsi, K. Multiple myeloma: An update. Oman Med. J. 2013, 28, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Soutar, R.; Lucraft, H.; Jackson, G.; Reece, A.; Bird, J.; Low, E.; Samson, D. Guidelines Working Group of the UK Myeloma Forum; British Committee for Standards in Haematology; British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Br. J. Haematol. 2004, 124, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Amita, R.; Sandhyamani, S.; Nair, S.; Kapilamoorthy, T.R. Plasmacytoma of the clivus. Asian J. Neurosurg. 2017, 12, 573–575. [Google Scholar] [PubMed]
- Omoti, A.E.; Omoti, C.E. Ophthalmic manifestations of multiple myeloma. West Afr. J. Med. 2007, 26, 265–268. [Google Scholar] [PubMed]
- Kashyap, R.; Kumar, R.; Kumar, S. Cranial nerve palsy in multiple myeloma and solitary plasmacytoma. Asia Pac. J. Clin. Oncol. 2010, 6, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Blade, J.; Fernandez de Larrea, C.; Rosinol, L.; Cibeira, M.T.; Jimenez, R.; Powles, R. Soft-tissue plasmacytomas in multiple myeloma: Incidence, mechanisms of extramedullary spread, and treatment approach. J. Clin. Oncol. 2011, 29, 3805–3812. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Rajkumar, S.V.; Ketterling, R.P.; Greipp, P.T.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Hayman, S.R.; Hwa, Y.L.; et al. Prognostic implications of abnormalities of chromosome 13 and the presence of multiple cytogenetic high-risk abnormalities in newly diagnosed multiple myeloma. Blood Cancer. J. 2017, 7, e600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.M.; Rajkumar, S.V. Interpretation of cytogenetic results in multiple myeloma for clinical practice. Blood Cancer. J. 2015, 5, e365. [Google Scholar] [CrossRef] [PubMed]
- Bryce, A.H.; Ketterling, R.P.; Gertz, M.A.; Lacy, M.; Knudson, R.A.; Zeldenrust, S.; Kumar, S.; Hayman, S.; Buadi, F.; Kyle, R.A.; et al. Translocation t(11;14) and survival of patients with light chain (AL) amyloidosis. Haematologica 2009, 94, 380–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, F.; Colla, S.; Wu, X.; Chen, B.; Stewart, J.P.; Kuehl, W.M.; Barlogie, B.; Shaughnessy, J.D., Jr. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2- and p27Kip1-dependent and -independent mechanisms. Blood 2007, 109, 4995–5001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durie, B.G.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibekwe, E.; Horsley, N.B.; Jiang, L.; Achenjang, N.-S.; Anudu, A.; Akhtar, Z.; Chornenka, K.G.; Monohan, G.P.; Chornenkyy, Y.G. Abducens Nerve Palsy as Initial Presentation of Multiple Myeloma and Intracranial Plasmacytoma. J. Clin. Med. 2018, 7, 253. https://doi.org/10.3390/jcm7090253
Ibekwe E, Horsley NB, Jiang L, Achenjang N-S, Anudu A, Akhtar Z, Chornenka KG, Monohan GP, Chornenkyy YG. Abducens Nerve Palsy as Initial Presentation of Multiple Myeloma and Intracranial Plasmacytoma. Journal of Clinical Medicine. 2018; 7(9):253. https://doi.org/10.3390/jcm7090253
Chicago/Turabian StyleIbekwe, Elochukwu, Neil B. Horsley, Lan Jiang, Nadine-Stella Achenjang, Azubuogu Anudu, Zeeshan Akhtar, Karina G. Chornenka, Gregory P. Monohan, and Yevgen G. Chornenkyy. 2018. "Abducens Nerve Palsy as Initial Presentation of Multiple Myeloma and Intracranial Plasmacytoma" Journal of Clinical Medicine 7, no. 9: 253. https://doi.org/10.3390/jcm7090253
APA StyleIbekwe, E., Horsley, N. B., Jiang, L., Achenjang, N.-S., Anudu, A., Akhtar, Z., Chornenka, K. G., Monohan, G. P., & Chornenkyy, Y. G. (2018). Abducens Nerve Palsy as Initial Presentation of Multiple Myeloma and Intracranial Plasmacytoma. Journal of Clinical Medicine, 7(9), 253. https://doi.org/10.3390/jcm7090253




