Association between Proton Pump Inhibitor Use and CNS Infection Risk: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Population
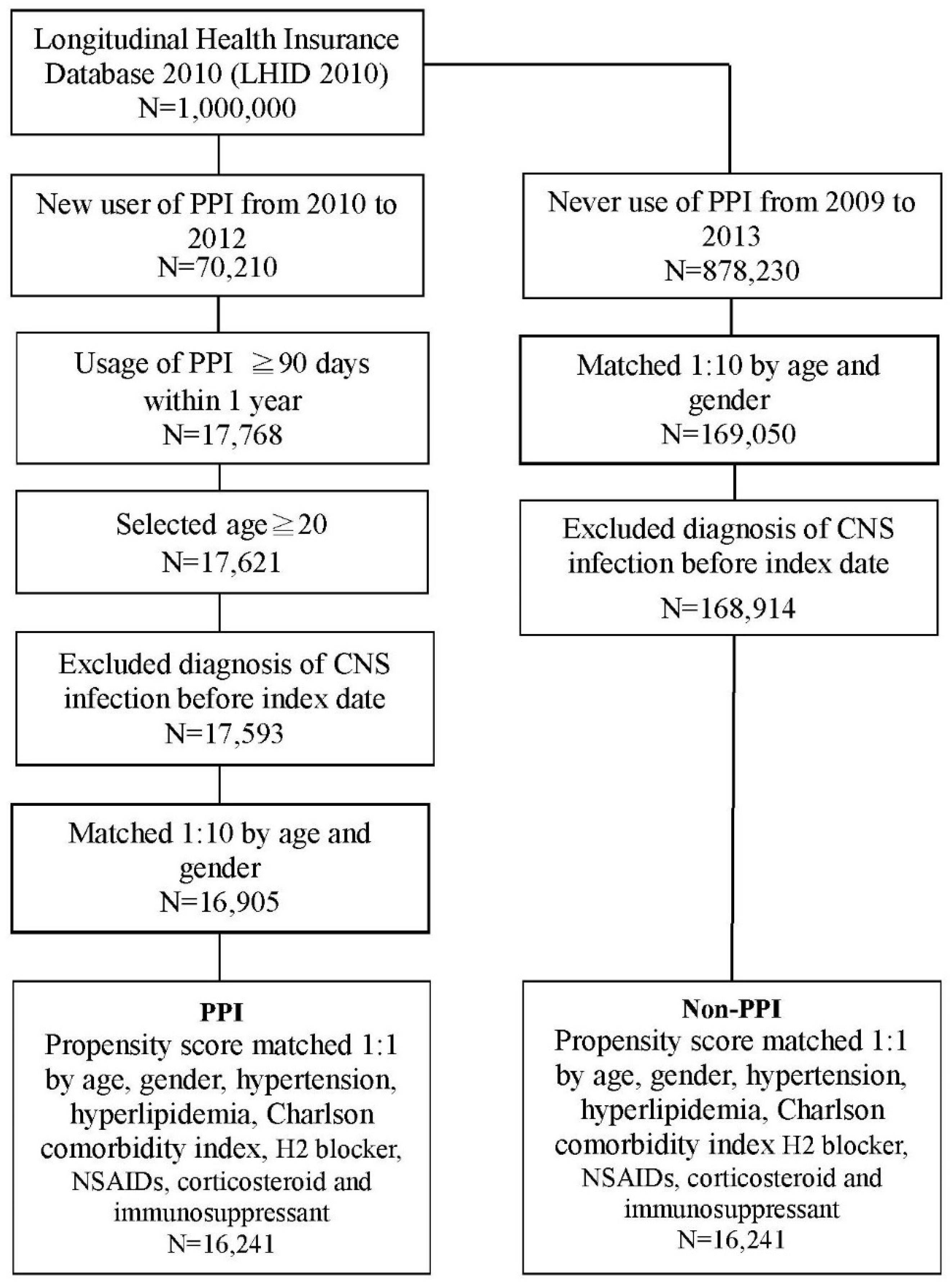
2.2. Study Design
2.3. Outcome Measurement
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
3.2. Risk of CNS Infection in PPI Users
3.3. Risk of CNS Infection in PPI Users and Nonusers and Subgroup Specific Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ward, R.M.; Kearns, G.L. Proton pump inhibitors in pediatrics: Mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr. Drugs 2013, 15, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Strand, D.S.; Kim, D.; Peura, D.A. 25 years of proton pump inhibitors: A comprehensive review. Gut Liver 2017, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Sachs, G.; Shin, J.M.; Howden, C.W. Review article: The clinical pharmacology of proton pump inhibitors. Aliment. Pharmacol. Ther. 2006, 23 (Suppl. 2), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Corleto, V.D.; Annibale, B.; Gibril, F.; Angeletti, S.; Serrano, J.; Venzon, D.J.; Delle Fave, G.; Jensen, R.T. Does the widespread use of proton pump inhibitors mask, complicate and/or delay the diagnosis of Zollinger-Ellison syndrome? Aliment. Pharmacol. Ther. 2001, 15, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Altman, K.W.; Haines, G.K.; Hammer, N.D.; Radosevich, J.A. The H+/K+-Atpase (proton) pump is expressed in human laryngeal submucosal glands. Laryngoscope 2003, 113, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Barbuskaite, D.; Tozzi, M.; Giannuzzo, A.; Sorensen, C.E.; Novak, I. Proton pump inhibitors inhibit pancreatic secretion: Role of gastric and non-gastric H+/K+-Atpases. PLoS ONE 2015, 10, e0126432. [Google Scholar] [CrossRef] [PubMed]
- Shiraev, T.P.; Bullen, A. Proton pump inhibitors and cardiovascular events: A systematic review. Heart Lung Circ. 2018, 27, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xie, Y.; Al-Aly, Z. The association of proton pump inhibitors and chronic kidney disease: Cause or confounding? Curr. Opin. Nephrol. Hypertens. 2018, 27, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Stanciu, C.; Girleanu, I.; Stoica, O.C.; Singeap, A.M.; Maxim, R.; Chiriac, S.A.; Ciobica, A.; Boiculese, L. Proton pump inhibitors therapy and risk of clostridium difficile infection: Systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 6500–6515. [Google Scholar] [CrossRef] [PubMed]
- Hafiz, R.A.; Wong, C.; Paynter, S.; David, M.; Peeters, G. The risk of community-acquired enteric infection in proton pump inhibitor therapy: Systematic review and meta-analysis. Ann. Pharmacother. 2018, 52, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.W.; Tsai, M.C.; Teng, Y.H.; Yeh, Y.T.; Wang, Y.H.; Yang, S.F.; Yeh, C.B. Population-based cohort study on the risk of pneumonia in patients with non-traumatic intracranial haemorrhage who use proton pump inhibitors. BMJ Open 2014, 4, e006710. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.W.; Teng, Y.H.; Yang, S.F.; Yeh, H.W.; Wang, Y.H.; Chou, M.C.; Yeh, C.B. Association of proton pump inhibitors usage with risk of pneumonia in dementia patients. J. Am. Geriatr. Soc. 2017, 65, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Fallahzadeh, M.K.; Borhani Haghighi, A.; Namazi, M.R. Proton pump inhibitors: Predisposers to alzheimer disease? J. Clin. Pharm. Ther. 2010, 35, 125–126. [Google Scholar] [CrossRef] [PubMed]
- Badiola, N.; Alcalde, V.; Pujol, A.; Munter, L.M.; Multhaup, G.; Lleo, A.; Coma, M.; Soler-Lopez, M.; Aloy, P. The proton-pump inhibitor lansoprazole enhances amyloid beta production. PLoS ONE 2013, 8, e58837. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, F.C.; Steenland, K.; Zhao, L.; Wharton, W.; Levey, A.I.; Hajjar, I. Proton pump inhibitors and risk of mild cognitive impairment and dementia. J. Am. Geriatr. Soc. 2017, 65, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.F.; Chen, M.H.; Wang, Y.P.; Chu, C.J.; Huang, Y.H.; Lin, H.C.; Hou, M.C.; Lee, F.Y.; Su, T.P.; Lu, C.L. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology 2017, 152, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Riddell, J.T.; Shuman, E.K. Epidemiology of central nervous system infection. Neuroimaging Clin. N. Am. 2012, 22, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.; Thwaites, G. Infections of the nervous system. Pract. Neurol. 2011, 11, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Agastya, G.; West, B.C.; Callahan, J.M. Omeprazole inhibits phagocytosis and acidification of phagolysosomes of normal human neutrophils in vitro. Immunopharmacol. Immunotoxicol. 2000, 22, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, K.; Akamatsu, H.; Nishina, K.; Niwa, Y. Effects of pirenzepine, omeprazole, lansoprazole, and rabeprazole on human neutrophil functions. Can. J. Anaesth. 2001, 48, 421–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zedtwitz-Liebenstein, K.; Wenisch, C.; Patruta, S.; Parschalk, B.; Daxbock, F.; Graninger, W. Omeprazole treatment diminishes intra-and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit. Care Med. 2002, 30, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, I.; Frances, R.; Zapater, P.; Gimenez, P.; Gomez-Hurtado, I.; Moratalla, A.; Lozano-Ruiz, B.; Bellot, P.; Gonzalez-Navajas, J.M.; Such, J. Use of proton pump inhibitors decrease cellular oxidative burst in patients with decompensated cirrhosis. J. Gastroenterol. Hepatol. 2015, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Siegrist, H.; Frei, R.; Froehlich, F.; Duroux, P.; Thorens, J.; Blum, A.; Bille, J.; Gonvers, J.J.; Gyr, K. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut 1994, 35, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Thorens, J.; Froehlich, F.; Schwizer, W.; Saraga, E.; Bille, J.; Gyr, K.; Duroux, P.; Nicolet, M.; Pignatelli, B.; Blum, A.L.; et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: A prospective randomised double blind study. Gut 1996, 39, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Adriani, K.S.; Brouwer, M.C.; van de Beek, D. Risk factors for community-acquired bacterial meningitis in adults. Neth. J. Med. 2015, 73, 53–60. [Google Scholar] [PubMed]
- Lundbo, L.F.; Benfield, T. Risk factors for community-acquired bacterial meningitis. Infect. Dis. 2017, 49, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Gowin, E.; Januszkiewicz-Lewandowska, D. Genes and their single nucleotide polymorphism involved in innate immune response in central nervous system in bacterial meningitis: Review of literature data. Inflamm. Res. 2018, 67, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, B.; von Holt, K.; Wiese, B.; Prokein, J.; Lange, C.; Ernst, A.; Brettschneider, C.; Konig, H.H.; Werle, J.; Weyerer, S.; et al. Risk of dementia in elderly patients with the use of proton pump inhibitors. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Panjawatanan, P.; Ungprasert, P. Proton pump inhibitors and risk of dementia. Ann. Transl. Med. 2016, 4, 240. [Google Scholar] [CrossRef] [PubMed]
- Tai, S.Y.; Chien, C.Y.; Wu, D.C.; Lin, K.D.; Ho, B.L.; Chang, Y.H.; Chang, Y.P. Risk of dementia from proton pump inhibitor use in Asian population: A nationwide cohort study in Taiwan. PLoS ONE 2017, 12, e0171006. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Guerrero, G.; Amador-Munoz, D.; Calderon-Ospina, C.A.; Lopez-Fuentes, D.; Nava Mesa, M.O. Proton pump inhibitors and dementia: Physiopathological mechanisms and clinical consequences. Neural Plast. 2018, 2018, 5257285. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.C.; Chang, J.; Park, S.M. A nationwide population-based cohort study of dementia risk among acid suppressant users. Am. J. Geriatr. Psychiatry 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, R.; Gilmartin, J.F.; Kemp, W.; Hopper, I.; Liew, D. Dementia, cognitive impairment and proton pump inhibitor therapy: A systematic review. J. Gastroenterol. Hepatol. 2017, 32, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Baker, S.S.; Trinidad, J.; Burlingame, A.L.; Baker, R.D.; Forte, J.G.; Virtuoso, L.P.; Egilmez, N.K.; Zhu, L. Inhibition of lysosomal enzyme activities by proton pump inhibitors. J. Gastroenterol. 2013, 48, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Peddicord, T.E.; Olsen, K.M.; Collier, D.S. Effect of omeprazole, lansoprazole, and ranitidine on the DNA synthesis of mononuclear cells. Crit. Care Med. 1999, 27, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Arakawa, T. Lansoprazole decreases peripheral blood monocytes and intercellular adhesion molecule-1-positive mononuclear cells. Dig. Dis. Sci. 1999, 44, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoorn, M.M.C.; Tett, S.E.; de Vries, O.J.; Dobson, A.J.; Peeters, G. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone 2015, 81, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Park, E.; Ahn, S.B.; Jo, Y.K.; Son, B.; Kim, S.H.; Park, Y.S.; Kim, H.J. A proton pump inhibitor’s effect on bone metabolism mediated by osteoclast action in old age: A prospective randomized study. Gut Liver 2015, 9, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.N.; Johansen, P.B.; Abrahamsen, B. Proton pump inhibitors and osteoporosis. Curr. Opin. Rheumatol. 2016, 28, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Reis, S.; Teixeira, S.; Lopes, S.; Fernandes, M.H. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J. 2013, 280, 5052–5064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prause, M.; Seeliger, C.; Unger, M.; Rosado Balmayor, E.; van Griensven, M.; Haug, A.T. Pantoprazole decreases cell viability and function of human osteoclasts in vitro. Mediat. Inflamm. 2015, 2015, 413097. [Google Scholar] [CrossRef] [PubMed]
- Motegi, H.; Abe, S.; Tansho, S.; Suzuki, D.; Yamaguchi, H.; Hoshino, E. Suppressive effect of lansoprazole on anti-Candida activity of murine macrophages. Kansenshogaku Zasshi 2001, 75, 137–143. [Google Scholar] [CrossRef] [PubMed]

| Unmatched | Matched | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PPI (N = 16,905) | Non-PPI (N = 168,914) | PPI (N = 16,241) | Non-PPI (N = 16,241) | |||||||
| n | % | n | % | p-Value | n | % | n | % | p-Value | |
| Age | 1 | 0.144 | ||||||||
| 20–40 | 2301 | 13.6 | 22,999 | 13.6 | 2123 | 13.1 | 2005 | 12.3 | ||
| 40–65 | 9029 | 53.4 | 90,223 | 53.4 | 8557 | 52.7 | 8637 | 53.2 | ||
| ≥65 | 5575 | 33.0 | 55,692 | 33.0 | 5561 | 34.2 | 5599 | 34.5 | ||
| Mean ± SD | 57.8 ± 15.3 | 57.8 ± 15.3 | 0.977 | 58.2 ± 15.3 | 59.6 ± 14.9 | <0.001 ** | ||||
| Gender | 0.997 | 0.991 | ||||||||
| Female | 8242 | 48.8 | 82,356 | 48.8 | 7911 | 48.7 | 7912 | 48.7 | ||
| Male | 8663 | 51.2 | 86,558 | 51.2 | 8330 | 51.3 | 8329 | 51.3 | ||
| Hypertension | 6579 | 38.9 | 45726 | 27.1 | <0.001 ** | 6399 | 39.4 | 6393 | 39.4 | 0.946 |
| Hyperlipidemia | 3283 | 19.4 | 21,316 | 12.6 | <0.001 ** | 3202 | 19.7 | 3165 | 19.5 | 0.605 |
| CCI † | <0.001 ** | 0.327 | ||||||||
| 0 | 4618 | 27.3 | 122,220 | 72.4 | 4618 | 28.4 | 4592 | 28.3 | ||
| 1 | 9146 | 54.1 | 35,275 | 20.9 | 8664 | 53.3 | 8780 | 54.1 | ||
| ≥2 | 3141 | 18.6 | 11,419 | 6.8 | 2959 | 18.2 | 2869 | 17.7 | ||
| H2 Blocker | 4354 | 25.8 | 6190 | 3.7 | <0.001 ** | 3690 | 22.7 | 3683 | 22.7 | 0.926 |
| NSAIDs | 2309 | 13.7 | 11,587 | 6.9 | <0.001 ** | 2165 | 13.3 | 2283 | 14.1 | 0.057 |
| Corticosteroid | 2576 | 15.2 | 7651 | 4.5 | <0.001 ** | 2368 | 14.6 | 2457 | 15.1 | 0.165 |
| Immunosuppressant | 99 | 0.6 | 193 | 0.1 | <0.001 ** | 77 | 0.5 | 74 | 0.5 | 0.807 |
| Propensity score | ||||||||||
| Mean ± SD | 0.22 ± 0.2 | 0.08 ± 0.09 | 0.50 ± 0.01 | 0.50 ± 0.01 | ||||||
| Min, Max | 0.02, 0.93 | 0.02, 0.91 | 0.47, 0.54 | 0.47, 0.53 | ||||||
| No. of CNS Infection Event | Observed Person-Years | Incidence Density (Per 1000 Person-Years) | Crude HR | 95% CI | Adjusted HR ‡ | 95% CI | |
|---|---|---|---|---|---|---|---|
| PPI | |||||||
| No | 18 | 41,444 | 0.4 | 1 | 1 | ||
| Yes | 36 | 38,454 | 0.9 | 2.14 ** | 1.22–3.78 | 2.23 ** | 1.27–3.94 |
| Age | |||||||
| 20–40 | 5 | 10,235 | 0.5 | 1 | 1 | ||
| 40–65 | 30 | 42,944 | 0.7 | 1.43 | 0.56–3.69 | 1.25 | 0.47–3.32 |
| ≥65 | 19 | 26,718 | 0.7 | 1.46 | 0.54–3.90 | 0.95 | 0.32–2.83 |
| Gender | |||||||
| Female | 25 | 39,057 | 0.6 | 1 | 1 | ||
| Male | 29 | 40,841 | 0.7 | 1.11 | 0.65–1.89 | 1.08 | 0.63–1.84 |
| Hypertension | 22 | 31,116 | 0.7 | 1.08 | 0.63–1.85 | 0.91 | 0.50–1.66 |
| Hyperlipidemia | 8 | 15,831 | 0.5 | 0.70 | 0.33–1.49 | 0.63 | 0.29–1.35 |
| CCI † | |||||||
| 0 | 10 | 23,373 | 0.4 | 1 | 1 | ||
| 1 | 26 | 43,469 | 0.6 | 1.40 | 0.67–2.90 | 1.36 | 0.64–2.89 |
| ≥2 | 18 | 13,056 | 1.4 | 3.21 ** | 1.48–6.95 | 3.07 ** | 1.33–7.08 |
| H2 Blocker | 13 | 19,341 | 0.7 | 1.00 | 0.54–1.87 | 0.82 | 0.42–1.57 |
| NSAIDs | 14 | 13,025 | 1.1 | 1.85 * | 1.00–3.41 | 1.57 | 0.81–3.04 |
| Corticosteroid | 18 | 13,045 | 1.4 | 2.62 ** | 1.48–4.61 | 2.17 * | 1.17–4.03 |
| PPI | Non-PPI | |||||
|---|---|---|---|---|---|---|
| N | No. of CNS Infection Event | N | No. of CNS Infection Event | HR | 95% CI | |
| Age a | ||||||
| 20–40 | 2123 | 2 | 2005 | 3 | 0.59 | 0.10–3.57 |
| 40–65 | 8557 | 20 | 8637 | 10 | 2.23 * | 1.04–4.76 |
| ≥65 | 5561 | 14 | 5599 | 5 | 3.23 * | 1.16–8.99 |
| Gender b | ||||||
| Female | 7911 | 16 | 7912 | 9 | 1.98 | 0.88–4.50 |
| Male | 8330 | 20 | 8329 | 9 | 2.39 * | 1.09–5.28 |
| Hypertension c | ||||||
| No | 9842 | 19 | 9848 | 13 | 1.59 | 0.78–3.23 |
| Yes | 6399 | 17 | 6393 | 5 | 3.80 ** | 1.40–10.32 |
| Hyperlipidemia d | ||||||
| No | 13,039 | 30 | 13,076 | 16 | 2.09 * | 1.13–3.83 |
| Yes | 3202 | 6 | 3165 | 2 | 3.09 | 0.62–15.40 |
| Charlson comorbidity index e | ||||||
| 0 | 4618 | 6 | 4592 | 4 | 1.57 | 0.44–5.59 |
| 1 | 8664 | 18 | 8780 | 8 | 2.47 * | 1.07–5.70 |
| ≥2 | 2959 | 12 | 2869 | 6 | 2.25 | 0.84–6.04 |
| H2 Blocker f | ||||||
| No | 12,551 | 27 | 12,558 | 15 | 2.17 * | 1.14–4.15 |
| Yes | 3690 | 9 | 3683 | 3 | 2.34 | 0.71–7.68 |
| NSAIDs g | ||||||
| No | 14,076 | 25 | 13,958 | 15 | 1.83 | 0.96–3.48 |
| Yes | 2165 | 11 | 2283 | 3 | 4.08 * | 1.13–14.7 |
| Corticosteroid h | ||||||
| No | 13,873 | 23 | 13,784 | 13 | 1.92 | 0.97–3.80 |
| Yes | 2368 | 13 | 2457 | 5 | 2.91 * | 1.03–8.2 |
| Immunosuppressant i | ||||||
| No | 16,164 | 36 | 16,167 | 18 | 2.20 ** | 1.25–3.89 |
| Yes | 77 | 0 | 74 | 0 | NA | NA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, W.-T.; Teng, Y.-H.; Yang, S.-F.; Yeh, H.-W.; Yeh, Y.-T.; Wang, Y.-H.; Chou, M.-Y.; Chou, M.-C.; Chan, C.-H.; Yeh, C.-B. Association between Proton Pump Inhibitor Use and CNS Infection Risk: A Retrospective Cohort Study. J. Clin. Med. 2018, 7, 252. https://doi.org/10.3390/jcm7090252
Hung W-T, Teng Y-H, Yang S-F, Yeh H-W, Yeh Y-T, Wang Y-H, Chou M-Y, Chou M-C, Chan C-H, Yeh C-B. Association between Proton Pump Inhibitor Use and CNS Infection Risk: A Retrospective Cohort Study. Journal of Clinical Medicine. 2018; 7(9):252. https://doi.org/10.3390/jcm7090252
Chicago/Turabian StyleHung, Wei-Te, Ying-Hock Teng, Shun-Fa Yang, Han-Wei Yeh, Ying-Tung Yeh, Yu-Hsun Wang, Ming-Yung Chou, Ming-Chih Chou, Chi-Ho Chan, and Chao-Bin Yeh. 2018. "Association between Proton Pump Inhibitor Use and CNS Infection Risk: A Retrospective Cohort Study" Journal of Clinical Medicine 7, no. 9: 252. https://doi.org/10.3390/jcm7090252






